Therapeutic Efficacy of Mesenchymal Stem Cells for Cardiovascular Diseases
Abstract
Introduction
Discussions
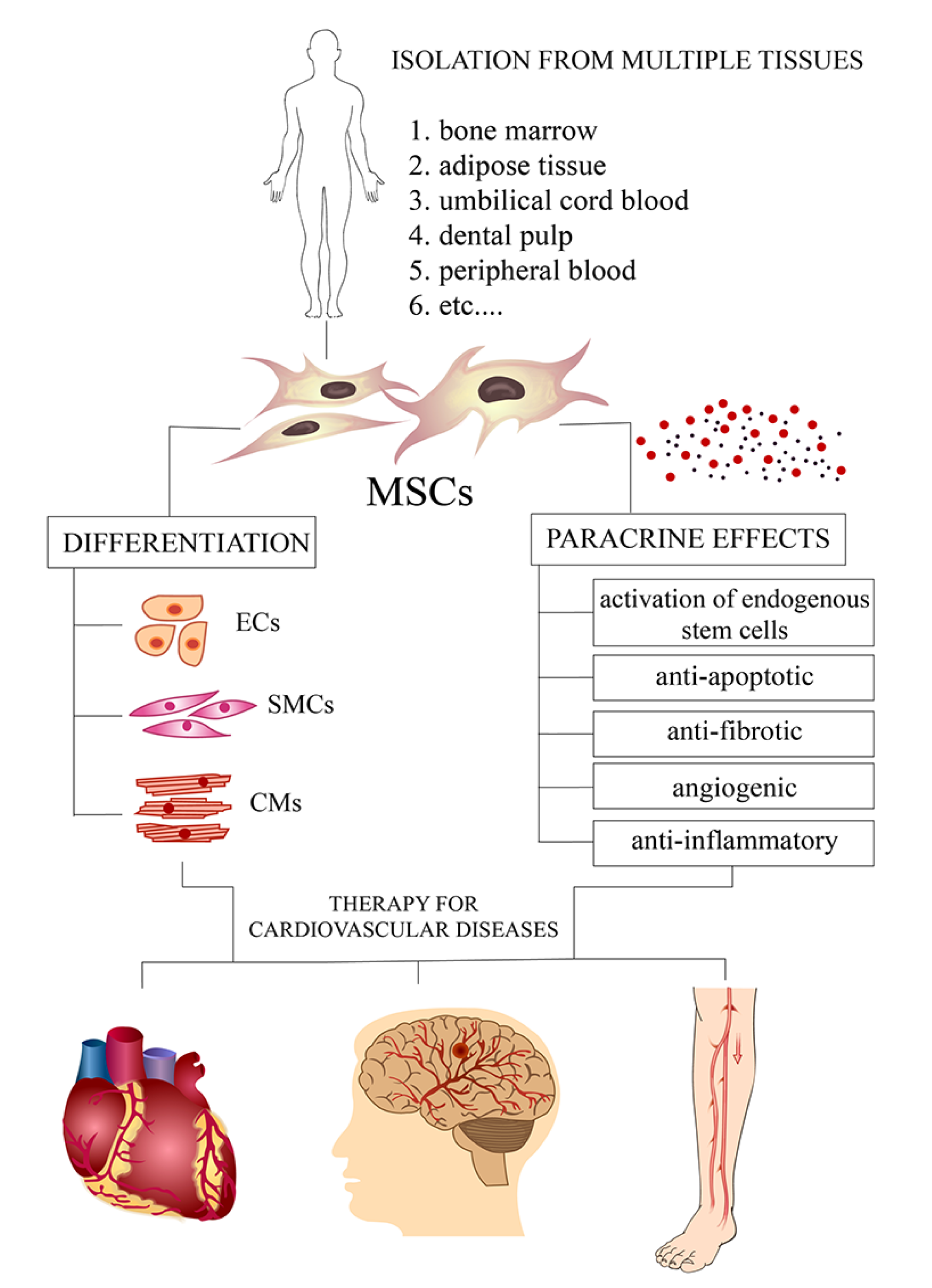
Mesenchymal stem cells - a new therapeutic strategy for cardiovascular diseases
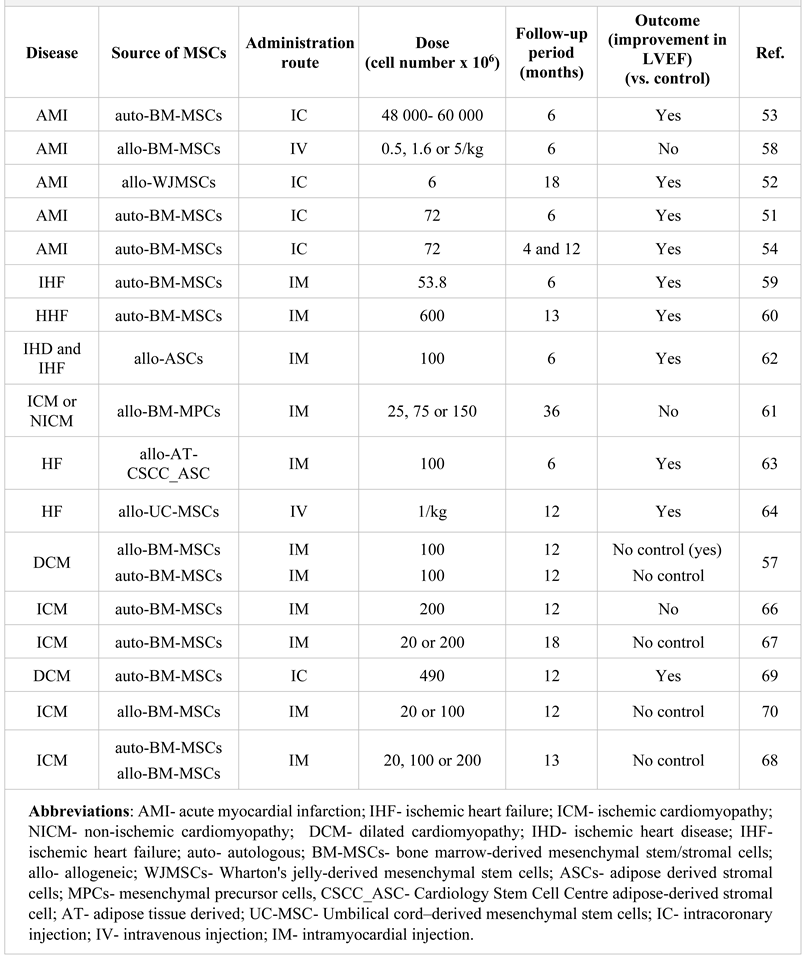
MSC-based therapy of coronary artery disease
MSCs as novel therapeutic agents in the treatment of heart failure
MSCs as new agents in cell-based therapy of cardiomyopathy
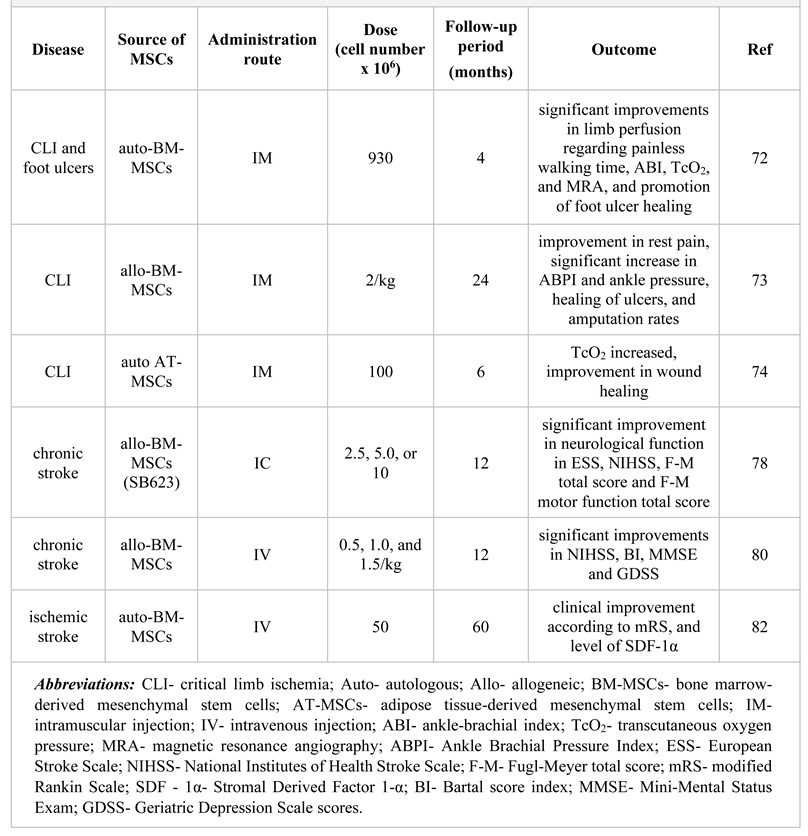
MSC-mediated modulation of peripheral artery disease
Mesenchymal stem cells therapy in stroke
Conclusions
Funding
Conflict of interest disclosure
Acknowledgments
References
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [PubMed]
- Flora, G.D.; Nayak, M.K. A Brief Review of Cardiovascular Diseases, Associated Risk Factors and Current Treatment Regimes. Curr Pharm Des. 2019, 25, 4063–4084. [Google Scholar] [CrossRef] [PubMed]
- Mladěnka, P.; Applová, L.; Patočka, J.; Costa, V.M.; Remiao, F.; Pourová, J.; Mladěnka, A.; Karlíčková, J.; Jahodář, L.; Vopršalová, M.; Varner, K.J.; Štěrba, M.; TOX-OER and CARDIOTOX Hradec Králové Researchers and Collaborators. Comprehensive review of cardiovascular toxicity of drugs and related agents. Med Res Rev. 2018, 38, 1332–1403. [Google Scholar] [CrossRef]
- Senst, B.; Kumar, A.; Diaz, R.R. Cardiac Surgery. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2021- forthcoming. https://www.ncbi.nlm.nih. Available online: https://www.ncbi.nlm.nih.gov/books/N BK532935/.
- Howitt, S.H.; Herring, M.; Malagon, I.; McCollum, C.N.; Grant, S.W. Incidence and outcomes of sepsis after cardiac surgery as defined by the Sepsis-3 guidelines. Br J Anaesth. 2018, 120, 509–516. [Google Scholar] [CrossRef]
- Volarevic, V.; Bojic, S.; Nurkovic, J.; Volarevic, A.; Ljujic, B.; Arsenijevic, N.; Lako, M.; Stojkovic, M. Stem cells as new agents for the treatment of infertility: current and future perspectives and challenges. Biomed Res Int. 2014, 2014, 507234. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Sadikot, R.; Pascual, J.; Fellabaum, C.; Jankovic, M.G.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Based Therapy of Inflammatory Lung Diseases: Current Understanding and Future Perspectives. Stem Cells Int. 2019, 2019, 4236973. [Google Scholar] [CrossRef]
- Otsuru, S.; Hofmann, T.J.; Olson, T.S.; Dominici, M.; Horwitz, E.M. Improved isolation and expansion of bone marrow mesenchymal stromal cells using a novel marrow filter device. Cytotherapy. 2013, 15, 146–153. [Google Scholar] [CrossRef]
- Guasti, L.; New, S.E.; Hadjidemetriou, I.; Palmiero, M.; Ferretti, P. Plasticity of human adipose-derived stem cells - relevance to tissue repair. Int J Dev Biol. 2018, 62, 431–439. [Google Scholar] [CrossRef]
- Tondreau, T.; Meuleman, N.; Delforge, A.; Dejeneffe, M.; Leroy, R.; Massy, M.; Mortier, C.; Bron, D.; Lagneaux, L. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression, and plasticity. Stem Cells. 2005, 23, 1105–1112. [Google Scholar] [CrossRef]
- Tatullo, M.; Codispoti, B.; Pacifici, A.; Palmieri, F.; Marrelli, M.; Pacifici, L.; Paduano, F. Potential Use of Human Periapical Cyst-Mesenchymal Stem Cells (hPCy- MSCs) as a Novel Stem Cell Source for Regenerative Medicine Applications. Front Cell Dev Biol. 2017, 5, 103. [Google Scholar] [CrossRef]
- Cagliani, J.; Grande, D.; Molmenti, E.P.; Miller, E.J.; Rilo, H.L.R. Immunomodulation by Mesenchymal Stromal Cells and Their Clinical Applications. J Stem Cell Regen Biol. 2017, 3, 10.15436/2471-0598.17.022. [Google Scholar] [CrossRef]
- Niezgoda, A.; Niezgoda, P.; Nowowiejska, L.; Białecka, A.; Męcińska-Jundziłł, K.; Adamska, U.; Czajkowski, R. Properties of skin stem cells and their potential clinical applications in modern dermatology. Eur J Dermatol. 2017, 27, 227–236. [Google Scholar] [CrossRef]
- Bojic, S.; Volarevic, V.; Ljujic, B.; Stojkovic, M. Dental stem cells--characteristics and potential. Histol Histopathol. 2014, 29, 699–706. [Google Scholar] [CrossRef]
- Majka, M.; Sułkowski, M.; Badyra, B.; Musiałek, P. Concise Review: Mesenchymal Stem Cells in Cardiovascular Regeneration: Emerging Research Directions and Clinical Applications. Stem Cells Transl Med. 2017, 6, 1859–1867. [Google Scholar] [CrossRef]
- Alves da Silva, M.L.; Costa-Pinto, A.R.; Martins, A.; Correlo, V.M.; Sol, P.; Bhattacharya, M.; Faria, S.; Reis, R.L.; Neves, N.M. Conditioned medium as a strategy for human stem cells chondrogenic differentiation. J Tissue Eng Regen Med. 2015, 9, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Koike, C.; Zhou, K.; Takeda, Y.; Fathy, M.; Okabe, M.; Yoshida, T.; Nakamura, Y.; Kato, Y.; Nikaido, T. Characterization of amniotic stem cells. Cell Reprogram. 2014, 16, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, N.; Shabani, R.; Ebrahimi, M.; Baghestani, A.; Dehdashtian, E.; Vahabzadeh, G.; Soleimani, M.; Moradi, F.; Katebi, M. Comparative phenotypic characterization of human colostrum and breast milk-derived stem cells. Hum Cell. 2020, 33, 308–317. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhu, W.; Cao, Q.; Shen, Y.; Zhou, Q.; Yu, P.; Liu, X.; Ma, J.; Li, Y.; Hong, K. Generation of Mesenchymal-Like Stem Cells From Urine in Pediatric Patients. Transplant Proc. 2016, 48, 2181–2185. [Google Scholar] [CrossRef]
- Harrell, C.R.; Gazdic, M.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Therapeutic Potential of Amniotic Fluid Derived Mesenchymal Stem Cells Based on their Differentiation Capacity and Immunomodulatory Properties. Curr Stem Cell Res Ther. 2019, 14, 327–336. [Google Scholar] [CrossRef]
- Gazdic, M.; Arsenijevic, A.; Markovic, B.S.; Volarevic, A.; Dimova, I.; Djonov, V.; Arsenijevic, N.; Stojkovic, M.; Volarevic, V. Mesenchymal Stem Cell-Dependent Modulation of Liver Diseases. Int J Biol Sci. 2017, 13, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.j.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Toma, C.; Pittenger, M.F.; Cahill, K.S.; Byrne, B.J.; Kessler, P.D. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002, 105, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, S.; Yanagawa, B.; Tanaka, K.; Miyahara, Y.; Obata, H.; Kataoka, M.; Kodama, M.; Ishibashi-Ueda, H.; Kangawa, K.; Kitamura, S.; Nagaya, N. Transplantation of mesenchymal stem cells attenuates myocardial injury and dysfunction in a rat model of acute myocarditis. J Mol Cell Cardiol. 2007, 42, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Musiał-Wysocka, A.; Kot, M.; Sułkowski, M.; Majka, M. Regenerative Potential of the Product "CardioCell" Derived from the Wharton's Jelly Mesenchymal Stem Cells for Treating Hindlimb Ischemia. Int J Mol Sci. 2019, 20, 4632. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Wang, P.; Wu, Q.; Wang, S.; Yu, L.; Wang, G. Human umbilical cord blood derived mesenchymal stem cells improve cardiac function in cTnT(R141W) transgenic mouse of dilated cardiomyopathy. Eur J Cell Biol. 2016, 95, 57–67. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Zhu, D.; Kong, Y. Mesenchymal stem cells rejuvenate cardiac muscle after ischemic injury. Aging (Albany NY). 2019, 11, 63–72. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, L.; Qu, M.; Liang, H.; Li, W.; Li, Y.; Deng, L.; Zhang, Z.; Yang, G.Y. Mesenchymal stem cells attenuate blood-brain barrier leakage after cerebral ischemia in mice. J Neuroinflammation. 2018, 15, 135. [Google Scholar] [CrossRef]
- Yao, Y.; Huang, J.; Geng, Y.; Qian, H.; Wang, F.; Liu, X.; Shang, M.; Nie, S.; Liu, N.; Du, X.; Dong, J.; Ma, C. Paracrine action of mesenchymal stem cells revealed by single cell gene profiling in infarcted murine hearts. PLoS One. 2015, 10, e0129164. [Google Scholar] [CrossRef] [PubMed]
- Luger, D.; Lipinski, M.J.; Westman, P.C.; Glover, D.K.; Dimastromatteo, J.; Frias, J.C.; Albelda, M.T.; Sikora, S.; Kharazi, A.; Vertelov, G.; Waksman, R.; Epstein, S.E. Intravenously Delivered Mesenchymal Stem Cells: Systemic Anti-Inflammatory Effects Improve Left Ventricular Dysfunction in Acute Myocardial Infarction and Ischemic Cardiomyopathy. Circ Res. 2017, 120, 1598–1613. [Google Scholar] [CrossRef]
- Huang, Y.S.; Li, I.H.; Chueh, S.H.; Hueng, D.Y.; Tai, M.C.; Liang, C.M.; Lien, S.B.; Sytwu, H.K.; Ma, K.H. Mesenchymal stem cells from rat olfactory bulbs can differentiate into cells with cardiomyocyte characteristics. J Tissue Eng Regen Med. 2015, 9, E191–E201. [Google Scholar] [CrossRef]
- Soltani, L.; Rahmani, H.R.; Daliri Joupari, M.; Ghaneialvar, H.; Mahdavi, A.H.; Shamsara, M. Ovine fetal mesenchymal stem cell differentiation to cardiomyocytes, effects of co-culture, role of small molecules; reversine and 5-azacytidine. Cell Biochem Funct. 2016, 34, 250–261. [Google Scholar] [CrossRef]
- Hafez, P.; Jose, S.; Chowdhury, S.R.; Ng, M.H.; Ruszymah, B.H.; Abdul Rahman Mohd, R. Cardiomyogenic differentiation of human sternal bone marrow mesenchymal stem cells using a combination of basic fibroblast growth factor and hydrocortisone. Cell Biol Int. 2016, 40, 55–64. [Google Scholar] [CrossRef]
- Joddar, B.; Kumar, S.A.; Kumar, A. A Contact-Based Method for Differentiation of Human Mesenchymal Stem Cells into an Endothelial Cell-Phenotype. Cell Biochem Biophys. 2018, 76, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Hong, X.; Le Bras, A.; Nowak, W.N.; Issa Bhaloo, S.; Deng, J.; Xie, Y.; Hu, Y.; Ruan, X.Z.; Xu, Q. Smooth muscle cells differentiated from mesenchymal stem cells are regulated by microRNAs and suitable for vascular tissue grafts. J Biol Chem. 2018, 293, 8089–8102. [Google Scholar] [CrossRef]
- Zhang, X.; Bendeck, M.P.; Simmons, C.A.; Santerre, J.P. Deriving vascular smooth muscle cells from mesenchymal stromal cells: Evolving differentiation strategies and current understanding of their mechanisms. Biomaterials. 2017, 145, 9–22. [Google Scholar] [CrossRef]
- Gu, W.; Hong, X.; Le Bras, A.; Nowak, W.N.; Issa Bhaloo, S.; Deng, J.; Xie, Y.; Hu, Y.; Ruan, X.Z.; Xu, Q. Smooth muscle cells differentiated from mesenchymal stem cells are regulated by microRNAs and suitable for vascular tissue grafts. J Biol Chem. 2018, 293, 8089–8102. [Google Scholar] [CrossRef]
- White, I.A.; Sanina, C.; Balkan, W.; Hare, J.M. Mesenchymal Stem Cells in Cardiology. Methods Mol Biol. 2016, 1416, 55–87. [Google Scholar] [CrossRef] [PubMed]
- Volarevic, V.; Gazdic, M.; Simovic Markovic, B.; Jovicic, N.; Djonov, V.; Arsenijevic, N. Mesenchymal stem cell- derived factors: Immuno-modulatory effects and therapeutic potential. Biofactors. 2017, 43, 633–644. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.; Chen, A.; Zhao, Q. Mesenchymal stem cells promote cardiac muscle repair via enhanced neovascularization. Cell Physiol Biochem. 2015, 35, 1219–1229. [Google Scholar] [CrossRef]
- Ohnishi, S.; Yanagawa, B.; Tanaka, K.; Miyahara, Y.; Obata, H.; Kataoka, M.; Kodama, M.; Ishibashi-Ueda, H.; Kangawa, K.; Kitamura, S.; Nagaya, N. Transplantation of mesenchymal stem cells attenuates myocardial injury and dysfunction in a rat model of acute myocarditis. J Mol Cell Cardiol. 2007, 42, 88–97. [Google Scholar] [CrossRef]
- Markel, T.A.; Wang, Y.; Herrmann, J.L.; Crisostomo, P.R.; Wang, M.; Novotny, N.M.; Herring, C.M.; Tan, J.; Lahm, T.; Meldrum, D.R. VEGF is critical for stem cell-mediated cardioprotection and a crucial paracrine factor for defining the age threshold in adult and neonatal stem cell function. Am J Physiol Heart Circ Physiol. 2008, 295, H2308–H2314. [Google Scholar] [CrossRef] [PubMed]
- Hatzistergos, K.E.; Quevedo, H.; Oskouei, B.N.; Hu, Q.; Feigenbaum, G.S.; Margitich, I.S.; Mazhari, R.; Boyle, A.J.; Zambrano, J.P.; Rodriguez, J.E.; Dulce, R.; Pattany, P.M.; Valdes, D.; Revilla, C.; Heldman, A.W.; McNiece, I.; Hare, J.M. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010, 107, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Hatzistergos, K.E.; Saur, D.; Seidler, B.; Balkan, W.; Breton, M.; Valasaki, K.; Takeuchi, L.M.; Landin, A.M.; Khan, A.; Hare, J.M. Stimulatory Effects of Mesenchymal Stem Cells on cKit+ Cardiac Stem Cells Are Mediated by SDF1/CXCR4 and SCF/cKit Signaling Pathways. Circ Res. 2016, 119, 921–930. [Google Scholar] [CrossRef]
- Beigi, F.; Schmeckpeper, J.; Pow-Anpongkul, P.; Payne, J.A.; Zhang, L.; Zhang, Z.; Huang, J.; Mirotsou, M.; Dzau, V.J. C3orf58, a novel paracrine protein, stimulates cardiomyocyte cell-cycle progression through the PI3K-AKT-CDK7 pathway. Circ Res. 2013, 113, 372–380. [Google Scholar] [CrossRef]
- Liu, X.; Chen, H.; Zhu, W.; Chen, H.; Hu, X.; Jiang, Z.; Xu, Y.; Zhou, Y.; Wang, K.; Wang, L.; Chen, P.; Hu, H.; Wang, C.; Zhang, N.; Ma, Q.; Huang, M.; Hu, D.; Zhang, L.; Wu, R.; Wang, Y.; Xu, Q.; Yu, H.; Wang, J. Transplantation of SIRT1-engineered aged mesenchymal stem cells improves cardiac function in a rat myocardial infarction model. J Heart Lung Transplant. 2014, 33, 1083–1092. [Google Scholar] [CrossRef]
- Harrell, C.R.; Jankovic, M.G.; Fellabaum, C.; Volarevic, A.; Djonov, V.; Arsenijevic, A.; Volarevic, V. Molecular Mechanisms Responsible for Anti-inflammatory and Immunosuppressive Effects of Mesenchymal Stem Cell-Derived Factors. Adv Exp Med Biol. 2019, 1084, 187–206. [Google Scholar] [CrossRef]
- Gazdic, M.; Volarevic, V.; Arsenijevic, N.; Stojkovic, M. Mesenchymal stem cells: a friend or foe in immune- mediated diseases. Stem Cell Rev Rep. 2015, 11, 280–287. [Google Scholar] [CrossRef]
- Markovic, B.S.; Kanjevac, T.; Harrell, C.R.; Gazdic, M.; Fellabaum, C.; Arsenijevic, N.; Volarevic, V. Molecular and Cellular Mechanisms Involved in Mesenchymal Stem Cell-Based Therapy of Inflammatory Bowel Diseases. Stem Cell Rev Rep. 2018, 14, 153–165. [Google Scholar] [CrossRef]
- Molina, E.J.; Palma, J.; Gupta, D.; Torres, D.; Gaughan, J.P.; Houser, S.; Macha, M. Reverse remodeling is associated with changes in extracellular matrix proteases and tissue inhibitors after mesenchymal stem cell (MSC) treatment of pressure overload hypertrophy. J Tissue Eng Regen Med. 2009, 3, 85–91. [Google Scholar] [CrossRef][Green Version]
- Lee, J.W.; Lee, S.H.; Youn, Y.J.; Ahn, M.S.; Kim, J.Y.; Yoo, B.S.; Yoon, J.; Kwon, W.; Hong, I.S.; Lee, K.; Kwan, J.; Park, K.S.; Choi, D.; Jang, Y.S.; Hong, M.K. A randomized, open- label, multicenter trial for the safety and efficacy of adult mesenchymal stem cells after acute myocardial infarction. J Korean Med Sci. 2014, 29, 23–31. [Google Scholar] [CrossRef]
- Gao, L.R.; Chen, Y.; Zhang, N.K.; Yang, X.L.; Liu, H.L.; Wang, Z.G.; Yan, X.Y.; Wang, Y.; Zhu, Z.M.; Li, T.C.; Wang, L.H.; Chen, H.Y.; Chen, Y.D.; Huang, C.L.; Qu, P.; Yao, C.; Wang, B.; Chen, G.H.; Wang, Z.M.; Xu, Z.Y.; Bai, J.; Lu, D.; Shen, Y.H.; Guo, F.; Liu, M.Y.; Yang, Y.; Ding, Y.C.; Yang, Y.; Tian, H.T.; Ding, Q.A.; Li, L.N.; Yang, X.C.; Hu, X. Intracoronary infusion of Wharton's jelly-derived mesenchymal stem cells in acute myocardial infarction: double-blind, randomized controlled trial. BMC Med. 2015, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Fang, W.W.; Ye, F.; Liu, Y.H.; Qian, J.; Shan, S.J.; Zhang, J.J.; Chunhua, R.Z.; Liao, L.M.; Lin, S.; Sun, J.P. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004, 94, 92–95. [Google Scholar] [CrossRef]
- Kim, S.H.; Cho, J.H.; Lee, Y.H.; Lee, J.H.; Kim, S.S.; Kim, M.Y.; Lee, M.G.; Kang, W.Y.; Lee, K.S.; Ahn, Y.K.; Jeong, M.H.; Kim, H.S. Improvement in Left Ventricular Function with Intracoronary Mesenchymal Stem Cell Therapy in a Patient with Anterior Wall ST-Segment Elevation Myocardial Infarction. Cardiovasc Drugs Ther. 2018, 32, 329–338. [Google Scholar] [CrossRef]
- Katritsis, D.G.; Sotiropoulou, P.; Giazitzoglou, E.; Karvouni, E.; Papamichail, M. Electrophysiological effects of intracoronary transplantation of autologous mesenchymal and endothelial progenitor cells. Europace. 2007, 9, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Golpanian, S.; Wolf, A.; Hatzistergos, K.E.; Hare, J.M. Rebuilding the Damaged Heart: Mesenchymal Stem Cells, Cell-Based Therapy, and Engineered Heart Tissue. Physiol Rev. 2016, 96, 1127–1168. [Google Scholar] [CrossRef]
- Hare, J.M.; DiFede, D.L.; Rieger, A.C.; Florea, V.; Landin, A.M.; El-Khorazaty, J.; Khan, A.; Mushtaq, M.; Lowery, M.H.; Byrnes, J.J.; Hendel, R.C.; Cohen, M.G.; Alfonso, C.E.; Valasaki, K.; Pujol, M.V.; Golpanian, S.; Ghersin, E.; Fishman, J.E.; Pattany, P.; Gomes, S.A.; Delgado, C.; Miki, R.; Abuzeid, F.; Vidro-Casiano, M.; Premer, C.; Medina, A.; Porras, V.; Hatzistergos, K.E.; Anderson, E.; Mendizabal, A.; Mitrani, R.; Heldman, A.W. Randomized Comparison of Allogeneic Versus Autologous Mesenchymal Stem Cells for Nonischemic Dilated Cardiomyopathy: POSEIDON-DCM Trial. J Am Coll Cardiol. 2017, 69, 526–537. [Google Scholar] [CrossRef]
- Hare, J.M.; Traverse, J.H.; Henry, T.D.; Dib, N.; Strumpf, R.K.; Schulman, S.P.; Gerstenblith, G.; DeMaria, A.N.; Denktas, A.E.; Gammon, R.S.; Hermiller, J.B., Jr.; Reisman, M.A.; Schaer, G.L.; Sherman, W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009, 54, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- Mathiasen, A.B.; Qayyum, A.A.; Jørgensen, E.; Helqvist, S.; Fischer-Nielsen, A.; Kofoed, K.F.; Haack-Sørensen, M.; Ekblond, A.; Kastrup, J. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo- controlled trial (MSC-HF trial). Eur Heart J. 2015, 36, 1744–1753. [Google Scholar] [CrossRef]
- Teerlink, J.R.; Metra, M.; Filippatos, G.S.; Davison, B.A.; Bartunek, J.; Terzic, A.; Gersh, B.J.; Povsic, T.J.; Henry, T.D.; Alexandre, B.; Homsy, C.; Edwards, C.; Seron, A.; Wijns, W.; Cotter, G.; CHART Investigators. Benefit of cardiopoietic mesenchymal stem cell therapy on left ventricular remodelling: results from the Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART-1) study. Eur J Heart Fail. 2017, 19, 1520–1529. [Google Scholar] [CrossRef]
- Perin, E.C.; Borow, K.M.; Silva, G.V.; DeMaria, A.N.; Marroquin, O.C.; Huang, P.P.; Traverse, J.H.; Krum, H.; Skerrett, D.; Zheng, Y.; Willerson, J.T.; Itescu, S.; Henry, T.D. A Phase II Dose-Escalation Study of Allogeneic Mesenchymal Precursor Cells in Patients With Ischemic or Nonischemic Heart Failure. Circ Res. 2015, 117, 576–584. [Google Scholar] [CrossRef]
- Kastrup, J.; Haack-Sørensen, M.; Juhl, M.; Harary Søndergaard, R.; Follin, B.; Drozd Lund, L.; Mønsted Johansen, E.; Ali Qayyum, A.; Bruun Mathiasen, A.; Jørgensen, E.; Helqvist, S.; Jørgen Elberg, J.; Bruunsgaard, H.; Ekblond, A. Cryopreserved Off-the-Shelf Allogeneic Adipose-Derived Stromal Cells for Therapy in Patients with Ischemic Heart Disease and Heart Failure-A Safety Study. Stem Cells Transl Med. 2017, 6, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Kastrup, J.; Schou, M.; Gustafsson, I.; Nielsen, O.W.; Møgelvang, R.; Kofoed, K.F.; Kragelund, C.; Hove, J.D.; Fabricius-Bjerre, A.; Heitman, M.; Haack-Sørensen, M.; Lund, L.D.; Johansen, E.M.; Qayyum, A.A.; Mathiasen, A.B.; Ekblond, A. Rationale and Design of the First Double- Blind, Placebo-Controlled Trial with Allogeneic Adipose Tissue-Derived Stromal Cell Therapy in Patients with Ischemic Heart Failure: A Phase II Danish Multicentre Study. Stem Cells Int. 2017, 2017, 8506370. [Google Scholar] [CrossRef]
- Bartolucci, J.; Verdugo, F.J.; González, P.L.; Larrea, R.E.; Abarzua, E.; Goset, C.; Rojo, P.; Palma, I.; Lamich, R.; Pedreros, P.A.; Valdivia, G.; Lopez, V.M.; Nazzal, C.; Alcayaga-Miranda, F.; Cuenca, J.; Brobeck, M.J.; Patel, A.N.; Figueroa, F.E.; Khoury, M. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]). Circ Res. 2017, 121, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, B.A.; Rieger, A.C.; Florea, V.; Banerjee, M.N.; Natsumeda, M.; Nigh, E.D.; Landin, A.M.; Rodriguez, G.M.; Hatzistergos, K.E.; Schulman, I.H.; Hare, J.M. Comparison of Mesenchymal Stem Cell Efficacy in Ischemic Versus Nonischemic Dilated Cardiomyopathy. J Am Heart Assoc. 2018, 7, e008460. [Google Scholar] [CrossRef] [PubMed]
- Heldman, A.W.; DiFede, D.L.; Fishman, J.E.; Zambrano, J.P.; Trachtenberg, B.H.; Karantalis, V.; Mushtaq, M.; Williams, A.R.; Suncion, V.Y.; McNiece, I.K.; Ghersin, E.; Soto, V.; Lopera, G.; Miki, R.; Willens, H.; Hendel, R.; Mitrani, R.; Pattany, P.; Feigenbaum, G.; Oskouei, B.; Byrnes, J.; Lowery, M.H.; Sierra, J.; Pujol, M.V.; Delgado, C.; Gonzalez, P.J.; Rodriguez, J.E.; Bagno, L.L.; Rouy, D.; Altman, P.; Foo, C.W.; da Silva, J.; Anderson, E.; Schwarz, R.; Mendizabal, A.; Hare, J.M. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014, 311, 62–73. [Google Scholar] [CrossRef]
- Karantalis, V.; DiFede, D.L.; Gerstenblith, G.; Pham, S.; Symes, J.; Zambrano, J.P.; Fishman, J.; Pattany, P.; McNiece, I.; Conte, J.; Schulman, S.; Wu, K.; Shah, A.; Breton, E.; Davis- Sproul, J.; Schwarz, R.; Feigenbaum, G.; Mushtaq, M.; Suncion, V.Y.; Lardo, A.C.; Borrello, I.; Mendizabal, A.; Karas, T.Z.; Byrnes, J.; Lowery, M.; Heldman, A.W.; Hare, J.M. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ Res. 2014, 114, 1302–1310. [Google Scholar] [CrossRef]
- Hare, J.M.; Fishman, J.E.; Gerstenblith, G.; DiFede Velazquez, D.L.; Zambrano, J.P.; Suncion, V.Y.; Tracy, M.; Ghersin, E.; Johnston, P.V.; Brinker, J.A.; Breton, E.; Davis- Sproul, J.; Schulman, I.H.; Byrnes, J.; Mendizabal, A.M.; Lowery, M.H.; Rouy, D.; Altman, P.; Wong Po Foo, C.; Ruiz, P.; Amador, A.; Da Silva, J.; McNiece, I.K.; Heldman, A.W.; George, R.; Lardo, A. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012, 308, 2369–2379. [Google Scholar] [CrossRef]
- Xiao, W.; Guo, S.; Gao, C.; Dai, G.; Gao, Y.; Li, M.; Wang, X.; Hu, D. A Randomized Comparative Study on the Efficacy of Intracoronary Infusion of Autologous Bone Marrow Mononuclear Cells and Mesenchymal Stem Cells in Patients With Dilated Cardiomyopathy. Int Heart J. 2017, 58, 238–244. [Google Scholar] [CrossRef]
- Florea, V.; Rieger, A.C.; DiFede, D.L.; El-Khorazaty, J.; Natsumeda, M.; Banerjee, M.N.; Tompkins, B.A.; Khan, A.; Schulman, I.H.; Landin, A.M.; Mushtaq, M.; Golpanian, S.; Lowery, M.H.; Byrnes, J.J.; Hendel, R.C.; Cohen, M.G.; Valasaki, K.; Pujol, M.V.; Ghersin, E.; Miki, R.; Delgado, C.; Abuzeid, F.; Vidro-Casiano, M.; Saltzman, R.G.; DaFonseca, D.; Caceres, L.V.; Ramdas, K.N.; Mendizabal, A.; Heldman, A.W.; Mitrani, R.D.; Hare, J.M. Dose Comparison Study of Allogeneic Mesenchymal Stem Cells in Patients With Ischemic Cardiomyopathy (The TRIDENT Study). Circ Res. 2017, 121, 1279–1290. [Google Scholar] [CrossRef]
- Subherwal, S.; Patel, M.R.; Kober, L.; Peterson, E.D.; Bhatt, D.L.; Gislason, G.H.; Olsen, A.M.; Jones, W.S.; Torp- Pedersen, C.; Fosbol, E.L. Peripheral artery disease is a coronary heart disease risk equivalent among both men and women: results from a nationwide study. Eur J Prev Cardiol. 2015, 22, 317–325. [Google Scholar] [CrossRef]
- Lu, D.; Chen, B.; Liang, Z.; Deng, W.; Jiang, Y.; Li, S.; Xu, J.; Wu, Q.; Zhang, Z.; Xie, B.; Chen, S. Comparison of bone marrow mesenchymal stem cells with bone marrow- derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract. 2011, 92, 26–36. [Google Scholar] [CrossRef]
- Gupta, P.K.; Chullikana, A.; Parakh, R.; Desai, S.; Das, A.; Gottipamula, S.; Krishnamurthy, S.; Anthony, N.; Pherwani, A.; Majumdar, A.S. A double blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal stem cell in critical limb ischemia. J Transl Med. 2013, 11, 143. [Google Scholar] [CrossRef]
- Bura, A.; Planat-Benard, V.; Bourin, P.; Silvestre, J.S.; Gross, F.; Grolleau, J.L.; Saint-Lebese, B.; Peyrafitte, J.A.; Fleury, S.; Gadelorge, M.; Taurand, M.; Dupuis-Coronas, S.; Leobon, B.; Casteilla, L. Phase I trial: the use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy. 2014, 16, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Luo, H.; Zhang, Y.; Wang, Q.; Zhou, C.; Xu, D. Autologous Stem Cell Therapy in Critical Limb Ischemia: A Meta-Analysis of Randomized Controlled Trials. Stem Cells Int. 2018, 2018, 7528464. [Google Scholar] [CrossRef]
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; Hoh, B.L.; Janis, L.S.; Kase, C.S.; Kleindorfer, D.O.; Lee, J.M.; Moseley, M.E.; Peterson, E.D.; Turan, T.N.; Valderrama, A.L.; Vinters, H.V.; American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013, 44, 2064–2089. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.; Onuma, O.; Owolabi, M.; Sachdev, S. Stroke: a global response is needed. Bull World Health Organ. 2016, 94, 634-634A. [Google Scholar] [CrossRef]
- Steinberg, G.K.; Kondziolka, D.; Wechsler, L.R.; Lunsford, L.D.; Coburn, M.L.; Billigen, J.B.; Kim, A.S.; Johnson, J.N.; Bates, D.; King, B.; Case, C.; McGrogan, M.; Yankee, E.W.; Schwartz, N.E. Clinical Outcomes of Transplanted Modified Bone Marrow-Derived Mesenchymal Stem Cells in Stroke: A Phase 1/2a Study. Stroke. 2016, 47, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.C.; Wechsler, L.R.; Clark, W.M.; Savitz, S.I.; Ford, G.A.; Chiu, D.; Yavagal, D.R.; Uchino, K.; Liebeskind, D.S.; Auchus, A.P.; Sen, S.; Sila, C.A.; Vest, J.D.; Mays, R.W. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017, 16, 360–368. [Google Scholar] [CrossRef]
- Levy, M.L.; Crawford, J.R.; Dib, N.; Verkh, L.; Tankovich, N.; Cramer, S.C. Phase I/II Study of Safety and Preliminary Efficacy of Intravenous Allogeneic Mesenchymal Stem Cells in Chronic Stroke. Stroke. 2019, 50, 2835–2841. [Google Scholar] [CrossRef] [PubMed]
- Savitz, S.I.; Yavagal, D.; Rappard, G.; Likosky, W.; Rutledge, N.; Graffagnino, C.; Alderazi, Y.; Elder, J.A.; Chen, P.R.; Budzik RFJr Tarrel, R.; Huang, D.Y.; Hinson, J.M., Jr. A Phase 2 Randomized, Sham-Controlled Trial of Internal Carotid Artery Infusion of Autologous Bone Marrow-Derived ALD-401 Cells in Patients With Recent Stable Ischemic Stroke (RECOVER-Stroke). Circulation. 2019, 139, 192–205. [Google Scholar] [CrossRef]
- Lee, J.S.; Hong, J.M.; Moon, G.J.; Lee, P.H.; Ahn, Y.H.; Bang, O.Y.; STARTING collaborators. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010, 28, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Ljujic, B.; Milovanovic, M.; Volarevic, V.; Murray, B.; Bugarski, D.; Przyborski, S.; Arsenijevic, N.; Lukic, M.L.; Stojkovic, M. Human mesenchymal stem cells creating an immunosuppressive environment and promote breast cancer in mice. Sci Rep. 2013, 3, 2298. [Google Scholar] [CrossRef]
- Gazdic, M.; Simovic Markovic, B.; Jovicic, N.; Misirkic- Marjanovic, M.; Djonov, V.; Jakovljevic, V.; Arsenijevic, N.; Lukic, M.L.; Volarevic, V. Mesenchymal Stem Cells Promote Metastasis of Lung Cancer Cells by Downregulating Systemic Antitumor Immune Response. Stem Cells Int. 2017, 2017, 6294717. [Google Scholar] [CrossRef] [PubMed]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int J Med Sci. 2018, 15, 36–45. [Google Scholar] [CrossRef]
- Miloradovic, D.; Miloradovic, D.; Markovic, B.S.; Acovic, A.; Harrell, C.R.; Djonov, V.; Arsenijevic, N.; Volarevic, V. The Effects of Mesenchymal Stem Cells on Antimelanoma Immunity Depend on the Timing of Their Administration. Stem Cells Int. 2020, 2020, 8842659. [Google Scholar] [CrossRef]
 |
 |
© 2021 by the author. 2021 Dragana Radoje Miloradovic, Dragica Radoje Pavlovic, Miodrag Bozidar Stojkovic, Sanja Bratislav Bojic, Vladislav Bogdan Volarevic, Marina Milosav Gazdic Jankovic, Biljana Tomislav Ljujic
Share and Cite
Miloradovic, D.R.; Pavlovic, D.R.; Stojkovic, M.B.; Bojic, S.B.; Volarevic, V.B.; Jankovic, M.M.G.; Ljujic, B.T. Therapeutic Efficacy of Mesenchymal Stem Cells for Cardiovascular Diseases. J. Mind Med. Sci. 2021, 8, 179-190. https://doi.org/10.22543/7674.82.P179190
Miloradovic DR, Pavlovic DR, Stojkovic MB, Bojic SB, Volarevic VB, Jankovic MMG, Ljujic BT. Therapeutic Efficacy of Mesenchymal Stem Cells for Cardiovascular Diseases. Journal of Mind and Medical Sciences. 2021; 8(2):179-190. https://doi.org/10.22543/7674.82.P179190
Chicago/Turabian StyleMiloradovic, Dragana Radoje, Dragica Radoje Pavlovic, Miodrag Bozidar Stojkovic, Sanja Bratislav Bojic, Vladislav Bogdan Volarevic, Marina Milosav Gazdic Jankovic, and Biljana Tomislav Ljujic. 2021. "Therapeutic Efficacy of Mesenchymal Stem Cells for Cardiovascular Diseases" Journal of Mind and Medical Sciences 8, no. 2: 179-190. https://doi.org/10.22543/7674.82.P179190
APA StyleMiloradovic, D. R., Pavlovic, D. R., Stojkovic, M. B., Bojic, S. B., Volarevic, V. B., Jankovic, M. M. G., & Ljujic, B. T. (2021). Therapeutic Efficacy of Mesenchymal Stem Cells for Cardiovascular Diseases. Journal of Mind and Medical Sciences, 8(2), 179-190. https://doi.org/10.22543/7674.82.P179190



