Abstract
The SARS-CoV-2 mutations that have occurred have alarmed the entire medical community. Thus, global concerns have been expressed regarding the transmission, pathogenicity and immune evasion of the Omicron strain, which shares mutations with the variants of concern Alpha, Beta, and Gamma strains. Still, Omicron transmission has crossed numerous borders worldwide compared to other types of SARS-CoV-2. The number of confirmed cases has increased and is reappearing in India. Given the worrisome situation created by the Omicron outbreak, scientists and postgraduates have had to make decisions about current research programs at institutions around the globe. The Omicron variants under development have significantly heightened worldwide pandemic concerns. In this review, the authors will outline the molecular features and efficacy of vaccination, highlight possible omicron impacts on scientific research, and provide precautions, procedural guidelines for organizing scientific meetings during a pandemic.
Introduction
The global World Health Organization (WHO) has designated the coronavirus outbreak as a pandemic, the SARS-CoV-2 Omicron variant (B.1.1.529) being one of the most prevalent variants of concerns (VOCs) [1]. The first genomic surveillance teams in South Africa and Botswana found a new VOC after a rapid resurgence of SARS-CoV-2 outbreaks in the Gauteng region of South Africa [2]. Numerous VOCs have been found to have similar alterations to Omicrons such as alpha and beta. With more than twice as many mutated proteins in the spike protein as the already highly contagious delta variant [3], other viral replication-related proteins, such as two non-structural proteins (NSP12, NSP14), show substantial variation across variants [4]. Omicron SARS-CoV-2 was three times more infectious than SARS-CoV-2. The new Omicron variant is found in 38 countries throughout the world, including Hong Kong, Israel, Colombia, Australia, Botswana, South Africa, Singapore, India, China, Saudi Arabia, the US and other developing countries [5]. As 2022 draws to a close, people with vaccine-induced immunity have surged and many of them believed the pandemic was over. So far, 150 countries have closed schools, universities and research institutes, affecting 80% of students worldwide. Several countries have implemented specific measures in university and research institutions. which must be closed [6]. Reopening institutions in a pandemic situation can cause concern and anxiety among people [7]. This review article presents information on omicron characteristics, vaccine efficacy, and addresses the implications of omicron outbreaks for academic research.
Discussion
SARS-CoV-2 Omicron's (B.1.1.529) Havoc
The impact of the recent omicron growth in India on academic research has yet to be assessed. One of the longest periods of university, research and school closures in the world has probably occurred in India compared to other countries [7]. Omicron struck as the nation prepared to start colleges, schools and research organizations and regularize routine operations. As the system prepares to deal with the omicron's various issues, the reopening of institutions could be put on hold again. As before, complications are more of a concern these days, especially learning and mental health difficulties for researchers and students. As a result of the current omicron outbreak in India [8], there has been a significant rise in the number of secondary school kids and Ph.D. researchers hospitalized for Omicron infections, both in raw numbers and as a share of all Omicron hospitalizations. However, it is unclear whether these hospitalizations result from serious illnesses or researchers who were treated for other reasons and unintentionally found to be infected with COVID-19. Apart from the direct implications, the indirect consequences of omicron growth from closing research institutions, closing universities and economic consequences can exacerbate the world's physical, emotional and social health problems [9]. Many nations such as the US, Canada, Europe, the UK and the Middle East (with Sinopharm) have launched immunization of children and adolescents [10,11]. Two vaccines have received approval from the Drug Controller General of India: Covaxin, an inactivated vaccine for ages 18 and above, and ZyCoV-D, a DNA-based vaccination for children aged between 12 and 18. The Indian government has stated that starting on January 3, 2022, all children and adults aged 15 and older will get the Covaxin vaccine [12]. Since research has revealed the appearance of the omicron after its peak, progress should be monitored over the next few weeks.
Omicron's Structural Features
Omicron variant is derived from Pango lineage B.1.1.529, and differs from the originating strain in 21 amino acids associated with the spike protein, mostly in the receptor-binding domain (RBD) (residues 319–541) [13,14]. SARS-CoV-2 has undergone a number of changes that were not previously observed [15]. Mutations in the spike (S) protein, where antibodies attach, contribute to the increased infectivity and transmissibility of the Omicron variant [16,17]. The Omicron S protein contains 30 amino acid alterations, three deletions, and one small insertion [18]. About half (15) of the amino acid alterations are in the RBD [19]. The N501Y change is one of the most important and Q498R has a stronger affinity for ACE-2 receptors than the other 15 RBD variants, thus indicating its enhanced transmissibility [13,17]. Figure 1 shows the domain layout of the 32 spike proteins identified in Omicron, which contains more mutations than any other Delta COVID-19 variant. These mutations include A67V, T95I, Y145D, G339D, K417N, N440K, G446S, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, Y505H, D614G and K618 [18]. Mutations at H655Y, N679K and P681H in the S1S2 furin cleavage site of the omicron form may enhance transmissibility [19]. A P681H mutation was also detected in the alpha variant [20,21]. In the delta variant, Q498R and N501Y alterations boosted ACE2 binding [22] in which this mutation increases SARS-CoV-2 infectivity [23]. ACE-2 receptors are key factors in COVID-19, which may cause organ failure severely [20].
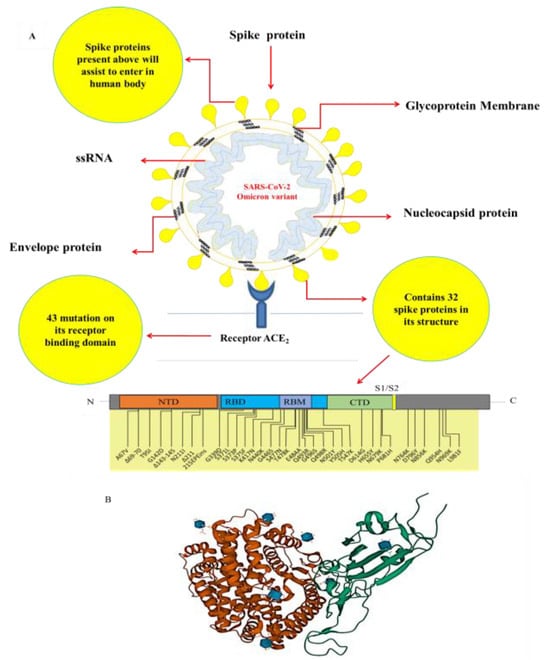
Figure 1.
A) The schematic diagram illustrating the domain arrangement of the 32-spike protein found in Omicron. B) The Cryo-EM structure of Omicron spike protein in complex with human ACE2.
Symptoms, complications and diagnosis
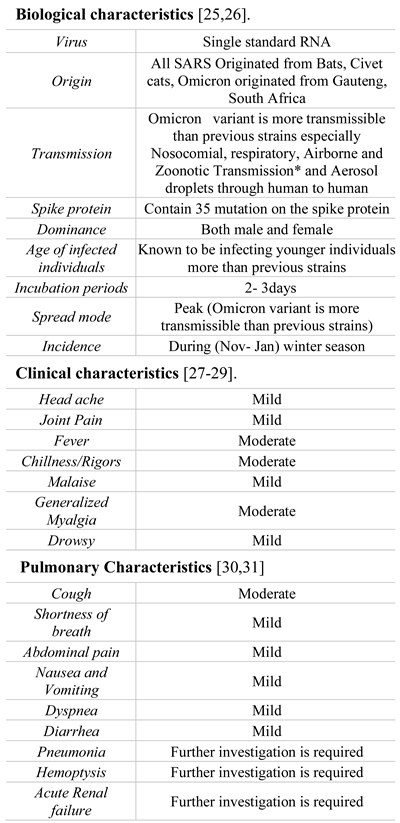
Omicron showed modified spike proteins compared to Delta variant COVID-19. The Delta variation (B.1.617.2) has eight spike protein mutations, whereas the Omicron variant (B.1.1.529) has 32 mutations. The delta variant was identified in India in 2021, along with eight other alterations [24]. The standard incubation time for the previous virus was five days, while the new virus may have a median incubation period of just two to three days [25]. Omicron symptoms include a runny nose, sore throat, headache, mild-to-severe fatigue, sneezing, and night sweats [25]. Fever, cough, and loss of taste/odor are far less common. Symptoms remain for several days before disappearing. Omicron-positive patients have no respiratory problems, and their saturation levels remained normal. The biological and clinical characteristics o of the Omicron are remarkably similar to the COVID-19 infections, several general characteristics being described in Table 1. RT-PCR, high-throughput genome sequencing, anti-viral immunoglobulin M (IgM) and G (IgG) antibody testing, as well as lung X-ray (CT scan value) were used to identify SARS-Covid-2 infection [32]. Both the SARS-CoV-2 infection diagnostic tests have been authorized by the FDA, including tests for PCR (also known as molecular testing). This is the most often used method while the another one is the antigen assay diagnostic test.

Table 1.
The general characteristics of Omicron.
Role of Booster Vaccination
Omicron improves vaccine-induced immunity more than delta variant. This is the most controversial issue being discussed in the world at present. In vitro investigations demonstrate that BNT162b2 and AZD1222 generate reduced neutralizing antibody titers against Omicron [33,34]. Several vaccines such as mRNA-1273 (Moderna), Sputnik, Sinopharm, and Ad26.COV2.S (Janssen) have had lower neutralizing antibody titers against Omicron. Pfizer reported that after the third BNT162b2 injection, the titer of neutralizing antibodies was raised by 25-fold, reaching the level of two vaccination doses to prevent the original viral strain [35]. Additionally, the levels of neutralizing antibodies produced in response to the Ad26.COV2.S vaccination was also increased. No impact on the Omicron form was seen in an mRNA investigation of the ChAdOx1immunization [36]. After the second BNT162b2 immunization, the vaccine's effectiveness dropped to 88% (CI: 64.9 percent – 95.8 percent) and steadily deteriorated over the next 2–9 weeks. It also decreased from 48.5 to 34.2 percent (5%–58.7%) from 10–14 weeks to 25 weeks after the second immunization. The two BNT162b2 or AZD1222 vaccines were ineffective in protecting against symptomatic illness and have resulted in fewer hospitalizations for omicrons [37]. During the omicron surge, two doses of BNT162b2 were 70% protective against hospitalization compared to 93% before the peak [38]. With the number of cases rising and concerns about an omicron surge, India has also stated that health care personnel, frontline employees, and people over 60 would will start receiving booster doses from January 10, 2022 [39]. Studies demonstrate that heterologous boosters perform better than homologous ones [40].
According to specific estimations, the Omicron distribution in India may be quite different from other countries. According to the worldwide data population, fully and partially vaccinated globally are shown in Figure 2. Just 73% of India's population is fully vaccinated including boosters, this percentage is much lower than the global average of Only 73% of India's population is fully vaccinated (including boosters), much lower than the United Arab Emirates global average of 100% [41]. Omicron may spread quicker in India, highlighting the urgent need to increase vaccine coverage. Despite the FDA-approved COVID-19 vaccines' reduced efficacy against variant viruses [42,43,44,45,46,47,48], they have been shown to protect against significant illness, hospitalization, and death [49,50,51].
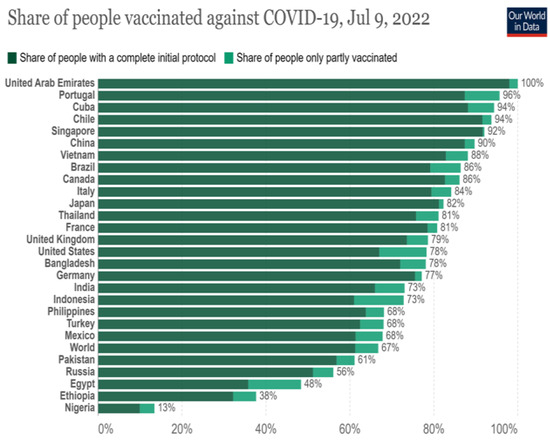
Figure 2.
World wide data of fully vaccinated and partially vaccinated for COVID-19 Vaccine.
On the other hand, sera from people who got the vaccine showed that the Omicron variant was 40% less effective at neutralizing the virus than the SARS-CoV-2 wild type [52]. Based on these results, the current COVID-19 vaccine might not work as well against the Omicron variant as it does against other COVID-19 variants. As there is no vaccine for Omicron, vaccines for SARS-CoV-2 variants that include Omicron are used as a preventive measure to make the disease less severe and less likely to lead to death [53].
However, three critical concerns remain unanswered:
- 1)
- Will this variant lead to more severe and fatal diseases?
- 2)
- Will prior vaccination protect against this new variant?
- 3)
- Will previous medicines be effective against this new variant?
The most critical issue is whether boosters are necessary and beneficial. Price-justified? Omicron is now widespread in the nations with the highest rates of booster immunization (the United States, the United Kingdom, and Europe). Still, cases have begun to decline considerably in South Africa, where only 30% of the population has had two doses of the vaccine.
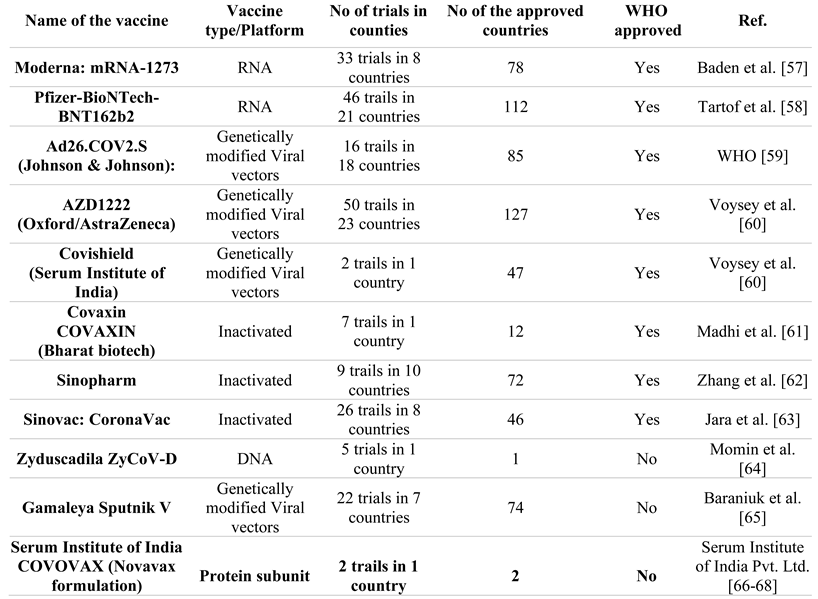
Several studies demonstrate that a COVID-19 booster dosage may maintain and improve vaccine effectiveness [54]. Since India last manufactured a billion vaccine doses, it can now analyze over one million samples each day [55]. Globally, the WHO is actively collaborating with researchers to investigate the transmission, severity, and effectiveness of Omicron vaccines and the availability of clinical trials for the disease [56]. Table 2 shows the list of vaccines approved in India b the WHO. Also, more research is needed to find out how well COVID-19, Omicron, and other variants in India work.

Table 2.
The list of vaccines approved in India for COVID-19 by WHO.
Impact on biomedical research studies
Most developed countries have implemented strict national isolation measures to stop viral transmission and spread in response to the increase in omicron cases worldwide. Similarly, India has also implemented state and district-wise boarders' closure of universities, schools, colleges, and research organizations, resulting in strict limitations on people's mobility [69]. As a result, universities and research institutions have been compelled to carefully identify and allocate essential research activities, while other industrial companies have been completely suspended [70]. Basic scientific research often involves wet lab work, making it difficult for scientists to work from home such an activity that requires their physical presence in the laboratory [71]. The Omicron controversy had a negative influence on scientific research on animals. In light of personnel shortages (caused by disease and quarantine) and supply chain interruptions, concerns have been expressed about the treatment of laboratory animals [72]. Over a hundred rodents were killed in an animal house lab at the Vinayaga Mission Research Foundation. This scientifically valuable resource might take years to rebuild [72]. In their study of genetically modified organisms, scientists from the Max Delbrück Center in Germany and Tsinghua University killed more than a thousand mice and rats.
Disruption induced by a pandemic and time passage rendered these organisms unsustainable in both circumstances [73]. Conversely, during the pandemic period, laboratories were instructed to scale down their animal care by research groups, pharmaceutical companies, and universities around the globe. As a result, ethical questions were inevitably raised. Similarly, in research involving the use of stem cells, researchers have had similar difficulties. In the Aaruapdai Veedu medical college and hospital cell culture lab, OG specialists, Ph.D. students, and research scientists froze hundreds of cell lines due to facility closures. After thawing, the experimental cell line is analyzed at to ensure it is still good. In addition, there is a risk that issues related to an integrity breach may occur, which would affect any application that follows [74].
Additionally, researchers may need to spend a lot of time thawing materials. For labs to resume their pre-Omicron level of research, it can take months or even years, and if there is damaged material it can take much longer. Basic scientific research has also been affected by supply restrictions. The majority of researchers need to use personal protective equipment (PPE) to protect themselves against hazards in the lab [75]. Numerous lab supplies, including cell culture media and chemicals for RNA extraction, have been documented to be short-handed [76]. When new and ongoing non-COVID-19 research is stopped and more professionals move to COVID-19 research, there is a growing fear that basic scientific research on other important diseases, like cardiometabolic disorders and cancer, could be hurt in a way that can't be fixed [77].
Impact on life sciences studies
Omicron had a particularly devastating effect on early-career researchers (ECRs) and Ph.D. students [78]. Research budgets are generally limited, making both parties' incomes highly susceptible to economic fluctuations. Most Ph.D. students depend on fellowships that last from one to four years. Additional tuition and consumable research costs may be incurred due to time wasted via the lack of productivity caused by omicron. Short-term contracts of one to two years are anticipated from ECRs.
Job applications may be negatively affected if researchers cannot meet the goals outlined in their initial research plan [78]. More than half of postdoctoral researchers polled by Nature between June and July 2020 indicated anxiety about the COVID-19 pandemic's influence on their future career prospects [79,80,81]. In addition, many more variables have raised worries on research aspects professional advancement, as follows:
- Due to university and research organization closures, researchers may be unable to complete investigations in time for publication.
- Many academic accomplishments remain unreported owing to a lack of funding since their research output has been used in funding decision.
- In a scientific meeting, many academics may lose key career development opportunities for networking or research dissemination as most global scientific meetings move to a virtual platform. Also, young researchers may feel especially exposed when they share sensitive information and new ideas online.
- Employment closures and funding issues several institutions are facing financial difficulties due to a historic drop in foreign student enrollment and the associated drop in university income. Many have eliminated jobs and slowed or frozen hiring.
Globally, omicron and other viral variants have complicated academic and professional work for many scientists involved in research [82]. Many international researchers are forced to limit or even abandon their studies or jobs due to visa term restrictions [79]. Due to visa time constraints, a group of researchers may have difficulty acquiring a position on the following academic career ladder, particularly if they desire to remain in their current location [80]. Many international researchers have been unable to take jobs abroad due to travel restrictions imposed by the pandemic [79]. Figure 3 shows the overall impact of Omicron on various academic research studies.
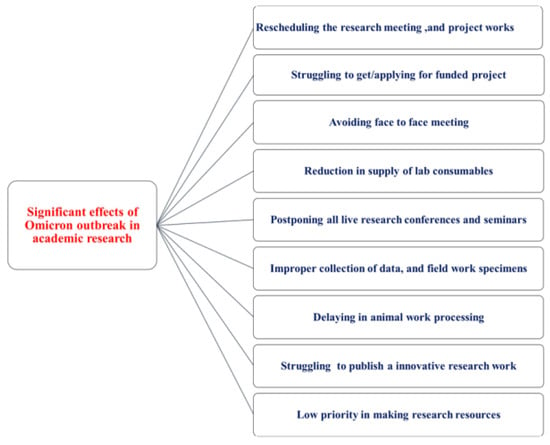
Figure 3.
Effects of the Omicron outbreak in academic research.
Impact on clinical studies
The omicron scenario will make it difficult for clinical studies to meet their research and teaching commitments and secure funding [83]. Omicron has also affected the research industry, forcing scientists and researchers to return to regular activities [84]. The lack of patient samples hampered due to the impact of the pandemic. Clinicians do not find any remarkable success in their regular research and development activities because companies lack scientific support, fewer specialists, and other equipment and chemicals [85]. Ensure that participants are regularly informed of any changes to the research protocol. For current trials, concerns include electronic data recording through telemedicine, phone interviews, or alternative sites for assessment. Determine whether to revise the original statistical analysis plan in light of potential missing data (e.g., from missed study visits or study termination). There is an urgent need to provide evidence-based suggestions for clinical practices that can be impacted by strict and effective Omicron infection control procedures [86]. So, following the rapid advice guidelines approach and the essential criteria of WHO guideline production, we developed this recommendation. For research purposes, this rapid mentoring guideline is for first-line scientists, researchers, research students, and healthcare professionals interested in the 2021 Omicron outbreak and learning more quickly [80]. Clinical and researchers working on the omicron pandemic outbreak period will benefit from our assessment, which sees this rapid review opinion measure as a sound scientific technique [71]. We address technology in this guideline, organize meetings during pandemic scenarios, and carry out current research projects within the organization. We also provide guidelines for physicians and researchers in preparation for their scientific meetings and also guidance for medical treatment. When it comes to creating future frontiers, they will need to know how to tackle the current situation.
Precautions and Procedural Guidelines
Requirements (Good Personal Hygiene Practices). Aspiring clinicians should fulfill these minimum requirements:
No employee with conjunctivitis, a severe cold, etc., will be not allowed in.
Each supervisor or department head shall monitor the omicron and notify the research committee.
Avoid crowded areas and direct contact with chronically ill or sick people.
Frequently clean your hands with soap (such as before eating, after going to the toilet, or when your hands become dirty with respiratory secretions after coughing or sneezing).
If you have a cough or a runny nose, use a mask.
Cover your mouth with tissue paper and discard the used tissue when coughing or sneezing; if you're sick, get medical attention immediately.
Discourage them from sharing their meals or personal hygiene supplies.
Avoid physical interaction such as handshaking and facial contact.
Personal hygiene is required for all researchers.
All clinicians will wash their hands after using the restroom and urinal.
Maintain short, clean nails and avoid facial hair crossing the seal of the respirator mask.
Personal items should be kept in lockers by all workers. Please store your items in the visiting lockers.
The clinicians must wash their mouths and hands after the meal/tea.
Research significance
The consequences of omicron have disrupted research efforts and current research programs worldwide, including in the United States and India. As explained below, several precautions and scientific considerations must be considered in the omicron scenarios.
- General guidelines with specifications and description data [87].Always maintain a social distance of at least one meter with other humans in all directions.Minimal touch with the article and human; no handshake.Sanitize your hands as much as possible.Do not use another person's laptop, mobile phone, or desktop.Conducting online meetings from a workstation using a laptop or a mobile device.Do not use the meeting room for any meetings.All employees will be informed that they will be under CCTV surveillance to monitor the above guidelines, and any non-compliance will be reported.
- During transit [88].Using a mask and starting from home.It is best to work while keeping a social distance. Say nothing until absolutely required.It is better not to bring food or other goods from home to work.
- During entry [88].After exiting your vehicle, keep social distance. Keeping track of visitors' names, addresses, ages, and contact information.Undertake a thermal screening with an OHC representative.Hand sanitizing with sanitizer.
Guideline end-user
Our preventive measures include researchers, scientists, academics and doctors working in hospitals and healthcare sectors. The measures also target healthy members of the community, public health officials and anyone interested in managing the coronavirus pandemic, in whatever capacity they act if it is considered acceptable and beneficial. For reasons of safety and prevention, the precautions mentioned above must therefore be followed when attending any kind of academic or research meeting during a pandemic period.
- During the meeting or event [88].The simplest method to accomplish this is to advice or inform participants about omicron and the procedures taken to ensure their safety.Encourage everyone to wash their hands often or use rubbing alcohol.Coughing or sneezing should be covered with a bent elbow or a handkerchief.Providing closed trash cans and wipes to dispose of them.Hand massagers with alcohol were conspicuously exhibited around the premises.Keep windows and doors open to provide proper ventilation during your performance.Providing a mask so they may safely return home or transport them to a recognized assessment facility.
- Before a meeting or event [87].Initially, identify and validate communication routes with key partners like public health and healthcare professionals.Ensure you have enough tissues, masks, and hand sanitizer for everyone attending.Ensure that all event organizers, participants, vendors, and visitors provide contact information, including mobile phone numbers, email addresses, as well as event locations.
Conclusions
Omicron is the most-mutated VOC in many countries. Science, research, and technology are progressing at the same level as the pandemic due to improved diagnosis, targeted vaccines, and medical treatments. However, further studies are needed to improve the understanding of viral pathogenesis, which may lead to better prevention and treatment measures. The CDC has cooperated with commercial labs and universities to perform genetic surveillance research. To develop a scientifically valid plan to prevent COVID-19, the WHO advises that countries increase their research infrastructure. For clinical and surgical trainees globally, COVID-19 has imposed several scientific restrictions. The state-wide lockdown has had a particularly negative impact on basic science research. Clinicians experienced comparable difficulties due to the suspension of healthcare services and a sudden return to full-time responsibilities.
Institutions should take more preventive measures to stop the spread of COVID-19 now and in the future. Students, Ph.D. researchers and teachers should often connect using email and university internet services. The mental health of students, faculty and staff at a university should be a priority, as should their safety on campus. It is the responsibility of the government as well as the individual institutions to provide food and accommodation for students traveling from other states or nations. The different impacts of the pandemic and the additional difficulties generated by the modified strains must be overcome, and ensuring equal access to healthcare is essential. Major global events have a profound impact on science, and overcoming these academic challenges require dedicated investigation. Finally, people will still be able to stop the spread of the disease by wearing masks, staying away from public places and getting vaccinated.
Author’s contributions
Prithiviraj Nagarajan wrote the initial draft, and Saravanaavel Kumar prepared the tables and figures as a graphical illustration. Anitha Vetrivel, Jayanthi Kumar, and Anusheela Howlader critically revised and organized the manuscript. Kumar Rangarajalu, Satheesh Kumar and Muthu Gopal provided intellectual input and an overall manuscript review. All authors contributed to manuscript revision, read, and approved the submitted version.
Acknowledgments
We sincerely thank Aarupadai Veedu Medical College and Hospital, Vinayaka Missions Research Foundation, for immense support in writing this manuscript during the pandemic lock-down period.
Conflict of interest disclosure
There are no known conflicts of interest in the publication of this article. The manuscript was read and approved by all authors.
Compliance with ethical standards
Any aspect of the work covered in this manuscript has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
References
- Viana, R.; Moyo, S.; Amoako, D.G.; Tegally, H.; Scheepers, C.; et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature. 2022, 603, 679–686. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus disease (COVID-19) pandemic. (2020). Accessed: March 20, 2020. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- Omicron variant detected in more countries as scientists race to find answers. Reuters 2021 Nov 28. Available online: https://www.reuters.com/world/new-coronavirus-variant-omicron-keeps-spreading-australia-detects-cases-2021-11-28/.
- Pascarella, S.; Ciccozzi, M.; Bianchi, M.; Benvenuto, D.; Cauda, R.; Cassone, A. The electrostatic potential of the Omicron variant spike is higher than in Delta and Delta-plus variants: A hint to higher transmissibility? J Med Virol. 2022, 94, 1277–1280. [Google Scholar] [CrossRef]
- Islam, M.R.; Hossain, M.J. Detection of SARS-CoV-2 Omicron (B.1.1.529) variant has created panic among the people across the world: What should we do right now? J Med Virol. 2022, 94, 1768–1769. [Google Scholar] [CrossRef] [PubMed]
- UNESCO. COVID-19 Educational disruption and response. (2020). Accessed: March 25, 2020. Available online: https://en.unesco.org/themes/education-emergencies/coronavirus-school-closures.
- Karim, S.S.A.; Karim, Q.A. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021, 398, 2126–2128. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.A.; Ahmed, S.; Mir, A.; Shinde, M.; Bender, O.; Alshammari, F.; Ansari, M.; Anwar, S. The SARS-CoV-2 mutations versus vaccine effectiveness: New opportunities to new challenges. J Infect Public Health. 2022, 15, 228–240. [Google Scholar] [CrossRef]
- Singhal, T. The Emergence of Omicron: Challenging Times Are Here Again! Indian J Pediatr. 2022, 89, 490–496. [Google Scholar] [CrossRef]
- Price, A.M.; Olson, S.M.; Newhams, M.M.; Halasa, N.B.; Boom, J.A.; et al.; Overcoming Covid-19 Investigators BNT162b2 Protection against the Omicron Variant in Children and Adolescents. N Engl J Med. 2022, 386, 1899–1909. [Google Scholar] [CrossRef]
- Fleming-Dutra, K.E.; Britton, A.; Shang, N.; Derado, G.; Link-Gelles, R.; Accorsi, E.K.; Smith, Z.R.; Miller, J.; Verani, J.R.; Schrag, S.J. Association of Prior BNT162b2 COVID-19 Vaccination With Symptomatic SARS-CoV-2 Infection in Children and Adolescents During Omicron Predominance. JAMA. 2022, 327, 2210–2219. [Google Scholar] [CrossRef]
- Shukla, S.C.; Pandit, S.; Soni, D.; Gogtay, N.J. Evaluation of Allergic Reactions following COVID-19 Vaccination in Patients with Documented Allergies. J Assoc Physicians India. 2021, 69, 11–12. [Google Scholar]
- Kannan, S.R.; Spratt, A.N.; Sharma, K.; Chand, H.S.; Byrareddy, S.N.; Singh, K. Omicron SARS-CoV-2 variant: Unique features and their impact on pre-existing antibodies. J Autoimmun. 2022, 126, 102779. [Google Scholar] [CrossRef]
- Lubin, J.H.; Markosian, C.; Balamurugan, D.; Pasqualini, R.; Arap, W.; Burley, S.K.; Khare, S.D. Structural models of SARS-CoV-2 Omicron variant in complex with ACE2 receptor or antibodies suggest altered binding interfaces. bioRxiv. 2021, 2021.12.12.472313. [Google Scholar] [CrossRef]
- Saxena, S.K.; Kumar, S.; Ansari, S.; Paweska, J.T.; Maurya, V.K.; Tripathi, A.K.; Abdel-Moneim, A.S. Characterization of the novel SARS-CoV-2 Omicron (B.1.1.529) variant of concern and its global perspective. J Med Virol. 2022, 94, 1738–1744. [Google Scholar] [CrossRef]
- Mannar, D.; Saville, J.W.; Zhu, X.; Srivastava, S.S.; Berezuk, A.M.; Tuttle, K.S.; Marquez, A.C.; Sekirov, I.; Subramaniam, S. SARS-CoV-2 Omicron variant: Antibody evasion and cryo-EM structure of spike protein-ACE2 complex. Science. 2022, 375, 760–764. [Google Scholar] [CrossRef]
- Kumar, S.; Thambiraja, T.S.; Karuppanan, K.; Subramaniam, G. Omicron and Delta variant of SARS-CoV-2: A comparative computational study of spike protein. J Med Virol. 2022, 94, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Rai, H.; Gautam, D.N.S.; Prajapati, P.K.; Sharma, R. Emerging evidence on Omicron (B.1.1.529) SARS-CoV-2 variant. J Med Virol. 2022, 94, 1876–1885. [Google Scholar] [CrossRef] [PubMed]
- Scialo, F.; Daniele, A.; Amato, F.; Pastore, L.; Matera, M.G.; Cazzola, M.; Castaldo, G.; Bianco, A. ACE2: The Major Cell Entry Receptor for SARS-CoV-2. Lung. 2020, 198, 867–877. [Google Scholar] [CrossRef]
- Joseph, S.; Nair, B.; Nath, L.R. The Ineluctable Role of ACE-2 Receptors in SARS COV-2 Infection and Drug Repurposing as a Plausible SARS COV-2 Therapy: A Concise Treatise. Curr Mol Med. 2021, 21, 888–913. [Google Scholar] [CrossRef]
- Tan, Z.; Chen, Z.; Yu, A.; Li, X.; Feng, Y.; Zhao, X.; Xu, W.; Su, X. The First Two Imported Cases of SARS-CoV-2 Omicron Variant-Tianjin Municipality, China, December 13, 2021. China CDC Wkly. 2022, 4, 76–77. [Google Scholar] [CrossRef]
- Tian, D.; Sun, Y.; Xu, H.; Ye, Q. The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 Omicron variant. J Med Virol. 2022, 94, 2376–2383. [Google Scholar] [CrossRef]
- Fehr, A.R.; Perlman, S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015, 1282, 1–23. [Google Scholar] [CrossRef]
- Barlan, A.; Zhao, J.; Sarkar, M.K.; Li, K.; McCray, P.B., Jr; Perlman, S.; Gallagher, T. Receptor variation and susceptibility to Middle East respiratory syndrome coronavirus infection. J Virol. 2014, 88, 4953–4961. [Google Scholar] [CrossRef] [PubMed]
- Del Águila-Mejía, J.; Wallmann, R.; Calvo-Montes, J.; Rodríguez-Lozano, J.; Valle-Madrazo, T.; Aginagalde-Llorente, A. Secondary Attack Rate, Transmission and Incubation Periods, and Serial Interval of SARS-CoV-2 Omicron Variant, Spain. Emerg Infect Dis. 2022, 28, 1224–1228. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; Yuan, M.L.; Zhang, Y.L.; Dai, F.H.; Liu, Y.; Wang, Q.M.; Zheng, J.J.; Xu, L.; Holmes, E.C.; Zhang, Y.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020, 579, 265–269. [Google Scholar] [CrossRef]
- de Wit, E.; van Doremalen, N.; Falzarano, D.; Munster, V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016, 14, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Cui, J.; Li, F.; Shi, Z.L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; Müller, M.A.; Drosten, C.; Pöhlmann, S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Chowell, G.; Abdirizak, F.; Lee, S.; Lee, J.; Jung, E.; Nishiura, H.; Viboud, C. Transmission characteristics of MERS and SARS in the healthcare setting: a comparative study. BMC Med. 2015, 13, 210. [Google Scholar] [CrossRef] [PubMed]
- Stroe, A.Z.; Stuparu, A.F.; Axelerad, S.D.; Axelerad, D.D.; Moraru, A. Neuropsychological symptoms related to the COVID-19 pandemic experienced by the general population and particularly by the healthcare personnel. J Mind Med Sci. 2021, 8, 197–208. [Google Scholar] [CrossRef]
- Cameroni, E.; Bowen, J.E.; Rosen, L.E.; Saliba, C.; Zepeda, S.K.; et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature. 2022, 602, 664–670. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Shaw, R.H.; Supasa, P.; Liu, C.; Stuart, A.S.; Pollard, A.J.; Liu, X.; Lambe, T.; Crook, D.; Stuart, D.I.; Mongkolsapaya, J.; Nguyen-Van-Tam, J.S.; Snape, M.D.; Screaton, G.R.; Com-COV2 study group. Reduced neutralisation of SARS-CoV-2 omicron B.1.1.529 variant by post-immunisation serum. Lancet. 2022, 399, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2022, 602, 654–656. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N Engl J Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021, 373, n1088. [Google Scholar] [CrossRef] [PubMed]
- Collie, S.; Champion, J.; Moultrie, H.; Bekker, L.G.; Gray, G. Effectiveness of BNT162b2 Vaccine against Omicron Variant in South Africa. N Engl J Med. 2022, 386, 494–496. [Google Scholar] [CrossRef]
- Mohapatra, R.K.; El-Shall, N.A.; Tiwari, R.; Nainu, F.; Kandi, V.; Sarangi, A.K.; Mohammed, T.A.; Desingu, P.A.; Chakraborty, C.; Dhama, K. Need of booster vaccine doses to counteract the emergence of SARS-CoV-2 variants in the context of the Omicron variant and increasing COVID-19 cases: An update. Hum Vaccin Immunother. 2022, 18, 2065824. [Google Scholar] [CrossRef]
- Chiu, N.C.; Chi, H.; Tu, Y.K.; Huang, Y.N.; Tai, Y.L.; Weng, S.L.; Chang, L.; Huang, D.T.; Huang, F.Y.; Lin, C.Y. To mix or not to mix? A rapid systematic review of heterologous prime-boost covid-19 vaccination. Expert Rev Vaccines. 2021, 20, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- AlKetbi, L.M.B.; Al Hosani, F.; Al Memari, S.; Al Mazrouei, S.; Al Shehhi, B.; AlShamsi, N.; AlKwuiti, M.M.; Saleheen, H.N.; Al Mutairi, H.; Al Hajeri, O.M. Parents' views on the acceptability of a COVID-19 vaccine for their children: A cross-sectional study in Abu Dhabi-United Arab Emirates. Vaccine. 2022, 40, 5562–5568. [Google Scholar] [CrossRef]
- Martínez-Baz, I.; Miqueleiz, A.; Casado, I.; Navascués, A.; Trobajo-Sanmartín, C.; Burgui, C.; Guevara, M.; Ezpeleta, C.; Castilla, J.; Working Group for the Study of COVID-19 in Navarra. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection and hospitalisation, Navarre, Spain, January to April 2021. Euro Surveill. 2021, 26, 2100438. [Google Scholar] [CrossRef]
- Martínez-Baz, I.; Trobajo-Sanmartín, C.; Miqueleiz, A.; Guevara, M.; Fernández-Huerta, M.; Burgui, C.; Casado, I.; Portillo, M.E.; Navascués, A.; Ezpeleta, C.; Castilla, J.; Working Group for the Study of COVID-19 in Navarre; Investigators, other members of the Working Group for the Study of COVID-19 in Navarre. Product-specific COVID-19 vaccine effectiveness against secondary infection in close contacts, Navarre, Spain, April to August 2021. Euro Surveill. 2021, 26, 2100894. [Google Scholar] [CrossRef]
- Sima, R.M.; Olaru, O.G.; Cazaceanu, A.; Scheau, C.; Dimitriu, M.T.; Popescu, M.; Ples, L. Stress and anxiety among physicians and nurses in Romania during the COVID-19 pandemic. J Mind Med Sci. 2021, 8, 252–258. [Google Scholar] [CrossRef]
- Keskin, A.; Karslioglu, B. Did Covid-19 pandemic narrow the spectrum of surgical indications? J Clin Invest Surg. 2021, 6, 58–63. [Google Scholar] [CrossRef]
- Tang, P.; Hasan, M.R.; Chemaitelly, H.; Yassine, H.M.; Benslimane, F.M.; et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat Med. 2021, 27, 2136–2143. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, K.B.; Pritchard, E.; Matthews, P.C.; Stoesser, N.; Eyre, D.W.; et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med. 2021, 27, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Pilishvili, T.; Gierke, R.; Fleming-Dutra, K.E.; Farrar, J.L.; Mohr, N.M.; et al.; Vaccine Effectiveness among Healthcare Personnel Study Team Effectiveness of mRNA Covid-19 Vaccine among U.S. Health Care Personnel. N Engl J Med. 2021, 385, e90. [Google Scholar] [CrossRef]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O'Brien, K.L.; Smith, P.G.; Wilder-Smith, A.; Zeger, S.; Deloria Knoll, M.; Patel, M.K. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Hitchings, M.D.T.; Ranzani, O.T.; Dorion, M.; D'Agostini, T.L.; de Paula, R.C.; et al. Effectiveness of ChAdOx1 vaccine in older adults during SARS-CoV-2 Gamma variant circulation in São Paulo. Nat Commun. 2021, 12, 6220. [Google Scholar] [CrossRef]
- Hall, V.; Foulkes, S.; Insalata, F.; Kirwan, P.; Saei, A.; et al.; SIREN Study Group Protection against SARS-CoV-2 after Covid-19 Vaccination and Previous Infection. N Engl J Med. 2022, 386, 1207–1220. [Google Scholar] [CrossRef]
- Cheng, S.M.S.; Mok, C.K.P.; Leung, Y.W.Y.; Ng, S.S.; Chan, K.C.K.; Ko, F.W.; Chen, C.; Yiu, K.; Lam, B.H.S.; Lau, E.H.Y.; Chan, K.K.P.; Luk, L.L.H.; Li, J.K.C.; Tsang, L.C.H.; Poon, L.L.M.; Hui, D.S.C.; Peiris, M. Neutralizing antibodies against the SARS-CoV-2 Omicron variant BA.1 following homologous and heterologous CoronaVac or BNT162b2 vaccination. Nat Med. 2022, 28, 486–489. [Google Scholar] [CrossRef]
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; et al. SARS-CoV-2 Omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. medRxiv 2021, 2021.12.08.21267417. [Google Scholar] [CrossRef]
- Voysey, M.; Costa Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; et al.; Oxford COVID Vaccine Trial Group Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021, 397, 881–891. [Google Scholar] [CrossRef]
- Beatty, A.L.; Peyser, N.D.; Butcher, X.E.; Cocohoba, J.M.; Lin, F.; Olgin, J.E.; Pletcher, M.J.; Marcus, G.M. Analysis of COVID-19 Vaccine Type and Adverse Effects Following Vaccination. JAMA Netw Open. 2021, 4, e2140364. [Google Scholar] [CrossRef] [PubMed]
- Burki, T.K. Omicron variant and booster COVID-19 vaccines. Lancet Respir Med. 2022, 10, e17. [Google Scholar] [CrossRef] [PubMed]
- LR, B.; HM, E.S.; Essink B; Kotloff K; Frey S; Novak R; Diemert, D. ; et al.; COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021, 398, 1407–1416. [Google Scholar] [CrossRef]
- Agency, E.M. WHO adds Janssen vaccine to list of safe and effective emergency tools against COVID-19. Saudi Med J. 2021, 42, 463–464. [Google Scholar]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; et al.; Oxford COVID Vaccine Trial Group Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Madhi, S.A.; Baillie, V.; Cutland, C.L.; Voysey, M.; Koen, A.L.; et al.; NGS-SA Group; Wits-VIDA COVID Group Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. N Engl J Med. 2021, 384, 1885–1898. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Jara, A.; Undurraga, E.A.; González, C.; Paredes, F.; Fontecilla, T.; Jara, G.; Pizarro, A.; Acevedo, J.; Leo, K.; Leon, F.; Sans, C.; Leighton, P.; Suárez, P.; García-Escorza, H.; Araos, R. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N Engl J Med. 2021, 385, 875–884. [Google Scholar] [CrossRef]
- Momin, T.; Kansagra, K.; Patel, H.; Sharma, S.; Sharma, B.; et al. Safety and Immunogenicity of a DNA SARS-CoV-2 vaccine (ZyCoV-D): Results of an open-label, non-randomized phase I part of phase I/II clinical study by intradermal route in healthy subjects in India. EClinicalMedicine. 2021, 38, 101020. [Google Scholar] [CrossRef] [PubMed]
- Baraniuk, C. Covid-19: What do we know about Sputnik V and other Russian vaccines? BMJ. 2021, 372, n743. [Google Scholar] [CrossRef]
- Serum Institute of India. News. Seruminstitute.com. [cited 2022 Jul 10]. Available online: https://www.seruminstitute.com/news.php.
- Komalatha, N.; Shilpa, G.; Laddha, K.S. Isolation of starch from curcuma longa and its characterization. Int J Pharm Sci Res. 2020, 11, 5712–5717. [Google Scholar] [CrossRef]
- Luo, W.; Li, Y.P.; He, Y.; Huang, S.L.; Li, D.; Gu, L.Q.; Huang, Z.S. Synthesis and evaluation of heterobivalent tacrine derivatives as potential multi-functional anti-Alzheimer agents. Eur J Med Chem. 2011, 46, 2609–2616. [Google Scholar] [CrossRef]
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int J Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Röhrig, B.; du Prel, J.B.; Wachtlin, D.; Blettner, M. Types of study in medical research: part 3 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2009, 106, 262–268. [Google Scholar] [CrossRef]
- Omary, M.B.; Hassan, M. Opinion: Here's how we restore productivity and vigor to the biomedical research workforce in the midst of COVID-19. Proc Natl Acad Sci U S A. 2020, 117, 19612–19614. [Google Scholar] [CrossRef]
- Ahmet, V.; Nedim, K.A. D-dimer levels and acute pulmonary embolism development in COVID-19 patients. J Mind Med Sci. 2021, 8, 133–138. [Google Scholar] [CrossRef]
- Sohrabi, C.; Mathew, G.; Franchi, T.; Kerwan, A.; Griffin, M.; Soleil CDel Mundo, J.; Ali, S.A.; Agha, M.; Agha, R. Impact of the coronavirus (COVID-19) pandemic on scientific research and implications for clinical academic training - A review. Int J Surg. 2021, 86, 57–63. [Google Scholar] [CrossRef]
- Madhusoodanan, J. Frozen cells and empty cages: researchers struggle to revive stalled experiments after the lockdown. Nature. 2020. [Google Scholar] [CrossRef]
- Emanuel, E.J.; Persad, G.; Upshur, R.; Thome, B.; Parker, M.; Glickman, A.; Zhang, C.; Boyle, C.; Smith, M.; Phillips, J.P. Fair Allocation of Scarce Medical Resources in the Time of Covid-19. N Engl J Med. 2020, 382, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, S. "Slow research" in the time of Covid-19. Indian J Med Ethics. 2020, V, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Zeggini, E.; Baumann, M.; Götz, M.; Herzig, S.; Hrabe de Angelis, M.; Tschöp, M.H. Biomedical Research Goes Viral: Dangers and Opportunities. Cell. 2020, 181, 1189–1193. [Google Scholar] [CrossRef]
- Paula, J.R. Lockdowns due to COVID-19 threaten PhD students' and early-career researchers' careers. Nat Ecol Evol. 2020, 4, 999. [Google Scholar] [CrossRef]
- Woolston, C. Pandemic darkens postdocs' work and career hopes. Nature. 2020, 585, 309–312. [Google Scholar] [CrossRef]
- Termini, C.M.; Traver, D. Impact of COVID-19 on early career scientists: an optimistic guide for the future. BMC Biol. 2020, 18, 95. [Google Scholar] [CrossRef]
- Woolston, C. Junior researchers hit by coronavirus-triggered hiring freezes. Nature. 2020, 582, 449–450. [Google Scholar] [CrossRef]
- Singh, J.A.; Bandewar, S.V.; Bukusi, E.A. The impact of the COVID-19 pandemic response on other health research. Bull World Health Organ. 2020, 98, 625–631. [Google Scholar] [CrossRef]
- Harrop, C.; Bal, V.; Carpenter, K.; Halladay, A. A lost generation? The impact of the COVID-19 pandemic on early career ASD researchers. Autism Res. 2021, 14, 1078–1087. [Google Scholar] [CrossRef]
- López-Vergès. S.; Urbani, B.; Fernández Rivas, D.; Kaur-Ghumaan, S.; Coussens, A.K.; et al. Mitigating losses: how scientific organisations can help address the impact of the COVID-19 pandemic on early-career researchers. Humanit Soc Sci Commun. 2021, 8, 284. [Google Scholar] [CrossRef]
- Chenneville, T.; Schwartz-Mette, R. Ethical considerations for psychologists in the time of COVID-19. Am Psychol. 2020, 75, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Coltey, E.; Alonso, D.; Vassigh, S.; Chen, S.C. Towards an AI-Driven Marketplace for Small Businesses During COVID-19. SN Comput Sci. 2022, 3, 441. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, S. Precautions for omicron virus - the new covid variant - PharmEasy. PharmEasy Blog. 2021 [cited 2022 Jul 10]. Available online: https://pharmeasy.in/blog/omicron-the-new-variant-of-covid-19-risks-and-safety-precautions/.
- Yamey, G.; Hanage, W.; Moultrie, T. Let’s not be fatalistic about Omicron. We know how to fight it. Time. 2021 Dec 15 [cited 2022 Jul 10]. Available online: https://time.com/6128506/omicron-covid-19-how-to-fight/.
© 2022 by the author. 2022 Prithiviraj Nagarajan, Anitha Vetrivel, Jayanthi Kumar, Anusheela Howlader, Kumar Rangarajalu, Satheesh Kumar Sabapathy, Muthu Gopal, Saravanaavel Kumar