Abstract
The aim of this study was to identify potentially toxic contaminants in milk powder. Powdered milk contains a range of toxic and non-toxic substances that are present in a wide variety, also having very different origins. A number of seven milk powder samples from different producers sold on the Romanian market were analyzed, the samples that were collected from the original packaging: P1, P2, P3, P4, P5, P6 and P7. The concentration of the following elements was analyzed using the X-ray (XRF) fluorescence method: potassium (K), chlorine (Cl), calcium (Ca), phosphorus (P) and aluminum (Al). The vast majority of the samples showed the levels of elements K, Ca, Cl, Al, P well above the maximum allowable limit (AML). In a single test, the elements potassium, calcium, chlorine, phosphorus showed levels below the maximum allowable limit, but the level of aluminum was much above. The experimental results showed that the market sells assortments of milk powder that exceed concentrations above the maximum limits established by the legislation in force for some constituent elements. Concentrations of constituent elements are not always specified on food labels, and if this information appears, they are not always the correct values.
Introduction
Milk and its derivatives have been the main food in human nutrition since ancient times. They are some of the most important products of animal origin, considered strategic products because they are complete foods, easily assimilated, with great nutritional value and antitoxic role [1,2,3].
Powdered milk is the main form of preserving milk, which has a significantly important role in human diet, due to its long preservation, removing the shortcomings of liquid milk which are very easily alterable. For newborns, powdered milk is the ideal food. Specialists recommend that, in the first four to six months of life, the baby's diet be exclusively natural, represented by breast milk. However, there are situations when this is not possible, and then mixed feeding (breast milk supplemented with powdered milk) or artificial feeding (powdered milk exclusively) are suggested. Powdered milk also contributes nutritionally, functionally and economically to a variety of food formulas that include pastries, confectionery, dairy, recombinant milk, meat, energy drinks and prepared foods [4,5,6].
Powdered milk is a dry product obtained by dehydration, with a high content of dry matter (about 97%), which gives it a high shelf life. Powdered milk manufacturing technology consists of three main phases: preliminary treatment, concentration and drying [4,6]. Powdered milk is a source of high-quality protein with high bioavailability of amino acids [7]. Powdered milk contains many soluble vitamins and minerals, including calcium, phosphorus and magnesium and can be used to fortify a wide range of foods especially due to its high calcium content [8]. Calcium plays an important role in maintaining bone health and preventing osteoporosis. Calcium is also crucial for nerve conditions, muscle contraction, heart rate, blood clotting, energy production and immune system maintenance [9,10,11,12,13].
Powdered milk may contain food additives such as stabilizers, emulsifiers and anti-caking agents. A good quality milk powder must have the following characteristics: easy reconstitution (homogeneous liquid) when dissolved in water without macroscopic particles, must be free of abnormal tastes and odors, must not contain pathogenic germs (Salmonella, Staphylococcus, etc.), must not contain antibiotics, must not contain various residues, which come from the production, harvesting and preservation of milk as a raw material, must not show changes in structure and physical-chemical composition, which reduce the nutritional value and technological processing skills [14,15,16].
Although powdered milk is a type of food with a high nutritional value, concentrated in a small volume, with a long storage capacity, it is not always free of physical, chemical and/ or microbiological toxins, which pose risks for the health of the consumers, thus requiring different improvements. The technological flow of processing by introducing new modern equipment as well as applying the procedures of the food safety system is common. Powdered milk can be contaminated with heavy metals during processing (from machines), as well as during storage, transportation and some packaging procedures. However, the highest risk is the quality of the milk used as a raw material. The degree of toxicity depends on the nature of the metal, its solubility and its compounds, the cumulative effect of some metals in some tissues, the degree of electrolyte imbalance produced in the body, the dose of the metal and its duration of action [17,18,19].
The use of modern methods for determining toxic metals has allowed their detection in most foods, establishing a close correlation between environmental pollution and the presence of heavy metals in food [20,21].
Milk and dairy products contain a range of toxic and non-toxic metals that are present in a wide variety, also having very different origins, including grazing cows on land that contains high levels of certain metals or it is contaminated by industrial or human activities. The main elements that are involved in altering the safety of the elements are: arsenic, cadmium, lead and mercury, sometimes copper, iron, selenium and zinc which are considered normal in milk and dairy products at certain concentrations, but which can cause technological problems at high concentrations [22,23,24,25]. Powdered milk is a major source of essential metals and any changes in the concentration of these elements in milk can lead to nutritional problems [26,27].
The present study, regarding the identification of contaminants with toxic potential in milk powder, was conducted on a number of seven milk powder samples from different producers sold on the Romanian market, samples that were collected from the original packaging: P1, P 2, P3, P4, P5, P6 and P7. Samples P2 and P6 are used in the diet of newborns, and the others are used in the nutrition of adults.
Materials and Methods
The X-ray fluorescence (XRF) method was used to determine some of the samples in the study. The quantitative analysis performed through the X-ray fluorescence (XRF) method was performed using an ED-XRF spectrometer ARL QUANT´X (Thermo Scientific). The software UniQuant 4 (Thermo Scientific) allows quantitative analysis without the use of standard or calibration curves when the sample matrix is known (for example: protein, water, metals/ alloys, wood, etc.). If the sample matrix is not provided in the program database, the only analytic solution is to use standard and calibration curves. The detection limit of the device depends on the atmosphere in which the sample is operated, which can be helium, vacuum or air.
In the present study, the samples were analyzed in vacuum. In this situation, the detection limit is 5 ppm. In order to identify elements below 5 ppm, it is necessary to approach another method, such as inductively coupled plasma spectroscopy (ICPS) or atomic absorption spectrometry. XRF spectroscopy is widely used for the analysis of elements, both qualitatively and quantitatively, environmental, geological, biological, industrial and other samples. Compared to other competitive techniques, such as atomic absorption spectroscopy (AAS) or inductively coupled plasma spectroscopy (ICPS), XRF has the advantage of being non-destructive, multi-element based, fast and efficient. In addition, it has a uniform detection limit for a large part of the periodic table and it is applied to a wide range of concentrations, from 100% to several parts per million (ppm). Its main disadvantage is that the assays are generally limited to elements heavier than fluorine [28,29].
The samples were analyzed in their raw state without chemical preparation, considering the matrix as a protein mixture, the same matrix provided in the program software. The water concentration was not taken into account.
Sample preparation: after calcination, the samples were homogenized by trituration with 0.5 g of graphite and then analyzed through X-ray fluorescence. Triplicate determinations were performed for each sample. The results are expressed as mean ± S.D (standard deviation) of triplicate analysis. For each element four standards were used: Standard 1:10 ppm, Standard 2: 500 ppm, Standard 3: 1 %, Standard 4: 10%.
Results
The concentration of the following elements was analyzed: potassium (K), chlorine (Cl), calcium (Ca), phosphorus (P) and aluminum (Al).
The characteristics of the milk powder samples studied according to the nutritional information submitted by the producers are presented in Table 1.

Table 1.
Characteristics of the analyzed milk powder samples.
The level of the elements determined in the analyzed milk powder samples are shown in Table 2.

Table 2.
Concentrations of the analyzed elements in powdered milk samples.
Concentrations were obtained as a protein mixture, but for which the mass ratio of hydrogen was not taken into account. In all analyzed samples, the average of the contaminant values far exceeds the AML (maximum allowable limit) for each element [30]. Respectively, the maximum values obtained not only exceed the maximum allowable limit of these elements, but they are also three or four times higher.
Powdered milk is rich in potassium, present in large quantities in all analyzed samples (Figure 1). The presence of potassium in the body highlights the many properties of this mineral element, namely: it ensures the functioning of osmotic pressure, it contributes to maintaining the acid-base balance of cells, revitalizing the body, thus maintaining its health, it increases diuresis and the elimination process of sodium, it promotes neuromuscular excitability, it has a plastic role, intervening in permeability processes, in carbohydrate metabolism, it is a cardiac tonic, stimulator of bowel movements, it contributes to the proper functioning of the adrenal glands, the elimination of organic waste and it ensures the water balance in the body [31,32,33].
Potassium toxicity is highlighted if administered more than 18g daily. Excess potassium can cause muscle fatigue, arrhythmias, spasmophilia, tetany and possibly cardiac arrest. Increasing the daily potassium intake in the presence of a high-salt diet can help decrease calcium excretion [34].
In Figure 1, it can be easily seen that the maximum limit of potassium value, which was recorded according to this study, is present in sample P4. Both sample P4 and samples P3, P5 and P6 show values of the potassium element two times higher than the AML of 3,500 ppm.
Sample P7 is the only sample with a potassium level of 1,730 ppm, which places it below the maximum limit allowed by the legislation in force for this element from the point of view of this indicator.
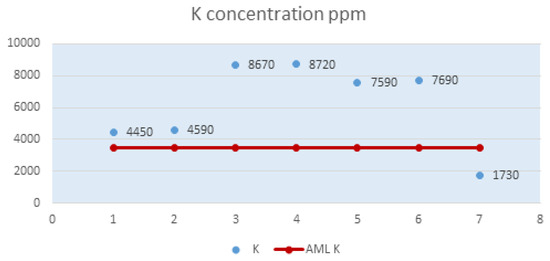
Figure 1.
Potassium level (K) in powdered milk samples.
The essential supplier of calcium for the children's body is milk and its derivatives, only 100 g of skimmed milk powder contains 1,300 mg of calcium. The currently available data support the recommendations for a daily intake of calcium between 1,200 and 1,500 mg for this category [35,36,37,38].
Figure 2 graphically shows the level of calcium in the seven milk powder samples, a level that exceeds the maximum allowable limit of 2,500 ppm in the first six samples.
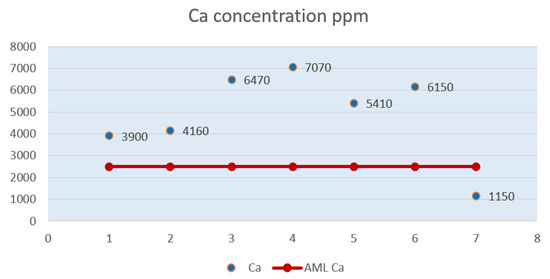
Figure 2.
Calcium (Ca) level in powdered milk samples.
Of the seven samples, P3 and P4 had a high Ca content, almost triple than that of AML.
Sample P7 had a lower Ca value than the other samples, below the maximum allowable limit of 2,500 ppm. For sample P5, the calcium value per 100 mg of milk powder displayed on the package was 5,140 ppm, twice as much as the maximum allowable limit. Also, the value resulting from the laboratory analysis does not coincide with the one displayed on the package, the latter being 2,187 ppm. Sample P6, with a concentration of 6,150 ppm Ca, has a level almost twice higher than the AML for calcium.
Sample P1 and sample P2 show calcium levels closer to the maximum allowable limit compared to samples P3 and P4.
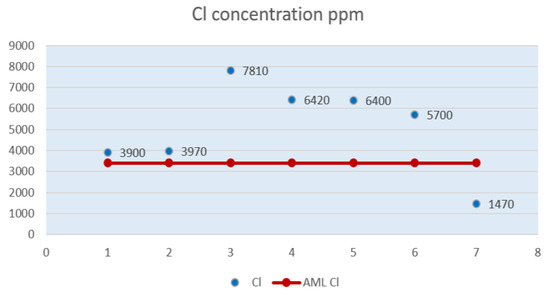
Figure 3.
Chlorine level (Cl) in powdered milk samples.
In the Figure 3, the highest chlorine values can be observed in samples P3, P4, P5, P6.
Sample P1 and sample P2 have chlorine levels close to the maximum allowable limit of 3,400 ppm. Sample P3 shows the highest chlorine level, more than twice the maximum allowable limit of 3,400 ppm. Samples P4 and sample P5 have chlorine levels close to the value obtained for chlorine in sample P6.
Sample P6, with a chlorine value of 5,700 ppm, exceeds the maximum allowable limit by 2,300 ppm. Sample P7 shows a chlorine level of 1,470 ppm, below the AML. Sample P5 is the only one that had displayed the presence of chlorine in the milk powder content on the packaging, in the ingredients, namely in 350 mg of milk powder there are 1,575 ppm chlorine, a value way below the one resulting from laboratory tests.
Aluminum can also be derived from aluminum and potassium silicate (E 555) and calcium and aluminum silicate (E 556), additives used by milk powder producers with the intention of "enriching" the quality of milk powder, which actually might trigger or worsen the evolution of Alzheimer's disease.
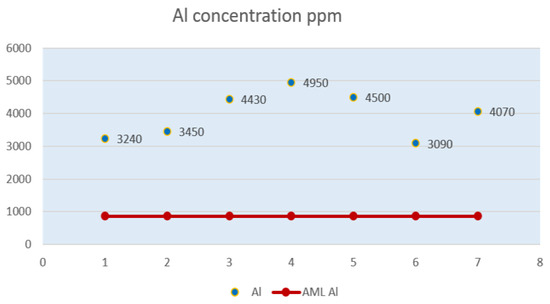
Figure 4.
Aluminum (Al) level in powdered milk samples.
Figure 4 shows how the level of aluminum in all powdered milk analyzed samples far exceeds the maximum allowable limit.
From the samples subjected to laboratory analysis, samples P3, P4 and P5 have the highest level of aluminum, respectively 4,430 ppm, 4,950 ppm, 4,500 ppm, values almost six times higher than the AML.
The value of aluminum in sample P1, close to that in sample P6, is more than three times higher than the AML. The level of aluminum in sample P2 is four times higher than the AML, and that in sample P7 is almost five times higher. Sample P6 shows the lowest level of aluminum in all seven samples, but with a value above the AML.
The values revealing the presence of aluminum in the milk powder content were not displayed on any of the samples.
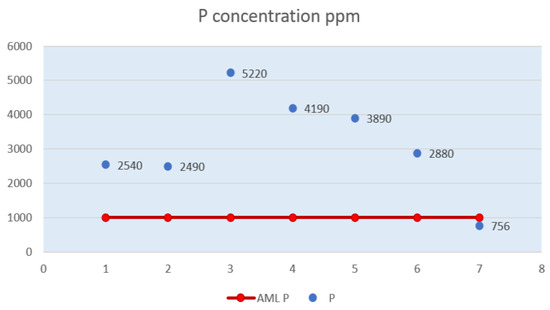
Figure 5.
Phosphorus level (P) in powdered milk samples.
Figure 5 shows a very high level of phosphorus in sample P3, a value more than five times higher than the maximum allowable limit.
At a difference of 1,030 ppm from sample P3, there is the phosphorus level in sample P4. A phosphorus concentration (3,890 ppm) close to that in sample P4 is also found in sample P5. Samples P3, P4, P5 have the highest values of phosphorus, exceeding the AML.
The phosphorus level in sample P6 is almost three times the maximum allowable limit. Sample P7 shows a lower value, of 756 ppm, below the AML of 1,000 ppm. Of the seven samples, sample P5 is the only one that has the same amount of phosphorus per 100 g of powdered milk and prepared milk as the one displayed on the package, obviously this time the values found exceed the declared value.
Discussions
With the exception of the aluminum level exceeding the maximum allowable limit of 867 ppm in all seven analyzed samples, the minimum levels of the other elements are below the maximum allowable limits.
In the case of sample P7, except for the aluminum element whose value exceeds the AML, it is the only one that shows levels for K, Ca, Cl, P below the AML (Table 1).
The highest levels for K, Ca and Al are found in sample P4. The highest levels for Cl and P are found in sample P3. Sample P7 represents the lowest powdered milk for the elements: K, Ca, Cl, P. Compared to the other samples, sample P1 and sample P2 show levels that fall within the average concentration recorded for these elements.
The levels of the five elements present in samples P5 and P6 are close to the values in sample P4, and in the case of samples P3 and P4, all five investigated elements showed increased values.
Of the seven analyzed samples, the only one that has the presence of potassium on the package is sample 5: 350 g of powder contains 2,030 ppm of potassium, a value much lower than that determined through laboratory analysis (7,590 ppm).
Potassium can be present in milk powder and in the form of aluminum and potassium silicate (E 555), potassium phosphate (E 340), potassium iodide, potassium chloride (which is known to cause gastric ulceration in large quantities).
During childhood and adolescence, the body uses mineral calcium to build strong bones, a process that ends after ten years. Calcium in the bones begins to decline in youth and even more begins to decline in old age, especially in women. Young people, especially girls, whose diets do not contain nutrients to strengthen their bones, are at a higher risk of developing osteoporosis, a bone disease that increases the risk of fractures. Younger children and babies with insufficient calcium and vitamin D (which helps the absorption of calcium) are at risk for rickets. Rickets is a disease that softens bones and can cause crooked legs, slow growth and sometimes cause muscle aches and weakness. Calcium plays an important role in muscle contraction, transmitting messages through nerves and releasing hormones. If the level of calcium in the blood is low (due to insufficient food intake), calcium will mobilize from the bones to ensure the proper functioning of the cells. Both insufficiency and excess are detrimental to the body's acid-base balance, deficiencies manifested through hypercalcemia and other diseases [39,40,41].
Chlorine is indicated in physical fatigue, anorexia, mild forms of diabetes, hypercholesterolemia, and atherosclerosis, as well as in gout (as a cure with chlorinated mineral waters), in regulating bowel movements and stimulating digestion. Excess chlorine causes: gastric hyperacidity, destruction of intestinal microflora, hypertension. By inhibiting the useful microflora, a series of disorders that lead to avitaminosis (many vitamins are synthesized in the large intestine) and constipation occur. Overloading the body with chlorine has harmful and irritating effects on the kidneys [42,43,44].
The daily requirement of chlorine is 2-3 grams for adults, which corresponds to 3-5 grams of salt, a chemical composed of 61% chlorine and 39% sodium [45].
The presence of chlorine in the analyzed powdered milk samples is justified by the addition of a certain amount of table salt to the formula in the form of sodium chloride. Sodium in the salt composition can only be calculated in a helium atmosphere, on "pastillate" samples, which is why it could not be identified in the case of powder samples.
Moreover, from the high values, far above the AML, we could deduce that the dishes, the appliances used in the powdered milk technology were not properly disinfected, washed with detergents that have the element chlorine in their composition.
At the European level, aluminum has been reported to be present excessively in the diet. This element is everywhere, in the air, water, soil, and in certain quantities is not harmful to the body. However, lately, the products we consume, from food to medicine, water and even the surrounding objects, put us in contact with an overdose of this toxic metal. The effects are among the most devastating: disorders of the nervous system, including Alzheimer's. A higher amount of aluminum in the body leads to intoxications that are manifested through headaches, nausea, vomiting. However, chronicity is even more harmful, and when specific manifestations occur, it may be too late. The most exposed are mainly children, pregnant women, patients with kidney disorders and the elderly. Aluminum is found in relatively small amounts in powdered milk, but the body retains a little each time [46,47].
Aluminum can reach milk powder through the packaging that is made of this element. It is also possible to use containers made of this material during the technological process of milk powder production.
Phosphorus is a macronutrient having the following properties: it controls the balance of calcium as well as acid-base, it enters many combinations with proteins, lipids and carbohydrates, it enters the constitution of bones, teeth and blood, it has a special importance in the production of the nervous, intellectual and sexual energy, regulating the heartbeat and helping the normal functioning of the kidneys [48,49].
Excess phosphorus has a direct effect on calcium, which is eliminated from the bones into the blood. This loss of calcium is at the origin of bone and dental fragility, the bones becoming more sensitive to fractures and osteoporosis. Excess phosphorus can cause low serum calcium concentration levels [50,51].
The daily requirement of phosphorus for adults is 1-2 grams, approximately equal to or slightly higher than the need for calcium, except for children, who require a calcium intake 20% higher than that of phosphorus. Pregnant women, as well as those who are breastfeeding, need 5 grams of phosphorus per day. Phosphorus poisoning is combated by increasing the intake of magnesium and potassium, antagonists of phosphorus, along with iron and aluminum in large quantities [52].
Conclusions
A number of seven milk powder samples from different producers sold on the Romanian market were analyzed.
Following the analyses performed, deviations of the concentrations of some constituent elements were found compared to the norms in force or to the product specifications for: potassium, calcium, chlorine, aluminum and phosphorus. In a single test, the elements potassium, calcium, chlorine, phosphorus showed levels below the maximum allowable limit. The most majority of samples analyzed had levels of elements K, Ca, Cl, Al, P well above the AML. The maximum level of potassium is more than twice the maximum allowable limit, which is found in four of the seven analyzed milk powder samples. Six of the samples exceeded the maximum allowable limit for calcium, of 2,500 ppm, of which four samples had a double calcium content, compared to the AML. The maximum allowable limit for chlorine is exceeded for six samples and even twice as high for sample P3. The level of aluminum resulting from laboratory analyses, in all milk powder samples, exceeds the AML, the maximum level obtained being almost six times above the maximum allowable limit, and the minimum level obtained was three times above the maximum allowable limit. The maximum phosphorus concentration (5,220 ppm) recorded in a single sample exceeds the maximum allowable limit five times. In general, the packaging does not show details about the powdered milk content, about the existence of the elements found through laboratory analysis.
The concentrations of constituents are not always specified on food labels, and if this information appears, they are not always the correct values, probably because the intake of food additives is not taken into account. Many potentially toxic elements can come from additives introduced by milk powder producers with the intention of "enriching" the quality of milk powder: aluminum from aluminum and potassium silicate (E 555), aluminum and calcium silicate (E 556), etc.
Conflict of interest disclosure
There are no known conflicts of interest in the publication of this article. The manuscript was read and approved by all authors.
Compliance with ethical standards
Any aspect of the work covered in this manuscript has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
References
- Dror, D.K.; Allen, L.H. Dairy product intake in children and adolescents in developed countries: trends, nutritional contribution, and a review of association with health outcomes. Nutr Rev. 2014, 72, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Vissers, P.A.; Streppel, M.T.; Feskens, E.J.; de Groot, L.C. The contribution of dairy products to micronutrient intake in the Netherlands. J Am Coll Nutr. 2011, 30 (Suppl. 1), 415S–421S. [Google Scholar] [CrossRef]
- Prentice, A.M. Dairy products in global public health. Am J Clin Nutr. 2014, 99 (Suppl. 5), 1212S–1216S. [Google Scholar] [CrossRef] [PubMed]
- Tamime, A.Y. Dried milk products. Dairy powders and concentrated milk products; Blackwell Pub. Ltd.: Oxford, UK, 2009; pp. 231–245. ISBN 978-1-4051-5764-3. [Google Scholar]
- Claeys, W.L.; Cardoen, S.; Daube, G.; De Block, J.; Dewettinck, K.; Dierick, K.; De Zutter, L.; Huyghebaert, A.; Imberechts, H.; Thiange, P.; Vandenplas, Y.; Herman, L. Raw or heated cow milk consumption: review of risks and benefits. Food Control. 2013, 31, 251–262. [Google Scholar] [CrossRef]
- Chandan, R.C.; Kilara, A.; Shan, N.P. Dairy Processing and Quality Assurance; Wiley-Blackwell, 2015; ISBN 978-1-118-81031-6. [Google Scholar]
- Sharma, A.; Jana, A.H.; Chavan, R.S. Functionality of milk powders and milk-based powders for end use applications-a review. Comprehensive Reviews in Food Science and Food Safety 2012, 11, 518–528. [Google Scholar] [CrossRef]
- McGregor, R.A.; Poppitt, S.D. Milk protein for improved metabolic health: a review of the evidence. Nutr Metab (Lond). 2013, 10, 46. [Google Scholar] [CrossRef]
- Caroli, A.; Poli, A.; Ricotta, D.; Banfi, G.; Cocchi, D. Invited review: dairy intake and bone health: a viewpoint from the state of the art. J Dairy Sci. 2011, 94, 5249–5262. [Google Scholar] [CrossRef]
- Goulding, A.; Rockell, J.E.; Black, R.E.; Grant, A.M.; Jones, I.E.; Williams, S.M. Children who avoid drinking cow's milk are at increased risk for prepubertal bone fractures. J Am Diet Assoc. 2004, 104, 250–253. [Google Scholar] [CrossRef]
- Rizzoli, R. Dairy products, yogurts, and bone health. Am J Clin Nutr. 2014, 99 (Suppl. 5), 1256S–1262S. [Google Scholar] [CrossRef]
- Skinner, M.L.; Simpson, J.A.; Buchholz, A.C. Dietary and total calcium intakes are associated with lower percentage total body and truncal fat in young, healthy adults. J Am Coll Nutr. 2011, 30, 484–490. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; Kovacs, C.S.; Mayne, S.T.; Rosen, C.J.; Shapses, S.A. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Dudriková, E.; Pol’aková, L.; Pukáčová, J. Health and hygienic conditions of Ewe’s milk processing from the aspect of food safety. Potravinarstvo. 2010, 4, 14–18. [Google Scholar] [CrossRef]
- FAO; WHO. Codex Alimentarius: Milk and Milk Products, 2nd ed.; Food and Agriculture Organization of the United Nations and World Health Organization: Rome, 2011; ISSN 0259-2916. [Google Scholar]
- EC (European Commission). Overview of Microbiological Criteria for Foodstuffs in Community Legislation in Force. Available online: https://ec.europa.eu/food/safety/biological-safety/food-hygiene/microbiological-criteria_en.
- Arianejad, M.; Alizadeh, M.; Bahrami, A.; Arefhoseini, S.R. Levels of Some Heavy Metals in Raw Cow's Milk from Selected Milk Production Sites in Iran: Is There any Health Concern? Health Promot Perspect. 2015, 5, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Năstăsescu, V.; Mititelu, M.; Goumenou, M.; Docea, A.O.; Renieri, E.; Udeanu, D.I.; Oprea, E.; Arsene, A.L.; Dinu-Pîrvu, C.E.; Ghica, M. Heavy metal and pesticide levels in dairy products: Evaluation of human health risk. Food Chem Toxicol. 2020, 146, 111844. [Google Scholar] [CrossRef]
- Rasane, P.; Jha, A.; Kumar, A.; Sharma, N. Reduction in phytic acid content and enhancement of antioxidant properties of nutricereals by processing for developing a fermented baby food. J Food Sci Technol. 2015, 52, 3219–3234. [Google Scholar] [CrossRef]
- Mititelu, M.; Moroşan, E.; Neacșu, S.M.; Ioniţă, E.I. Research regarding the pollution degree from Romanian Black Sea coast. Farmacia. 2018, 66, 1059–1063. [Google Scholar] [CrossRef]
- Mititelu, M.; Ghica, M.; Ionita, A.C.; Moroşan, E. The influence of heavy metals contamination in soil on the composition of some wild edible mushrooms. Farmacia 2019, 67, 398–404. [Google Scholar] [CrossRef]
- Awan, A.; Naseer, M.; Iqbal, A.; Ali, M.; Iqbal, R.; Iqbal, F. A study on chemical composition and detection of chemical adulteration in tetra pack milk samples commercially available in Multan. Pak J Pharm Sci. 2014, 27, 183–186. [Google Scholar]
- Motofei, I.G.; Rowland, D.L.; Georgescu, S.R.; Tampa, M.; Paunica, S.; Constantin, V.D.; Balalau, C.; Manea, M.; Baleanu, B.C.; Sinescu, I. Post-Finasteride Adverse Effects in Male Androgenic Alopecia: A Case Report of Vitiligo. Skin Pharmacol Physiol. 2017, 30, 42–45. [Google Scholar] [CrossRef]
- Qin, L.; Wang, X.; Li, W.; Tong, X.; Tong, W. The minerals and heavy metals in cow’s milk form China and Japan. Journal of Health Science 2009, 55, 300–305. [Google Scholar] [CrossRef]
- Tamime, A.Y. Milk Processing and Quality management; Wiley-Blackwell: Chichester, UK, 2008; ISBN 978-1-405-14530-5. [Google Scholar]
- Aumaître, A. Quality and safety of animal products. Livestock Production Science. 1999, 59, 113–124. [Google Scholar] [CrossRef]
- Bălălău, O.D.; Olaru, O.G.; Dumitru, A.V.; Păunică, I.; Stănescu, A.D. Maternal infections with an increased risk of transmission to the foetus; a literature review. J Clin Invest Surg. 2020, 5, 66–72. [Google Scholar] [CrossRef]
- Pashkova, G.V. X-ray Fluorescence Determination of Element Contents in Milk and Dairy Products. Food Analytical Methods 2009, 2, 303–310. [Google Scholar] [CrossRef]
- Stankey, J.A.; Akbulut, C.; Romero, J.E.; GovindasamyLucey, S. Evaluation of X-ray fluorescence spectroscopy as a method for the rapid and direct determination of sodium in cheese. J Dairy Sci. 2015, 98, 5040–5051. [Google Scholar] [CrossRef]
- FAO; WHO. Codex Alimentarius, Standard for Whole Milk Powder, Partly Skimmed Milk Powder and Skimmed Milk Powder (A-5-1971) Adopted in 1999. Amended in 2010, 2013, 2014, 2016, 2018. Available online: http://www.fao.org/fao-who-codexalimentarius/shproxy/en/?lnk=1&url=https%253A%252F%252Fwork space.fao.org%252Fsites%252Fcodex%252FStandard s%252FCXS%2B207-1999%252FCXS_207e.pdf.
- Shieh, C.C.; Coghlan, M.; Sullivan, J.P.; Gopalakrishnan, M. Potassium channels: molecular defects, diseases, and therapeutic opportunities. Pharmacol Rev. 2000, 52, 557–594. [Google Scholar] [CrossRef]
- Chiriță, C.; Ștefănescu, E.; Zbârcea, C.E.; Negreș, S.; Bratu, M.; Nuță, D.C.; Limban, C.; Chiriță, I.C.; Marineci, C.D. Experimental pharmacological research regarding some new quinazolin-4-ones derivatives. J Mind Med Sci. 2019, 6, 121–129. [Google Scholar] [CrossRef]
- Brophy, D.F. Disorders of potassium and magnesium homeostasis. In Pharmacotherapy: A Pathophysiologic Approach, 9th ed.; DiPiro, J.T., Talbert, R.L., Yee, G.C., et al., Eds.; McGraw-Hill: New York, NY, USA, 2014. [Google Scholar]
- WHO. Potassium intake for adults and children. Available online: https://www.who.int/nutrition/publications/guidelines/ potassium_intake_printversion.pdf.
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Press: Washington, DC, USA, 2011; ISBN 978-0-309-16394-1. [Google Scholar] [CrossRef]
- Zemel, B.S. Dietary calcium intake recommendations for children: are they too high? Am J Clin Nutr. 2017, 105, 1025–1026. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Motofei IG Rowland, D.L.; Baconi, D.L.; Georgescu, S.R.; Paunica, S.; Constantin, V.D.; Balalau, D.; Paunica, I.; Balalau, C.; Baston, C.; Sinescu, I. Therapeutic considerations related to finasteride administration in male androgenic alopecia and benign prostatic hyperplasia. Farmacia. 2017, 65, 660–666. [Google Scholar]
- Bailey, R.L.; Dodd, K.W.; Goldman, J.A.; Gahche, J.J.; Dwyer, J.T.; Moshfegh, A.J.; Sempos, C.T.; Picciano, M.F. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr. 2010, 140, 817–822. [Google Scholar] [CrossRef]
- Bonjour, J.P. Calcium and phosphate: a duet of ions playing for bone health. J Am Coll Nutr. 2011, 30 (Suppl. 1), 438S–48S. [Google Scholar] [CrossRef]
- Bălălău, O.D.; Bacalbașa, N.; Olaru, O.G.; Pleș, L.; Stănescu, D.A. Vaginal birth after cesarean section – literature review and modern guidelines. J Clin Invest Surg. 2020, 5, 13–17. [Google Scholar] [CrossRef]
- Gueguen, L. Calcium, phosphorus. Apports Nutritionnels Conseillees pour la Population Francaise (Recommended nutrient intakes for the French population); Tech & Doc: Paris, 2001; pp. 131–140. [Google Scholar]
- International Programme on Chemical Safet; World Health Organization. Chlorine and hydrogen chloride. World Health Organization. 1982. Available online: https://apps.who.int/iris/handle/10665/37072.
- Health and Safety Executive. EH40/2005 Workplace Exposure Limits; The Stationery Office: London, 2005. [Google Scholar]
- Winder, C. The toxicology of chlorine. Environ Res. 2001, 85, 105–114. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Chlorine in Drinkingwater, Background document for development of WHO Guidelines for Drinking-water Quality. 2003. Available online: https://www.who.int/water_sanitation_health/dwq/chlor ine.pdf.
- Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Food Contact Materials (AFC). Safety of aluminium from dietary intake. The EFSA Journal. 2008, 754, 1–34. Available online: https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j. efsa.2008.754.
- van Oers, H.; Schlebusch, L. Indicators of psychological distress and body image disorders in female patients with breast cancer. J Mind Med Sci. 2020, 7, 179–187. [Google Scholar] [CrossRef]
- Goretti Penido, M.; Alon, U.S. Phosphate homeostasis and its role in bone health. Pediatr Nephrol. 2012, 27, 2039–2048. [Google Scholar] [CrossRef]
- Moe, S.M.; Zidehsarai, M.P.; Chambers, M.A.; Jackman, L.A.; Radcliffe, J.S.; Trevino, L.L.; Donahue, S.E.; Asplin, J.R. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol. 2011, 6, 257–264. [Google Scholar] [CrossRef]
- Trautvetter, U.; Jahreis, G.; Kiehntopf, M.; Glei, M. Consequences of a high phosphorus intake on mineral metabolism and bone remodeling in dependence of calcium intake in healthy subjects - a randomized placebo-controlled human intervention study. Nutr, J. 2016, 15, 7. [Google Scholar] [CrossRef]
- Ismail, E.A.; Al-Mutairi, G.; Al-Anzy, H. A fatal small dose of phosphate enema in a young child with no renal or gastrointestinal abnormality. J Pediatr Gastroenterol Nutr. 2000, 30, 220–221. [Google Scholar] [CrossRef]
- Nutrition for Kidney Disease - UW Health. Available online: https://www.uwhealth.org/healthfacts/nutrition/320.pdf.
© 2021 by the author. 2021 Magdalena Mititelu, Lucian Hîncu, Emma Adriana Ozon, Daniela Luiza Baconi, Ioana Paunica, Oana Denisa Bălălău