An Algorithm for Indoor SARS-CoV-2 Transmission
Abstract
:Introduction
- the ability of the viral particles to linger in the air for hours in closed spaces and to infect susceptible people even after the source (the infected person) left the space [10].
- the use of restriction on opening times for various locations such as stores, bars or other businesses in an attempt to reduce the contact time among people [11].
Discussion
Remark 1
Population dynamics, epidemiology assumptions and the general algorithm
Population dynamics assumptions
- ➢
- At all times there is at least one location open.
- ➢
- The population is split into groups by preference ranking of locations. That means, for each group there is a list of locations in decreasing order of preference. If a given location is closed, individuals in that group will consider going to the next location in that list if open and so on.
- ➢
- Each individual, in a given hour of the day, is either at home or at one of these locations.
- ➢
- Each individual represents his/her family unit (thus, if one individual is infected, we consider the entire family infected).
- ➢
- Families rarely visit each other (this means infections at home will be rare).
- ➢
- The rate of moving from home to an open location depends on the time of day (consistent with the window of time when a typical individual has the time to visit a store, for example). The rate of returning home from an open location depends on the typical time spent for that type of location (such as the average shopping time).
The epidemiology assumptions
- ➢
- The viral load. Each location will have a current viral load and maximum viral load. The current viral load represents a measure of viral particles lingering in the air at a given moment of time which is proportional to the number of infected people who are inside that location at the present moment and up to several hours earlier (to account for the fact that the virus lingers in the air even after an infected person left the location). The maximum viral load represents the largest possible viral concentration in the air such that any additional infected person will not raise the infection risk in the air any further (in other words, the air is saturated as far as viral concentration is concerned).
- ➢
- The infection mechanism. A susceptible individual may become infected from the contact with the contaminated air inside a location at a maximum infection rate that can only happen if the air has a viral saturation level (i.e., it reached its maximum possible viral load). Thus, the infection term is implemented according to the following formula:
The overall steps of the algorithm
- ➢
- Step 1. Reading data from the input file. Initialize current time counter with 0.
- ➢
- Step 2. Transfer from susceptible to infected at each location to account for new infections. Transfer from infected to recovered.
- ➢
- Step 3. Transfer between home and open locations according to the preference ranking.
- ➢
- Step 4. Transfer from recovered to removed to account for death from disease.
- ➢
- Step 5. Writing the current size of susceptible and infected in the output file.
- ➢
- Step 6. Increment current time value by 1.
- ➢
- Step 7. If maximum running time is not reached go back to Step 2.
Remark 2
- they prefer to visit a large location with a large maximum viral load that is not easily reached,
- they find the most preferred location closed some or most of the time,
- their next choice in the preference ranking that is open happens to be a smaller location with a low maximum viral load that is easy to reach,
- they prefer to go to an alternative location rather than changing the shopping time,
- there is low or high masking quality and compliance.
Remark 3
Conclusions
Appendix
Notations and definitions


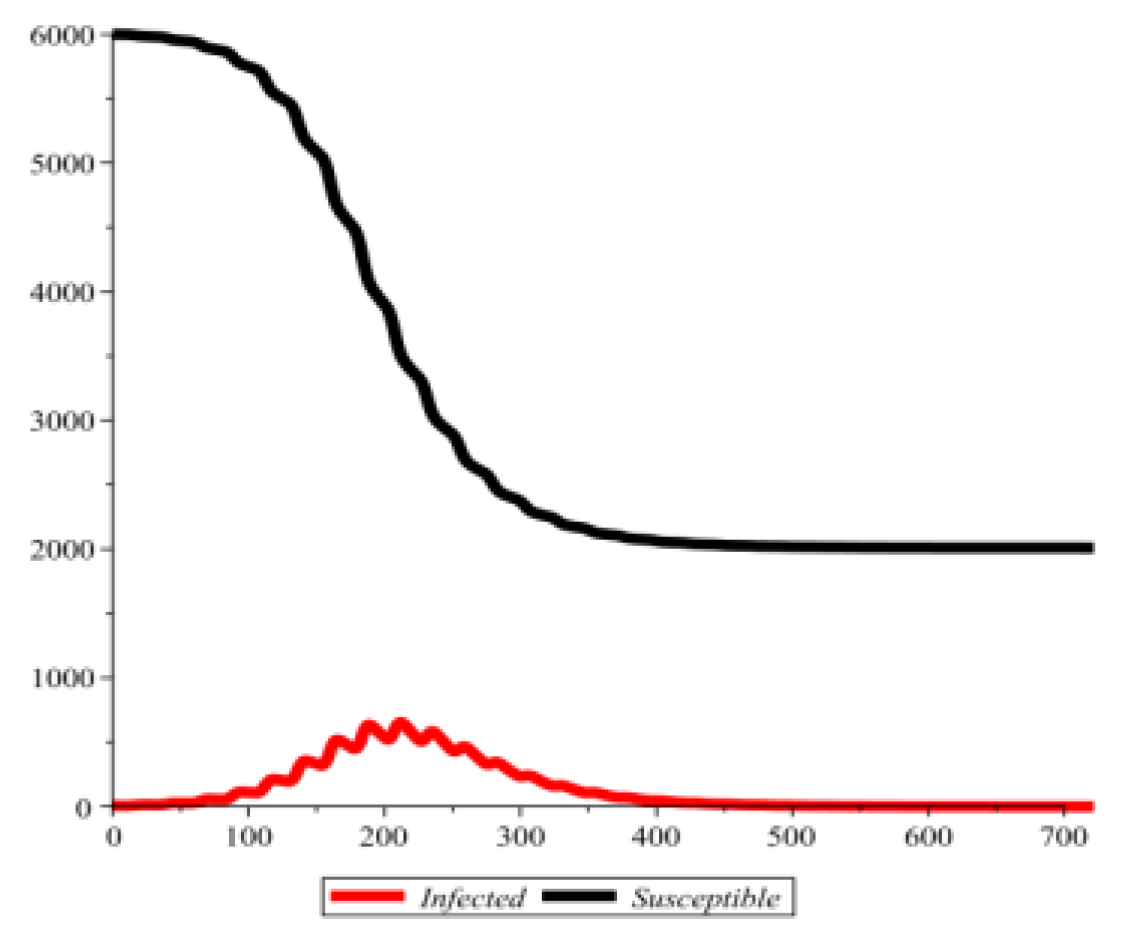

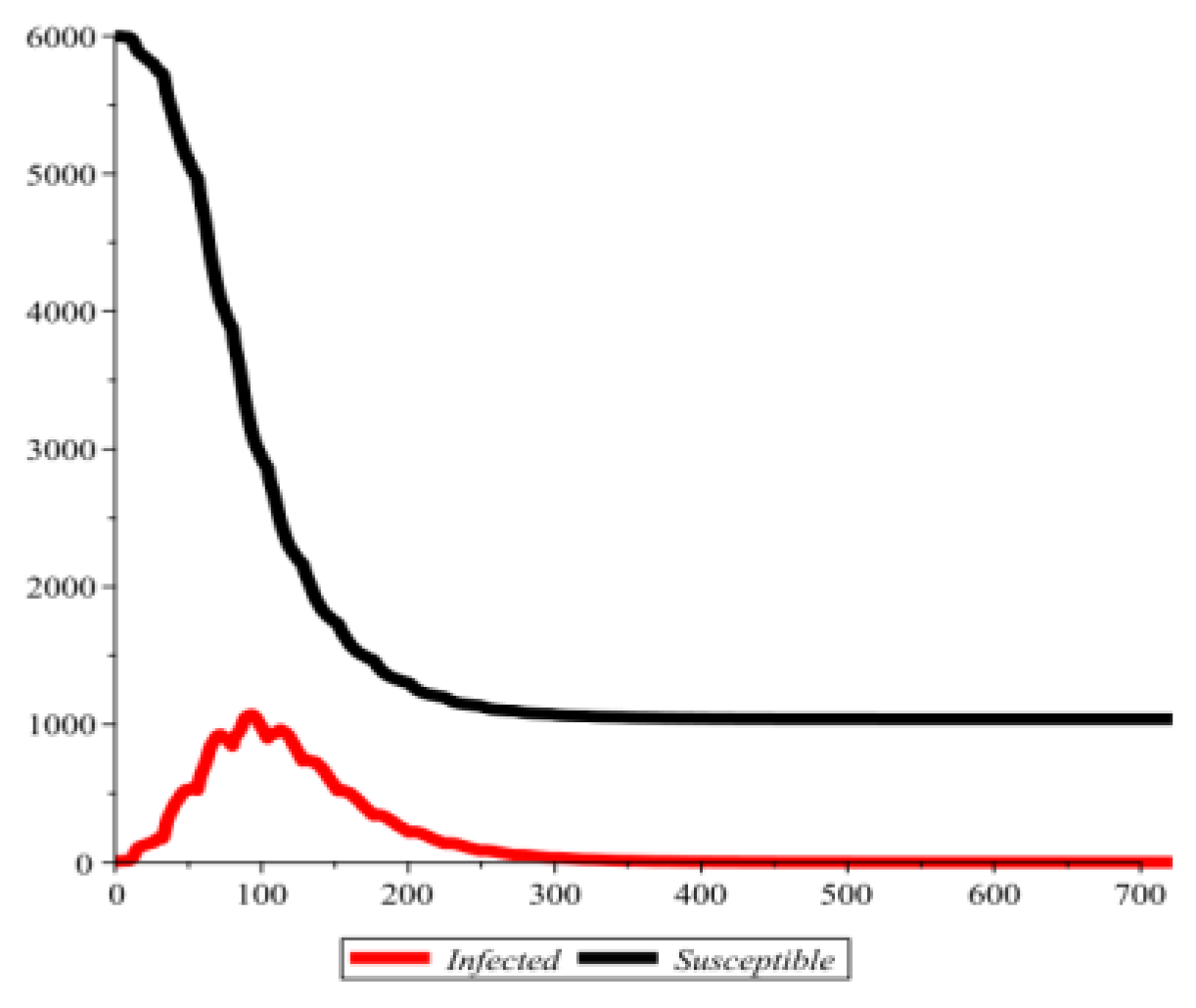

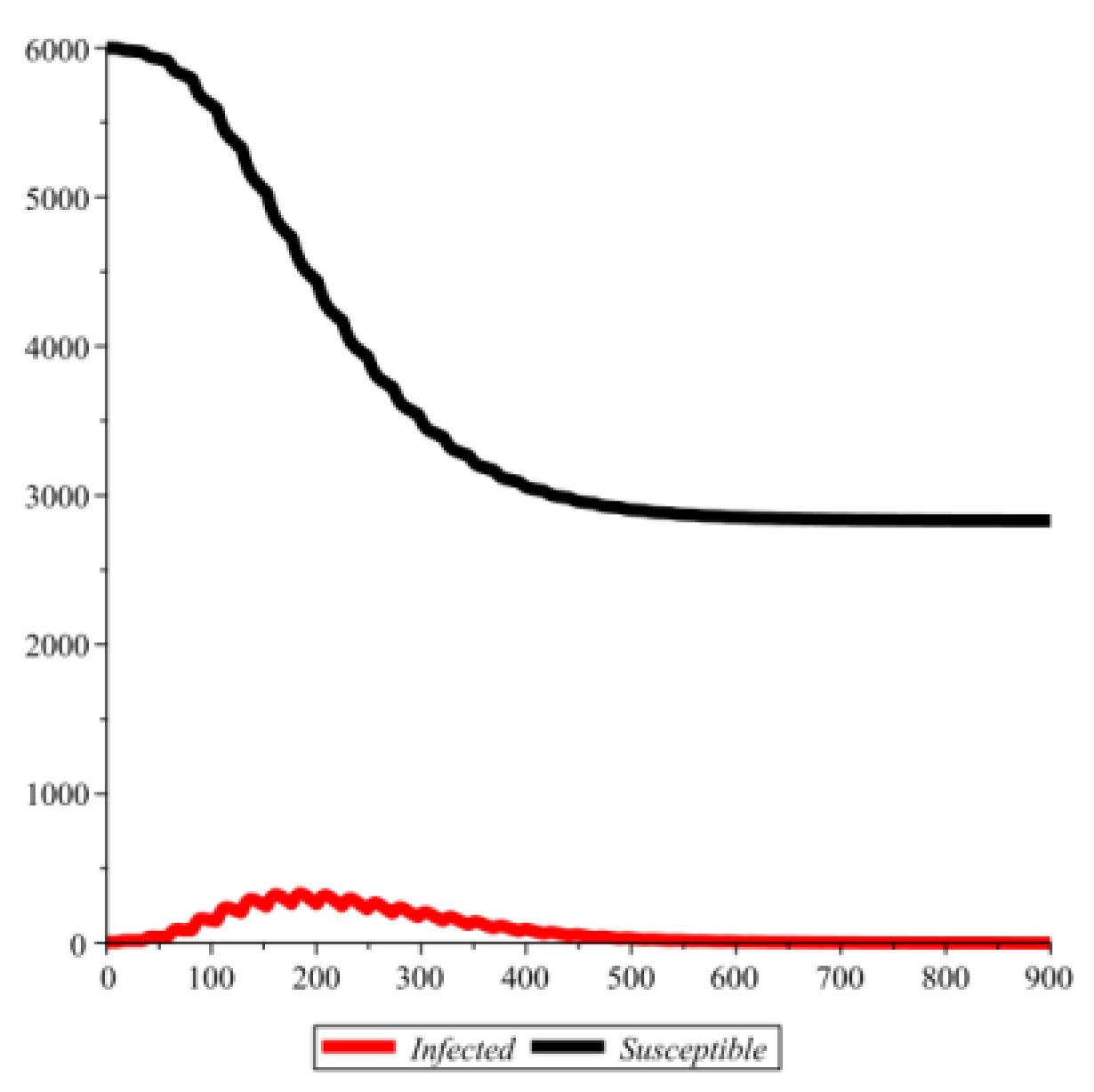
Conflict of interest disclosure
Compliance with ethical standards
References
- Carraturo, F.; Del Giudice, C.; Morelli, M.; Cerullo, V.; Libralato, G.; Galdiero, E.; Guida, M. Persistence of SARS-CoV-2 in the environment and COVID-19 transmission risk from environmental matrices and surfaces. Environ Pollut. 2020, 265 Pt B, 115010. [Google Scholar] [CrossRef]
- Al Huraimel, K.; Alhosani, M.; Kunhabdulla, S.; Stietiya, M.H. SARS-CoV-2 in the environment: Modes of transmission, early detection and potential role of pollutions. Sci Total Environ. 2020, 744, 140946. [Google Scholar] [CrossRef] [PubMed]
- Fisman, D.N.; Tuite, A.R. Evaluation of the relative virulence of novel SARS-CoV-2 variants: a retrospective cohort study in Ontario, Canada. CMAJ. 2021, 193, E1619–E1625. [Google Scholar] [CrossRef]
- Stroe, A.Z.; Stuparu, A.F.; Axelerad, S.D.; Axelerad, D.D.; Moraru, A. Neuropsychological symptoms related to the COVID-19 pandemic experienced by the general population and particularly by the healthcare personnel. J Mind Med Sci. 2021, 8, 197–208. [Google Scholar] [CrossRef]
- Oude Munnink, B.B.; Worp, N.; Nieuwenhuijse, D.F.; Sikkema, R.S.; Haagmans, B.; Fouchier, R.A.M.; Koopmans, M. The next phase of SARS-CoV-2 surveillance: real-time molecular epidemiology. Nat Med. 2021, 27, 1518–1524. [Google Scholar] [CrossRef]
- Giovanetti, M.; Benedetti, F.; Campisi, G.; Ciccozzi, A.; Fabris, S.; Ceccarelli, G.; Tambone, V.; Caruso, A.; Angeletti, S.; Zella, D.; Ciccozzi, M. Evolution patterns of SARS-CoV-2: Snapshot on its genome variants. Biochem Biophys Res Commun. 2021, 538, 88–91. [Google Scholar] [CrossRef]
- Meo, S.A.; Meo, A.S.; Al-Jassir, F.F.; Klonoff, D.C. Omicron SARS-CoV-2 new variant: global prevalence and biological and clinical characteristics. Eur Rev Med Pharmacol Sci. 2021, 25, 8012–8018. [Google Scholar] [CrossRef]
- Araújo, M.B.; Mestre, F.; Naimi, B. Ecological and epidemiological models are both useful for SARS-CoV-2. Nat Ecol Evol. 2020, 4, 1153–1154. [Google Scholar] [CrossRef]
- Koelle, K.; Martin, M.A.; Antia, R.; Lopman, B.; Dean, N.E. The changing epidemiology of SARS-CoV-2. Science. 2022, 375, 1116–1121. [Google Scholar] [CrossRef]
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. [Google Scholar] [CrossRef]
- Lipsitch, M.; Krammer, F.; Regev-Yochay, G.; Lustig, Y.; Balicer, R.D. SARS-CoV-2 breakthrough infections in vaccinated individuals: measurement, causes and impact. Nat Rev Immunol. 2022, 22, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Zonta, F.; Scaiewicz, A.; Levitt, M. The Gompertz Growth of COVID-19 Outbreaks is Caused by Super-Spreaders. arXiv 2021, arXiv:2111.02962v2. [Google Scholar]
- Bazant, M.Z.; Bush, J.W.M. A guideline to limit indoor airborne transmission of COVID19. Proceedings of the National Academy of Sciences 118, no. 17 (2021).
- Mendez-Brito, A.; Bcheraoui, E.C.; Pozo-Martin, F. Systematic review of empirical studies comparing the effectiveness of non-pharmaceutical interventions against COVID-19. Journal of Infection 2021, 83, 281–293. [Google Scholar] [PubMed]
- Azuma, K.; Yanagi, U.; Kagi, N.; Kim, H.; Ogata, M.; Hayashi, M. Environmental factors involved in SARS-CoV-2 transmission: effect and role of indoor environmental quality in the strategy for COVID-19 infection control. Environ Health Prev Med. 2020, 25, 66. [Google Scholar] [CrossRef]
- Rencken, G.K.; Rutherford, E.K.; Ghanta, N.; Kongoletos, J.; Glicksman, L. Patterns of SARS-CoV-2 aerosol spread in typical classrooms. Build Environ. 2021, 204, 108167. [Google Scholar] [CrossRef]
- Sima, R.M.; Olaru, O.G.; Cazaceanu, A.; Scheau, C.; Dimitriu, M.T.; Popescu, M.; Ples, L. Stress and anxiety among physicians and nurses in Romania during the COVID-19 pandemic. J Mind Med Sci. 2021, 8, 252–258. [Google Scholar] [CrossRef]
- Hong, L.X.; Lin, A.; He, Z.B.; Zhao, H.H.; Zhang, J.G.; Zhang, C.; Ying, L.J.; Ge, Z.M.; Zhang, X.; Han, Q.Y.; Chen, Q.Y.; Ye, Y.H.; Zhu, J.S.; Chen, H.X.; Yan, W.H. Mask wearing in pre-symptomatic patients prevents SARS-CoV-2 transmission: An epidemiological analysis. Travel Med Infect Dis. 2020, 36, 101803. [Google Scholar] [CrossRef]
- Shi, S.; Tanaka, S.; Ueno, R.; Gilmour, S.; Tanoue, Y.; Kawashima, T.; Nomura, S.; Eguchi, A.; Miyata, H.; Yoneoka, D. Travel restrictions and SARS-CoV-2 transmission: an effective distance approach to estimate impact. Bull World Health Organ. 2020, 98, 518–529. [Google Scholar] [CrossRef]
- López, V.; Čukić, M. A dynamical model of SARS-CoV-2 based on people flow networks. Saf Sci. 2021, 134, 105034. [Google Scholar] [CrossRef]
- Imborek, K.L.; Krasowski, M.D.; Natvig, P.; Merrill, A.E.; Diekema, D.J.; Ford, B.A. Experience With Pretravel Testing for SARS-CoV-2 at an Academic Medical Center. Acad Pathol. 2021, 8, 23742895211010247. [Google Scholar] [CrossRef]
- Al-Saleh, M.M.; Alamri, A.M.; Alhefzi, A.A.; Assiri, K.K.; Moshebah, A.Y. Population healthy lifestyle changes in Abha city during COVID-19 lockdown, Saudi Arabia. J Family Med Prim Care. 2021, 10, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Salway, R.; Su, T.T.; Ismail, R.; Glynis Armstrong, M.E.; Foster, C.; Johnson, L. The impact of COVID-19 movement restrictions on physical activity in a low-income semi-rural population in Malaysia: A longitudinal study. J Glob Health. 2021, 11, 05029. [Google Scholar] [CrossRef] [PubMed]
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int J Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef]
- Yang, H.; Rao, Z. Structural biology of SARS-CoV-2 and implications for therapeutic development. Nat Rev Microbiol. 2021, 19, 685–700. [Google Scholar] [CrossRef]
- Wilkin, P.J.; Al-Yozbaki, M.; George, A.; Gupta, G.K.; Wilson, CM. The Undiscovered Potential of Essential Oils for Treating SARS-CoV-2 (COVID-19). Curr Pharm Des. 2020, 26, 5261–5277. [Google Scholar] [CrossRef]
© 2022 by the author. 2022 Daniel Maxin, Spencer Gannon
Share and Cite
Maxin, D.; Gannon, S. An Algorithm for Indoor SARS-CoV-2 Transmission. J. Mind Med. Sci. 2022, 9, 242-248. https://doi.org/10.22543/2392-7674.1313
Maxin D, Gannon S. An Algorithm for Indoor SARS-CoV-2 Transmission. Journal of Mind and Medical Sciences. 2022; 9(2):242-248. https://doi.org/10.22543/2392-7674.1313
Chicago/Turabian StyleMaxin, Daniel, and Spencer Gannon. 2022. "An Algorithm for Indoor SARS-CoV-2 Transmission" Journal of Mind and Medical Sciences 9, no. 2: 242-248. https://doi.org/10.22543/2392-7674.1313
APA StyleMaxin, D., & Gannon, S. (2022). An Algorithm for Indoor SARS-CoV-2 Transmission. Journal of Mind and Medical Sciences, 9(2), 242-248. https://doi.org/10.22543/2392-7674.1313


