Abstract
The current treatment of Attention Deficit Disorder and Attention Deficit with Hyperactivity consists mainly in the administration of Straterra (Atomoxetine) Concerta and Ritalin (Methylphenidate). The FDA warned that the products might increase systolic, diastolic blood pressure, and lead to ventricular arrhythmias. Arrhythmic events and sudden cardiac death were described in adults with preexistent heart disease. However, studies on children have failed to demonstrate a clear association between the arrhythmic events and these drugs, as demonstrated in adults. What should the attitude of the pediatric psychiatrist be towards the administration of these products? What examination should be made by the psychiatrist before referring the child to a pediatric cardiologist? Which patients need a cardiology consultation before the administration of these products? What is the follow-up after drug initiation? These are some questions that this paper aims to answer.
Introduction
Attention deficit, with or without hyperactivity is a common condition during childhood, affecting 5% of the children [1,2]. This condition poses problems regarding the child’s adaptation to social life and educational activity during kindergarten, school or high-school. The most effective drugs in the treatment of these conditions are psychostimulants such as methylphenidate and non-stimulants: Atomoxetine [3,4], two drugs that have a phenyl-piperidine and phenyl-propane structure (Figure 1). Methylphenidate induces the release of norepinephrine and dopamine at the synaptic level and stimulates postsynaptic receptors (Figure 2), and Atomoxetine is a selective inhibitor of norepinephrine re-uptake (Figure 3). Increased plasma catecholamines will increase cardiac inotropism and heart rate through a beta-1 adrenergic effect and increase blood pressure through an alpha-adrenergic effect. In 2006, the FDA issued a major warning on psychostimulants that they can increase blood pressure and drew attention to other cardiac side effects such as ventricular arrhythmias and sudden cardiac death. For these reasons, the contraindication of psychostimulants has been formulated in children with known arrhythmias or known heart diseases such as congenital heart diseases, acquired myocarditis, dilated or hypertrophic cardiomyopathy. However, serious studies published in the New England Journal of Medicine [5] and the Journal of the American Medical Association [6] have shown that these psychostimulants do not increase blood pressure nor the risk of ventricular arrhythmias. According to these studies, which show the opposite of those formulated by the FDA, clinical guidelines developed by psychiatrists decided to perform a clinical screening of each child before the administration of the psychostimulant. In case of any clinical suspicion of known cardiac disease, the child should be referred to a cardiologist for an ECG and additional cardiac examinations. In a recent paper, David et al. [7] investigated the efficacy of a combined treatment: cognitive-behavioral therapy + Atomoxetine vs. psychotherapy vs. Atomoxetine for children with Attention-Deficit/Hyperactivity Disorder (ADHD). They found that the combined treatment was superior to medication alone on ADHD symptoms.
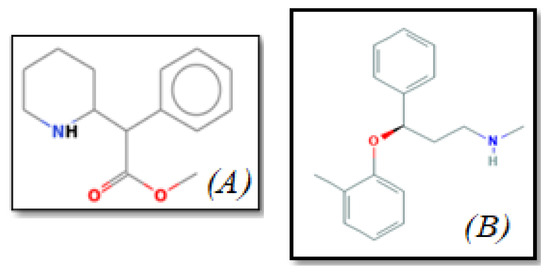
Figure 1.
The chemical structure of (A) Methylphenidate and (B) Atomoxetine.
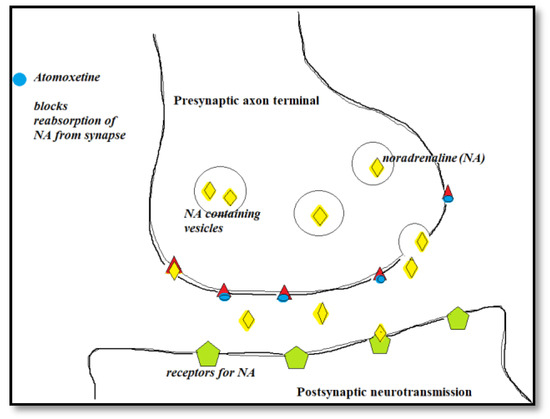
Figure 2.
The mechanism of action for Atomoxetine.
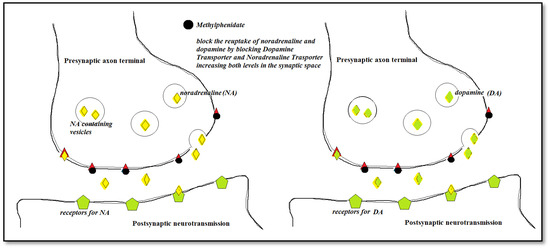
Figure 3.
The mechanism of action for Methylphenidate.
Discussions
Cardiac arrhythmias in children with Attention Deficit who have not received any medication
Polanczyk et al. have shown that the parasympathetic nervous system is not fully developed in children with attention deficit and hyperactivity, which translates into lower parasympathetic tone [8]. This may be responsible for a higher plasma concentration of adrenaline and norepinephrine, a higher excretion of catecholamines, as demonstrated by Dvorakova et al. [9] by measuring urinary catecholamines, increased heart rate, sinus tachycardia, and atrial or ventricular premature contractions. This explains why some children with attention deficit may also suffer from arrhythmias before the beginning of the treatment with Methylphenidate or Atomoxetine. This mechanism also explains the beneficial effect of the administration of beta-blockers when the treatment with Atomoxetine or Methylphenidate induces atrial or ventricular premature contractions, so that the psychostimulant treatment can be continued safely.
Considering that the two drugs can increase the endogenous concentration of catecholamines, we can explain the arrhythmic effects by a beta-1 adrenergic effect which increases inotropism and also the heart rate.
Prasad et al. [10] evaluated 5,289 children with attention deficit hyperactivity disorder with the aim of identifying arrhythmias that would contraindicate the administration of Atomoxetine or Methylphenidate. None of all these children suffered from ECG arrhythmias, so none of them had any contraindication in the administration of psychostimulants.
Arrhythmic side effects of Methylphenidate and Atomoxetine
The first mention of the arrhythmic side effects of Methylphenidate dates back to 2012, when Schellman et al. [11] demonstrated a 1.8-fold increased risk of ventricular arrhythmias and sudden cardiac death in the adults treated with Methylphenidate. Although the study was performed on a large number of individuals, it was non-randomized and the group in which ventricular arrhythmias were recorded had the peculiarity that it included many patients with pre-existing cardiac impairments. One year earlier, in a large-scale retrospective study including 150,000 individuals, Habel et al. [12] revealed that neither Methylphenidate nor Atomoxetine increases the risk of ventricular arrhythmias or the risk of sudden cardiac death. It is worth mentioning that the two above-mentioned studies were conducted on the adult population and not on children.
Two other large studies by the same author, one on 21,000 patients receiving Atomoxetine [13] and another on 44,000 patients receiving Methylphenidate [14], did not reveal an increased risk of ventricular arrhythmias or sudden cardiac death after drug administration.
Arj et al. administered Methylphenidate to 141 children with attention deficit and hyperactivity disorder and found no ECG changes in arrhythmias or significantly increased blood pressure levels in these children.
Tanidir et al. [15] administered Atomoxetine to 41 children and monitored them by means of ECG and Holter ECG. None of these children developed significant arrhythmias during the follow-up period.
In an experimental study, Scherer et al. [16] showed that Atomoxetine has the ability to block hERG channels. This study could not specify whether the blockage of hERG channels has clinical implications, as it was a study performed on renal embryonic cells. There are, however, reports on the prolongation of the QT interval by Atomoxetine with torsade de pointes in which this drug was incriminated, but obviously the value of these publications is small compared to studies performed on larger populations. Studies that have been performed on adults show that the short- or long-term administration of Methylphenidate or Atomoxetine does not increase the risk of QT prolongation [17,18,19].
The administration of Methylphenidate and Atomoxetine to Children with Cardiac Arrhythmias
Buchhorn et al. examined 100 children with Attention Deficit with and without Hyperactivity, not treated with drugs. Of these 100 cases, arrhythmias were observed in 9 children: 9 had premature atrial contractions, 4 had premature ventricular contractions and periods of accelerated idioventricular rhythm. Accelerated idioventricular rhythm is considered “physiological arrhythmia” in children and does not require treatment. Six of these children with arrhythmia received Methylphenidate and none experienced worsening of the clinical arrhythmia, therefore Methylphenidate could be continued for a long period of time.
There are also studies that evaluated the QT interval in children receiving Atomoxetine. It is worth mentioning that QT measurement differs from one cardiologist to another, the best overlapping values being found in arrhythmologists [20]. Thus, if QT interval measurements are made by different cardiologists, different values are obtained. This is the reason why 8.6% of the children who were administered the placebo drug had increased QT intervals of 30 ms in the meta-analysis by Wernicke et al [21].
Another problem with studies reporting medication-related arrhythmias is that they do not specify the type of arrhythmias: benign arrhythmias such as sinus tachycardia, premature atrial or ventricular contractions, QT prolongation, or malignant arrhythmias such as ventricular tachycardia and ventricular fibrillation [22,23]. The differentiation is important because the risk of sudden death differs depending on the type of arrhythmia. The study by Shin et al. [24] revealed a relatively increased risk of arrhythmias 1.6:1 in methylphenidate users compared to non-users. It is worth mentioning that there is no reference to the type of arrhythmia revealed during follow-up. This is a major drawback of a large study with 1,224 patients suffering from Attention Deficit and Hyperactivity.
Anamnestic screening for heart disease
This screening can be performed by a psychiatrist and it requires 3 important questions from the Anamnesis: 1) Does the child have a family history of sudden cardiac death? 2) Has the child ever been hospitalized for cardiac problems or has he undergone heart surgery? and 3) Has the child ever had palpitations with an increased heart rate > 180/ min or Syncope? This third question "opens the door" to other symptoms that can be reported by the patient such as palpitations that suggest premature beats, without an increased rate > 180/ min. The reason for this last question is to eliminate sinus tachycardia that is “physiological arrhythmia” and does not require any special attention.
If during Anamnesis, the psychiatrist receives any positive answer (YES) to any of the 3 questions, a cardiac consultation should be taken into account. This includes a clinical examination, an ECG and blood pressure measurement. During the physical examination, the cardiologist searches for signs of heart failure: edema, hepatomegaly, jugular distension, which may be signs of underlying heart disease. The presence of a heart murmur "per se" does not contraindicate the administration of Atomoxetine or Methylphenidate, but the cardiologist should differentiate between “physiological murmur” and the murmur caused by an underlying heart disease. A blood pressure level over 120/70 mmHg before the onset of the treatment involves regular measurement of the blood pressure during the treatment, at 1 week initially, then at 1-month follow-up. If the ECG examination does not reveal arrhythmias or prolonged QT interval, the treatment with Methylphenidate or Atomoxetine may be initiated. If arrhythmias are present on the initial ECG, treatment can be started, but it requires reassessment after 1 week and 1 month.
The reassessment estimates arrhythmia follow-up: is there any aggravation which requires the interruption of the treatment or is the arrhythmia stable and the treatment can be maintained? The presence of ventricular arrhythmia such as extrasystoles, bigeminies, couplets are not an absolute contraindication to Methylphenidate and Atomoxetine, but instead, the beta-blocker treatment should be initiated with re-assessment at 1 week and 1 month (Figure 4).
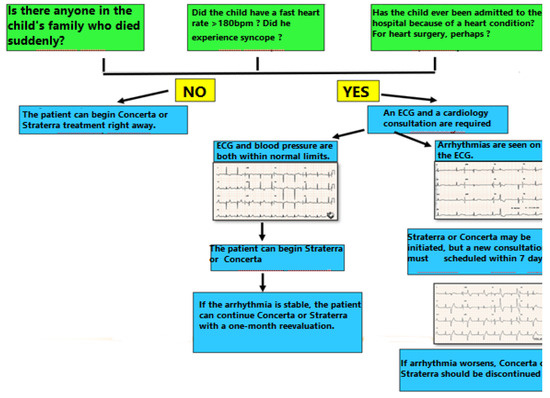
Figure 4.
Algorithm for screening before the beginning of the treatment with Concerta or Straterra.
Conclusions
To sum up, the risk of arrhythmic events due to Methylphenidate and Atomoxetine is extrapolated to children from studies performed on adults. Indeed, arrhythmic events were described in adults, some of them being fatal, especially if a pre-existing heart disease such as myocardial infarction, chronic pulmonary heart disease, were present. But these cardiac diseases are not found among children.
Studies on children have not revealed a clearly increased arrhythmic risk associated with these drugs. However, caution is required in the case of children who are known to have congenital heart disease or arrhythmogenic cardiomyopathies, and in such cases, a cardiac examination should be requested and medication should be administered under close ECG monitoring.
Conflict of interest disclosure
There are no known conflicts of interest in the publication of this article. The manuscript was read and approved by all authors.
Compliance with ethical standards
Any aspect of the work covered in this manuscript has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
References
- Polanczyk, G.; Rohde, L.A. Epidemiology of attention-deficit/hyperactivity disorder across the lifespan. Curr Opin Psychiatry. 2007, 20, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Schlander, M.; Trott, G.E.; Schwarz, O. Gesundheitsökonomie der Aufmerksamkeitsdefizit-/Hyperaktivitätsstörung in Deutschland Teil 1: Versorgungsepidemiologie und Krankheitskosten [The health economics of attention deficit hyperactivity disorder in Germany. Part 1: Health care utilization and cost of illness]. Nervenarzt. In German. 2010, 81, 289–300. [Google Scholar] [CrossRef]
- Faraone, S.V.; Spencer, T.; Aleardi, M.; Pagano, C.; Biederman, J. Meta-analysis of the efficacy of methylphenidate for treating adult attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2004, 24, 24–29. [Google Scholar] [CrossRef]
- Schubert, I.; Köster, I.; Lehmkuhl, G. The changing prevalence of attention-deficit/hyperactivity disorder and methylphenidate prescriptions: a study of data from a random sample of insurees of the AOK Health Insurance Company in the German State of Hesse, 2000-2007. Dtsch Arztebl Int. 2010, 107, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Cooper, W.O.; Habel, L.A.; Sox, C.M.; Chan, K.A.; Arbogast, P.G.; Cheetham, T.C.; Murray, K.T.; Quinn, V.P.; Stein, C.M.; Callahan, S.T.; Fireman, B.H.; Fish, F.A.; Kirshner, H.S.; O'Duffy, A.; Connell, F.A.; Ray, W.A. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011, 365, 1896–1904. [Google Scholar] [CrossRef] [PubMed]
- Habel, L.A.; Cooper, W.O.; Sox, C.M.; Chan, K.A.; Fireman, B.H.; Arbogast, P.G.; Cheetham, T.C.; Quinn, V.P.; Dublin, S.; Boudreau, D.M.; Andrade, S.E.; Pawloski, P.A.; Raebel, M.A.; Smith, D.H.; Achacoso, N.; Uratsu, C.; Go, A.S.; Sidney, S.; Nguyen-Huynh, M.N.; Ray, W.A.; Selby, J.V. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 2011, 306, 2673–2683. [Google Scholar] [CrossRef]
- David, D.; Dobrean, A.; Pasarelu, C.R.; Iftene, F.; Lupu, V.; Predescu, E.; Dopfner, M. Psychotherapy, Atomoxetine or Both? Preliminary Evidence from a Comparative Study of Three Types of Treatment for Attention-Deficit/Hyperactivity Disorder in Children. Cognitive Therapy and Research 2021, 45, 149–165. [Google Scholar] [CrossRef]
- Polanczyk, G.; Rohde, L.A. Epidemiology of attention-deficit/hyperactivity disorder across the lifespan. Curr Opin Psychiatry. 2007, 20, 386–392. [Google Scholar] [CrossRef]
- Dvoráková, M.; Jezová, D.; Blazícek, P.; Trebatická, J.; Skodácek, I.; Suba, J.; Iveta, W.; Rohdewald, P.; Duracková, Z. Urinary catecholamines in children with attention deficit hyperactivity disorder (ADHD): modulation by a polyphenolic extract from pine bark (pycnogenol). Nutr Neurosci. 2007, 10, 151–157. [Google Scholar] [CrossRef]
- Prasad, S.; Steer, C. Switching from neurostimulant therapy to atomoxetine in children and adolescents with attention-deficit hyperactivity disorder: clinical approaches and review of current available evidence. Paediatr Drugs. 2008, 10, 39–47. [Google Scholar] [CrossRef]
- Schelleman, H.; Bilker, W.B.; Kimmel, S.E.; Daniel, G.W.; Newcomb, C.; Guevara, J.P.; Cziraky, M.J.; Strom, B.L.; Hennessy, S. Methylphenidate and risk of serious cardiovascular events in adults. Am J Psychiatry. 2012, 169, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Habel, L.A.; Cooper, W.O.; Sox, C.M.; Chan, K.A.; Fireman, B.H.; Arbogast, P.G.; Cheetham, T.C.; Quinn, V.P.; Dublin, S.; Boudreau, D.M.; Andrade, S.E.; Pawloski, P.A.; Raebel, M.A.; Smith, D.H.; Achacoso, N.; Uratsu, C.; Go, A.S.; Sidney, S.; Nguyen-Huynh, M.N.; Ray, W.A.; Selby, J.V. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 2011, 306, 2673–2683. [Google Scholar] [CrossRef] [PubMed]
- Schelleman, H.; Bilker, W.B.; Kimmel, S.E.; Daniel, G.W.; Newcomb, C.; Guevara, J.P.; Cziraky, M.J.; Strom, B.L.; Hennessy, S. Amphetamines, atomoxetine and the risk of serious cardiovascular events in adults. PLoS One. 2013, 8, e52991. [Google Scholar] [CrossRef]
- Schelleman, H.; Bilker, W.B.; Kimmel, S.E.; Daniel, G.W.; Newcomb, C.; Guevara, J.P.; Cziraky, M.J.; Strom, B.L.; Hennessy, S. Methylphenidate and risk of serious cardiovascular events in adults. Am J Psychiatry. 2012, 169, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Tanidir, I.C.; Tanidir, C.; Ozturk, E.; Bahali, K.; Gunes, H.; Ergul, Y.; Uneri, O.S.; Akdeniz, C.; Tuzcu, V. Effects of atomoxetine on heart rhythm in children and adolescents. Pediatr Int. 2015, 57, 1078–1085. [Google Scholar] [CrossRef]
- Scherer, D.; Hassel, D.; Bloehs, R.; Zitron, E.; von Löwenstern, K.; Seyler, C.; Thomas, D.; Konrad, F.; Bürgers, H.F.; Seemann, G.; Rottbauer, W.; Katus, H.A.; Karle, C.A.; Scholz, E.P. Selective noradrenaline reuptake inhibitor atomoxetine directly blocks hERG currents. Br J Pharmacol. 2009, 156, 226–236. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Inanc, I.H.; Polat, E.; Coskun, F.Y. One-year clinical follow-up and outcomes in patients after drug-eluting stent implantation for unprotected left main coronary stenosis: A single center study from Turkey. J Clin Invest Surg. 2020, 5, 43–50. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Nagumo, K.; Nakashima, T.; Kinugawa, Y.; Kumaki, S. Life-threatening QT prolongation in a boy with attention-deficit/hyperactivity disorder on atomoxetine. Eur J Pediatr. 2014, 173, 1631–1634. [Google Scholar] [CrossRef]
- Stuhec, M.; Svab, V. Atomoxetine-induced life-threatening long QT syndrome. Ir J Med Sci. 2013, 182, 535–537. [Google Scholar] [CrossRef]
- Wylie Jones Jordan. Mental Health & Drugs; A Map the Mind. J Mind Med Sci. 2020, 7, 133–140. [Google Scholar] [CrossRef]
- Wernicke, J.F.; Faries, D.; Girod, D.; Brown, J.; Gao, H.; Kelsey, D.; Quintana, H.; Lipetz, R.; Michelson, D.; Heiligenstein, J. Cardiovascular effects of atomoxetine in children, adolescents, and adults. Drug Saf. 2003, 26, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Göçer, S.; Karaçalilar, M.; Yazici, S.; Aydin, C. Use of native Y-saphenous vein graft in multi-vessel coronary bypass surgery. J Clin Invest Surg. 2020, 5, 96–99. [Google Scholar] [CrossRef]
- Manea, M.; Bratu, O.G.; Bacalbasa, N.; Diaconu, C.C. Diagnosis and management of pericardial effusion. J Mind Med Sci. 2020, 7, 148–155. [Google Scholar] [CrossRef]
- Shin, J.Y.; Roughead, E.E.; Park, B.J.; Pratt, N.L. Cardiovascular safety of methylphenidate among children and young people with attention-deficit/hyperactivity disorder (ADHD): nationwide self controlled case series study. BMJ. 2016, 353, i2550. [Google Scholar] [CrossRef]
© 2021 by the author. 2021 Gabriel Cismaru, Viorel Lupu