Materials and Methods
The study group included 40 patients hospitalized to the Second Surgical Clinic of the Emergency County Hospital of Craiova, Romania, through the emergency service between 2015 and 2021. The age of the patients in the study group ranged from 39 to 93 years, most patients being over 70 years. Regarding the sex distribution of the patients with colonic diverticular disease studied, there was a predominance of males (26 males and 14 females). All the patients were hospitalized to the emergency department. The distribution of patients was as follows:
- -
22 patients with acute colonic diverticulitis (15 males and 7 females) out of which: 21 patients suffered from left acute colonic diverticulitis and 1 patient suffered from right acute colonic diverticulitis;
- -
12 patients suffered from perforated acute colonic diverticulitis (9 males and 3 females) out of which: 3 patients with acute left colonic diverticulitis perforated with perisigmoid abscess and 9 patients with left acute colonic diverticulitis perforated with generalized fecal peritonitis;
- -
3 patients with acute colonic hemorrhagic diverticular disease (2 females and 1 male);
- -
3 patients with fistulated colonic diverticular disease (2 males and 1 female)
Colocutaneous fistula (1 male);
Sigmoid-bladder fistula (1 female);
Sigmoid-bladder fistula and acute sigmoid diverticulitis perforated in the peritoneal cavity with generalized peritonitis (1 male).
Table 1.
The distribution of the cases of colonic diverticulosis and the type of disease.
Table 1.
The distribution of the cases of colonic diverticulosis and the type of disease.
| Type of colonic diverticulosis | Number of cases |
| Acute colonic diverticulitis | 22 |
| Perforated colonic diverticulosis | 12 |
| Acute hemorrhagic diverticulitis | 3 |
| Acute fistulized diverticulitis | 3 |
The clinical picture in patients with acute left colonic diverticulitis was pain in the left iliac fossa, fever, vomiting, constipation. On the objective clinical examination, spontaneous painful abdomen was found on palpation in the left iliac fossa, localized abdominal tenderness in the left iliac fossa. In the case of patients with acute right colonic diverticulitis, the clinical picture was represented by pain in the right iliac fossa and the right flank and on the objective clinical examination, localized abdominal tenderness in the right iliac fossa and the right flank. The paraclinical examinations revealed leukocytosis (L>15000/mm3) and the abdominal CT scan showed a thickening of the sigmoid colon wall and an infiltration of pericolic fat.
In the case of patients who presented with perforated acute colonic diverticulitis, the clinical picture was that of pelvic abscesses in 3 cases and in 9 patients the clinical picture was that of a generalized acute peritonitis manifested through diffuse abdominal pain, fever, vomiting and the clinical examination revealed muscle defense in the left and right lower quadrant of the abdomen, the digital rectal examination highlighted swelling and tenderness of the Douglas pouch. Laboratory tests revealed leukocytosis (L = 13,000-21,000/mm3). The CT examination revealed localized pneumoperitoneum under the form of gas bubbles and free intraperitoneal fluid.
In the case of patients with hemorrhagic colonic diverticulitis, the clinical picture was that of acute lower gastrointestinal bleeding externalized by massive rectal bleeding as bright red blood per rectum, manifested through the clinical signs of acute post-hemorrhagic anemia (lipothymy, syncope, dizziness, thirst). In 2 of the 3 cases, the clinical picture was that of a severe acute post-hemorrhagic anemia requiring emergency surgery for hemostatic purposes.
In patients with fistulized colonic diverticular disease, the symptomatology was purulent discharge through a fistulous orifice in the left flank (colonic diverticular disease complicated by colorocutaneous fistula), fecaluria (in patients with sigmoid-bladder fistula). The diagnosis was made by means of the abdominal CT, fistulography and cystoscopy.
Out of the 40 patients studied, more than 14 underwent colonoscopy in the outpatient clinic, which revealed colonic diverticulosis.
The treatment applied in the case of patients with uncomplicated acute colonic diverticulitis was conservatively represented by digestive rest and continued with water diet, antibiotic therapy (cephalosporin + metronidazole), non-steroidal anti-inflammatory drugs, heparin anticoagulant, local refrigeration. In the case of patients with acute perforated colonic diverticulitis, the applied surgical treatment was represented by Hartmann sigmoidectomy, peritoneal drainage and wound care. For the 2 patients with hemorrhagic diverticulosis with severe posthemorrhagic anemia, the applied treatment was total colectomy in one case and segmental colectomy in the other case.
We present 3 of the 40 cases that caused various problems in terms of diagnosis and treatment.
Case 1
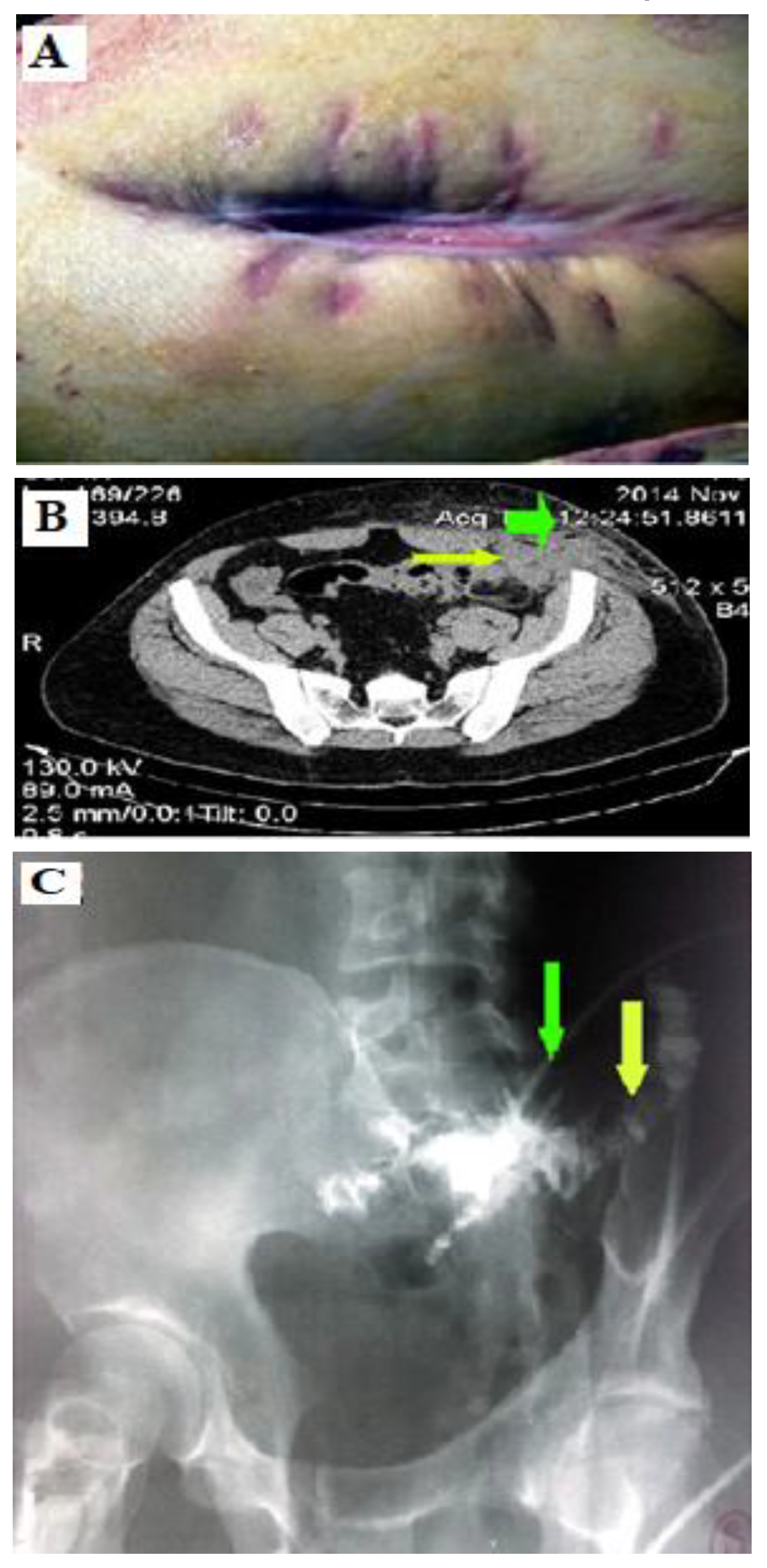
A 43-year-old man was hospitalized to the Second Surgical Clinic of the Emergency County Hospital in Craiova, in January 2015 with left lower quadrant pain, fecaluria, pneumaturia, hematuria accompanied by fever, whose onset was about 6 weeks ago. The physical examination revealed that the abdomen was sensitive to palpation in the left iliac fossa, and the rest of the abdomen was soft and painless. The rectal examination did not reveal any lesions. Laboratory investigations showed a change in the number of leukocytes (10,800/mm
3), and the examination of urine revealed the presence of a urinary tract infection, i.e. bacteriuria, numerous microbial flora, leukocytes and red blood cells. The tumor markers were: CEA 2.03 ng/mL (reference range: <4.7 ng/mL), CA 19-9 5.7 u/l, reference range: <37 u/l). Cystoscopy showed a fistulous orifice of 0.5 cm on the left posterolateral wall of the urinary bladder with the extravasation of feces. Mucosal biopsies were performed around the fistulous orifice and referred for histopathological examination. A biopsy was done and the frozen section revealed mainly uncertain inflammatory type tissue. The computed tomography revealed: the thickening of the urinary bladder walls with the presence of air bubbles (
Figure 1A) without lymphadenopathy.
The anatomy and morphology of the kidneys were preserved with permeable ureters. Colonoscopy examined the entire colon and the terminal ileum at a distance of 20 cm and revealed the presence of multiple diverticula on the sigmoid colon. The abdominal ultrasound showed a thickened posterior wall of the bladder and also an intestinal loop with thickened walls near the bladder and no fluid in the peritoneal cavity. The histopathological examination (biopsy fragment of the bladder mucosa around the fistulous orifice) revealed fibromuscular microscopic structure, granulation tissue and chronic inflammatory infiltrate. The tentative diagnosis was made given the clinical criteria and the computed tomography. The treatment consisted in the resection of the involved segment of the sigmoid colon with end-to-end colorectal anastomosis and partial cystectomy. Intraoperatively, the adhesion of the sigmoid loop to the posterior wall of the bladder was discovered (
Figure 1C) with a colo-bladder fistula of 0.4 cm in diameter (
Figure 1B) and 4 diverticula with sizes ranging from 1 and 1.5 cm in diameter were found nearby. The patient was discharged after 8 days without complications
Case 2
A 45-year-old male patient was hospitalized to the Second Surgical Clinic of the Emergency County Hospital in Craiova in January 2015 for cutaneous fistula and sero-purulent secretion through a fistulous orifice located in the left iliac fossa. The patient underwent surgery three months before for parietal abscess in the left iliac fossa when the collection was evacuated. The postoperative evolution was unfavorable with the formation of a fistulous orifice at the level of the postoperative scar in the left iliac fossa (
Figure 2A).
The physical examination indicated the presence of a postoperative scar with signs of inflammation and, at its lower pole, a fistulous orifice with a diameter of 0.2-0.3 cm through which sero-purulent fluid is evacuated. The fistulography of the left iliac fossa was performed using a small tube that was inserted into the fistulous orifice and showed the opacification of the sigmoid loop up to 2 cm in diameter with oblique-transverse trajectory, with ascending direction to the left flank; the sigmoid colonic mucosa also had inflammatory changes and incomplete luminal stenosis over a distance of about 10 cm (
Figure 2C).
The abdominal and pelvic CT showed the infiltration of the subcutaneous fat with inflammatory aspect in the anterior and left abdominal wall, extended to the pelvic rectum, external oblique, internal oblique and transverse. For the lower pole of the postoperative scar, the inflammatory process spread to the intrapelvic region without extending to the left iliac muscle, and without a cleavage plane to the ileal loops. It also showed the presence of a fluid collection and areas of air bubbles in the muscles of the abdominal wall (
Figure 2B). During surgery, an inflammatory process was found in the left iliac fossa involving the sigmoid colon, the anterior abdominal wall, and the large omentum (
Figure 3A). The release of the sigmoid loop was difficult and the presence of a fistulous orifice with a diameter of 0.4-0.5 cm was found, with a thickened wall and irregular edges. Sigmoidectomy was performed with end-to-end colorectal anastomosis. The postoperative piece consisted of a sigmoid colon segment with a length of 25 cm (
Figure 3C), which turned out to contain a perforated diverticulum (when sectioned) located antimezosthenically (
Figure 3B). The postoperative evolution was favorable. The patient was discharged after 7 days without complications.
Case 3
A 46-year-old male patient is hospitalized to the emergency department for fecaluria, diffuse abdominal pain, vomiting, constipation, fever, and chills. The objective clinical examination shows diffuse painful abdomen, diffuse tenderness of the abdominal wall, rebound tenderness. The digital rectal examination: rectal ampulla with thin walls without localized processes, prostates of normal volume, with a present median groove, violent pain from the digital rectal examination of the Douglas pouch. The paraclinical examinations showed: Hemoglobin (Hb) 11.7 g/dL, white blood cells (WBC) count 21,000/mm3, blood sugar levels 156 mg/dl, urea 76 mg/dl, creatinine 1.2 mg/dL, Na+ 137 mEg/L, K+ 3.6 mEg/L, Cl- 103 mEg/L, total bilirubin 1.2 mg%, oxaloacetic transaminase (SGOT) 41ui/l, alanine aminotransferase (ALAT)= 23 IU, amylasemia 21 IU. The abdominal CT highlights a thickened sigmoid wall at a distance of 12 cm, pneumoperitoneum located in the form of gas bubbles and free intraperitoneal fluid, dilated thin intestinal loops agglutinated in the bottom of the Douglas pouch. The diagnosis of generalized acute peritonitis probably caused by the perforation of the hollow organ, emergency surgery being performed and pelvic inflammatory block comprising the sigmoid colon, the terminal ileum, the posterior wall of the bladder and the large epiploon are found; turbid peritoneal fluid about 300 mL in the peritoneal cavity needles are collected for culture and antibiogram, false membranes on the terminal ileum and the sigmoid colon has a thickened wall at a distance of about 15 cm and it is closely adherent to the posterior wall of the bladder. It is difficult to dissect the inflammatory block and the presence of 2 diverticula in the sigmoid colon is found: one perforated in the peritoneal cavity and the second adherent to the posterior wall of the bladder with the presence of a sigmoid-bladder fistula. During sigmoid colon mobilization maneuvers, the terminal ileum adhering to the sigmoid colon is injured, requiring segmental enterectomy. Hartmann sigmoidectomy with temporary left iliac anus, segmental enterectomy with side-to-side entero-anastomosis, cystorafia, would cleaning, peritoneal drainage. The postoperative evolution is favorable except for the appearance of a superficial postoperative parietal suppuration. There were difficulties in suppressing the urethro-bladder probe, the Foley probe being clogged with feces. He is discharged on the 13th postoperative day by way of surgical healing, with the recommendation to return within 6 months for the reintegration of the colon in the digestive transit.
Results
The study group included 40 patients. The mean age of the examined patients was 70.3 years. There was a predominance of males (26 men and 14 women), the male percentage being 65% (
Figure 4A).
Out of the 40 cases studied, 22 cases presented acute diverticulitis, 12 cases presented with perforated colonic acute diverticulitis, 3 cases had hemorrhagic colonic diverticulosis and there were 3 cases of fistulated colonic diverticulosis (
Figure 4B).
The clinical picture of the patients with acute diverticulitis was represented by left lower quadrant abdominal pain, fever, +/- chills, and the objective clinical examination revealed the presence of a palpable tumor in the flank and left iliac fossa, localized abdominal tenderness in the left lower quadrant of the abdomen.
Laboratory tests showed an increase in the number of leukocytes (L = 12,000/mm3—15,000/mm3), increased C-reactive protein, increased ESR. The diagnosis of acute colonic diverticulitis was made by corroborating the data obtained on the objective clinical examination with the data obtained by exploring the abdomen by means of the computed tomography, which revealed a thickening of the colon wall (acute sigmoid diverticulitis predominated).
In the cases that we studied, acute left colonic diverticulitis predominated, right acute colonic diverticulitis being present in only 2 cases. In the case of patients with acute perforated colonic diverticulitis, the clinical picture was that of a localized or generalized acute peritonitis. In these cases, the diagnosis was made by corroborating the data obtained on the objective clinical examination and the data obtained on the abdominal CT (Pericolic air bubbles or small amounts of pericolic fluid in peritonitis located from acute perforated diverticulosis or fluid in the peritoneal cavity and distant gas bubbles in generalized peritonitis due to acute perforated diverticulitis). The biological paraclinical examinations showed the value of leukocytes > 15,000/mm3 and the C-reactive protein > 150 mg/dl.
In the case of hemorrhagic diverticulosis, the clinical picture was that of acute posthemorrhagic anemia and even hemorrhagic shock in one of the 3 studies cases. Colonoscopy played a very important role in making the diagnosis. It should be mentioned that all 3 patients with hemorrhagic diverticulosis were on anticoagulant treatment (plavix), the treatment being administered on the recommendation of the cardiologist for coronary heart disease accompanied by heart rhythm disorders.
In patients with acute fistulized diverticulitis, the clinical picture was represented by pneumaturia, fecaluria (in the case of sigmoid-bladder fistula) and the presence of a cutaneous fistulous orifice through which purulent content was externalized in the case of the initially treated parietal abscess). In making the diagnosis of colo-bladder fistula, the abdominal CT played a very important role along with cystoscopy. In the case of the patient with colo-cutaneous fistula, the diagnosis was made by means of fistulography and the CT examination.
The treatment administered to the patients with acute diverticulitis (
Figure 4C) was conservative: water diet, antibiotics (cephalosporins + metronidazole), non- steroidal anti-inflammatory drugs, hydro-electrolytic rebalancing, anticoagulant (low molecular weight heparin). In patients with acute perforated colonic diverticulitis, the administered treatment was the surgical one: Hartmann segmental colectomy with temporary left iliac anus (the reintegration of the colon in the digestive tract was done between 3 and 6 months), the antibiotic treatment for generalized acute peritonitis, anticoagulant low molecular weight), hydro-electrolytic and acid-base rebalancing. In patients with acute hemorrhagic diverticulitis, the treatment administered was subtotal colectomy with ileostomy, correction of anemia by means of whole blood transfusions. In patients with acute fistulized diverticulitis, the applied treatment was surgical and consisted in sigmoidectomy with side-to-side colo-rectoanastomosis, and cystography.
The postoperative complications were (
Figure 4D):
- -
Postoperative parietal suppuration—14 cases
- -
Acute urinary retention—15 cases
- -
Paralytic ileus—17 cases
- -
Intraperitoneal hemorrhage—2 cases
- -
Fixed evisceration—7 cases
- -
Multiple organ failure (MODS)—2 cases
The mortality rate was 3 out of the 40 cases (7.5%).
Discussion
Diverticular disease of the colon is one of the most common diseases, especially in the Western society, and it is one of the main reasons for hospitalization. Recently, colonic diverticular disease has shown an increased incidence in patients under the age of 40. Most commonly, colonic diverticular disease is diagnosed in the absence of symptoms, on a colonoscopic examination. Complications in colonic diverticulosis (inflammation, lower gastrointestinal bleeding, abscess formation, fistula, perforation, strictures and obstruction) are an important cause of morbidity and a significant economic burden [
1,
2]. Acute colonic diverticulitis is an entity that is found more and more frequently in emergency departments. An international group of experts from the World Society of Emergency Surgery (WSES) has updated the therapeutic guidelines for the therapeutic management of acute left colonic diverticulitis (ALCD). In some regions of the world (non-Western population, Japan, Hong Kong and Singapore), acute right colonic diverticulitis (ARCD) is more common. In ALCD, the sigmoid colon is most commonly involved, and in ARCD, one portion of the colon is most commonly involved [
3,
4,
5,
6].
Diverticulitis without significant complications accounts for more than 75% of the cases [
7,
8]. These patients usually have pain in the lower left quadrant, fever and leukocytosis, and the diagnosis is confirmed by means of computed tomography. In patients with uncomplicated diverticulitis, the main treatment is antibiotic therapy, intestinal rest or a clear, pain-controlled diet if needed [
9]. Antibiotic therapy should target Gram-positive and Gram-negative bacteria, as well as anaerobes. The usual outpatient regimens include oral ciprofloxacin and metronidazole or amoxicillin/clavulanate. For hospitalized patients, intravenous antibiotics covering a broad spectrum of bacteria should be selected and may include the following: ceftriaxone and metronidazole; beta-lactam/ beta-lactamase inhibitor monotherapy (e.g., piperacillin/ tazobactam); or meropenem [
10,
11]. The duration of the treatment ranges from 7 to 10 days.
In 2009, a randomized controlled trial of oral antibiotic therapy versus intravenous antibiotic therapy (ciprofloxacin and metronidazole) was carried out for patients with uncomplicated acute diverticulitis [
12]. Intravenous antibiotic therapy has not been shown to be more effective than oral antibiotic therapy in uncomplicated acute diverticulitis. For patients with uncomplicated ALCD and without comorbidities, it is suggested that the treatment be performed in a specialized outpatient setting. It is necessary to re-evaluate the patient within 7 days, and if the clinical condition deteriorates, the re-evaluation must be done earlier and for the patients with significant comorbidities who experience vomiting, the hydro-electrolytic rebalancing will be done during hospitalization [
12,
13,
14].
Up to 25% of the patients with acute diverticulitis develop complicated diseases [
13]. This includes abscess formation, fistulas, strictures/ obstruction and perforation. The abscess occurs with the perforation of a diverticulum (9). Small abscesses (less than 3 cm) can often be treated with antibiotics only [
14]. Larger abscesses (more than 4 cm) may require computerized tomographic-scan guided drainage followed by possible surgery after resolving the abscess [
15].
In patients with acute diverticulitis with findings on the CT examination, free gas at a distance without diffuse intra-abdominal fluid, a non-operative treatment is suggested, only if a careful and continuous monitoring of the patient can be performed. Out of these patients, about 25% treated non-operatively may require emergency surgery [
16]. If these patients show signs of acute peritonitis on the clinical examination, then emergency surgery, hydro-electrolytic rebalancing, antibiotic therapy are required.
Surgery in patients with clinical signs of acute peritonitis with acute perforated diverticulitis should comprise the surgical resection of the affected colon and anastomosis with or without a stoma, in stable patients without comorbidities and the Hartmann procedure (HP) in hemodynamically unstable patients or in patients with multiple comorbidities [
17].
In patients with multiple comorbidities, hemodynamic instability, the Hartmann (HP) procedure is recommended for the treatment of acute peritonitis caused by perforated colonic diverticulitis and in hemodynamically stable patients without comorbidities, colonic resection with primary anastomosis with or without a stoma is suggested. The HP is considered a therapeutic option in acute peritonitis caused by acute perforated colonic diverticulitis in patients presented to the emergency department with critical conditions and multiple comorbidities. However, restoration of the intestinal tract continuity is associated with significant morbidity [
18] and therefore many of these patients do not undergo digestive tract restoration surgery and thus remain with a permanent stoma [
19]. A study that included 2,729 patients [
20] evaluated patients with acute perforated diverticulitis who were treated either by means of colon resection with primary anastomosis and protective ileostomy or by means of HP. Most patients were treated by means of HP and only 208 (7.6%) patients were treated by means of colon resection with primary anastomosis and protective ileostomy. Mortality rates for patients undergoing HP and colon resection with primary anastomosis were 7.6% and 2.9%, respectively. The authors concluded that colon resection with primary anastomosis and protective ileostomy may be the optimal therapeutic strategy for carefully selected patients with acute peritonitis caused by perforated colonic diverticulitis.
It is recommended that after an acute episode of conservatively treated ALCD, a segmental resection of the affected colon be performed, especially in immunocompromised patients. Older clinical trials have reported that about one-third of patients with acute diverticulitis will have a recurrent attack, often within one year [
21,
22]. Currently, the decision for the segmental colectomy of the colon, performed after one or more episodes of acute colonic diverticulitis, must be made on a case-by-case basis taking into account the risk factors, the complications, the age, the severity of recurrent episodes, the personal pathological history and comorbidities. (e.g. immunosuppressed patients) [
23].
About one-third of the patients will have recurrent diverticulitis following an initial episode of uncomplicated diverticulitis [
24,
25]. The latest guidelines published by the American Society of Colon and Rectal Surgeons [
21,
26] recommend that the decision to perform a sigmoid colon resection after a second episode of acute diverticulitis be made on a case-by-case basis, taking into account the age and the comorbidities of the patient, as well as the frequency and severity of the attacks. These recommendations are in line with those of the Society for Tract Surgery [
27].
Perforating diverticular disease can also lead to fistulas, the most common locations being the colovesicular and colovaginal ones [
28,
29]. The complications of fistulas require surgical treatment. Recurrent episodes of diverticulitis can lead to fibrosis and stiffening of the colon, most often leading to the obstruction of the sigmoid colon [
9]. In the management of these strictures, malignancy must first be ruled out by means of colonoscopic examination [
21]. Endoscopic dilation can often provide temporary relief of symptoms and allow greater access to a stricture to do the biopsy [
30]. Moreover, the role of endoscopic stenting in diverticular disease has expanded in recent years. In a study on 23 patients who underwent stenting for diverticular strictures [
31], the procedure was successful in all individuals, allowing subsequent elective resection to be a single stage.
The diagnostic and treatment principles of ARCD are similar to those of ALCD. As a therapeutic option in ARCD, non-operative methods should be preferred in cases without acute diffuse peritonitis, although differentiation from malignant proliferative processes is difficult [
32,
33,
34]. The surgical treatment is usually performed in cases of complicated ARCD [
35,
36,
37,
38]. In experienced medical centers, laparoscopic resection of the affected colon with primary anastomosis can be performed.