The Molecular Mechanisms Linking Metabolic Syndrome to Endometrial and Breast Cancers
Abstract
Introduction
Discussions
Metabolic syndrome

Endometrial cancer
Epidemiology
Risk factors
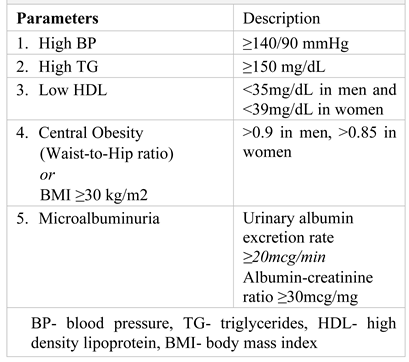
The metabolic syndrome
Obesity
Hyperestrogenism
Chronic inflammation
Growth factors
Cancer microenvironment
Pro-inflammatory cytokines and adipokines
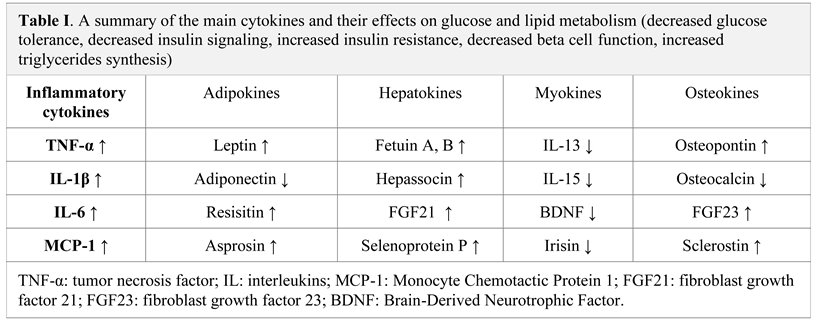
Diabetes mellitus
Hyperglycemia
Hyperinsulinemia and Insulin resistance
Dyslipidemia
Weight loss
Breast cancer
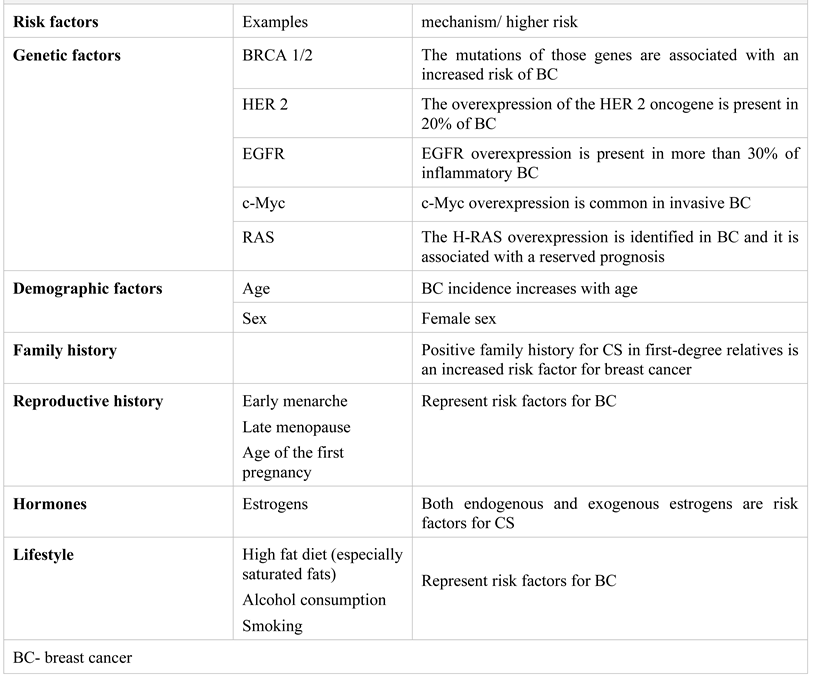
Risk factors
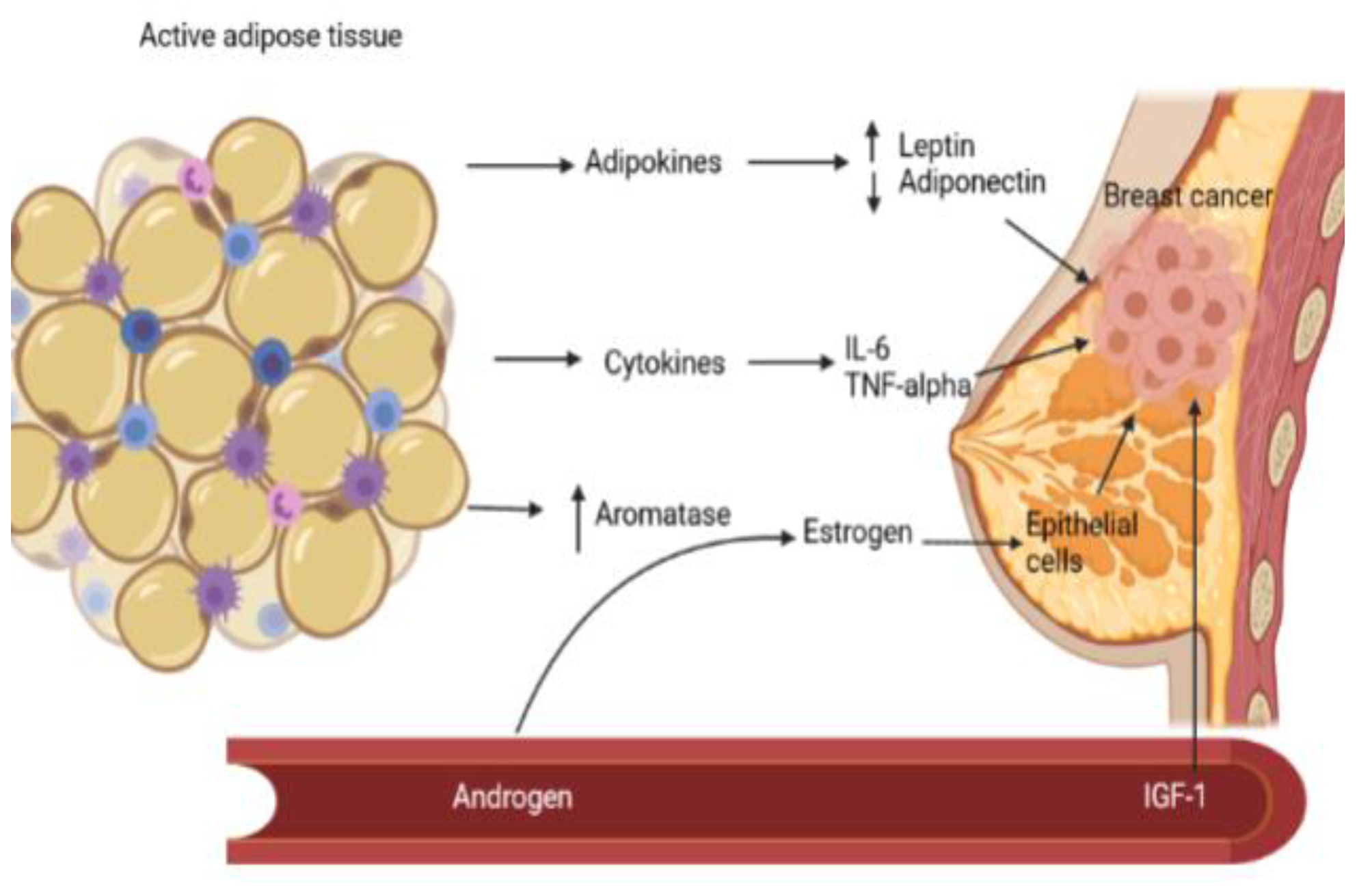
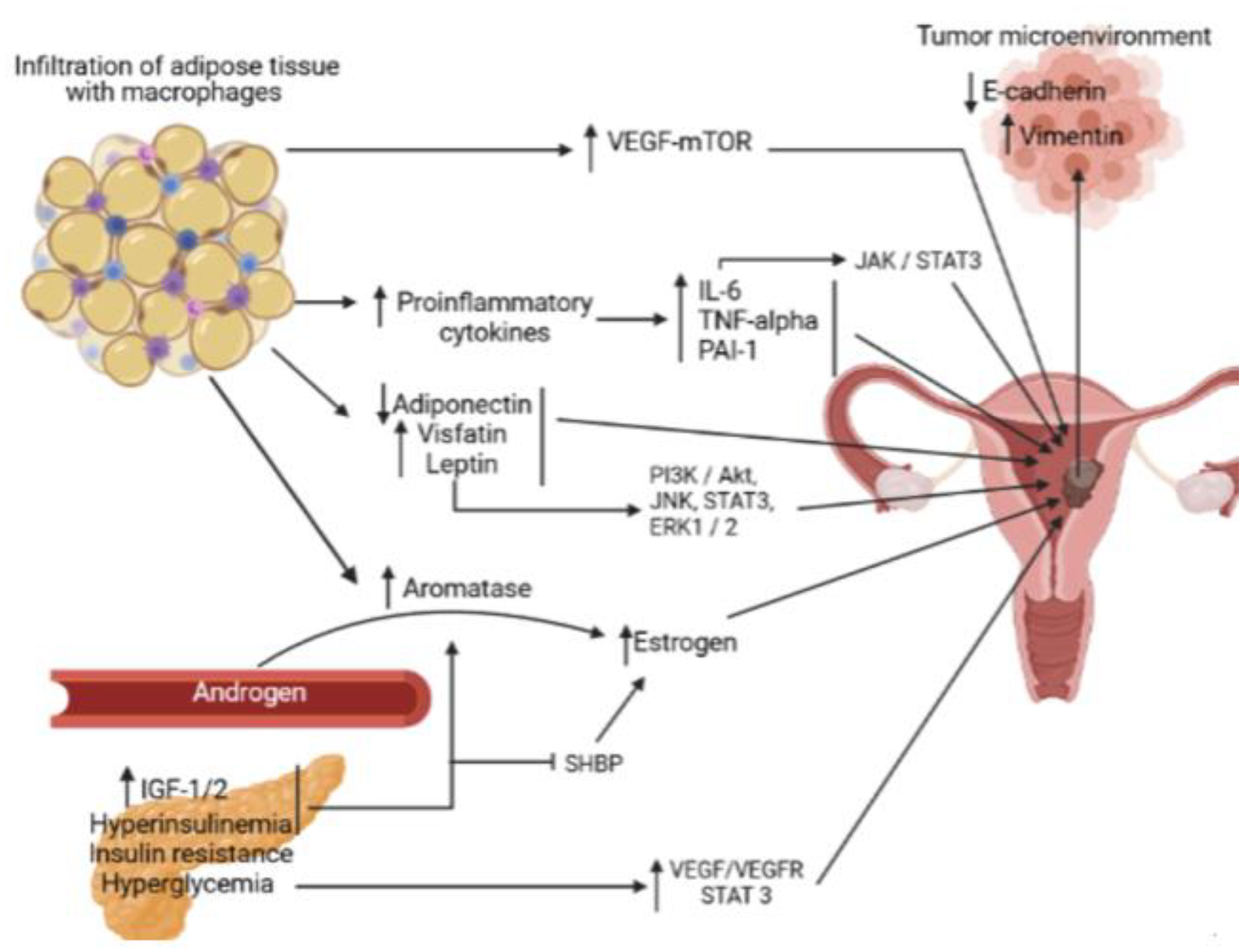
The metabolic syndrome and breast cancer
Mechanisms
Aromatase
Adipokines
Insulin resistance and diabetes mellitus
Chronic inflammation
The metabolic syndrome and various types of breast cancer
The metabolic syndrome and the therapeutic response in breast cancer
Preliminary data
Highlights
- ✓
- A comprehensive summary of the mechanisms underlying the link between the metabolic syndrome and endometrial and breast cancer
- ✓
- The implication of inflammatory changes associated with the metabolic syndrome in oncogenesis
Conclusions
Conflict of interest disclosure
Compliance with ethical standards
References
- World Health Organization. GLOBOCAN 2018- Romania. Available online: https://gco.iarc.fr/today/data/factsheets/populations/642-romania-fact-sheets.pdf.
- Bhandari, R.; Kelley, G.A.; Hartley, T.A.; et al. Metabolic syndrome is associated with increased breast cancer risk: a systematic review with meta-analysis. Int J Breast Cancer. 2014, 2014, 189384. [Google Scholar] [CrossRef] [PubMed]
- Bohîlțea, R.E.; Furtunescu, F.; Dosius, M.; et al. Evaluation of endometrial cancer epidemiology in Romania. J Med Life. 2015, 8, 218–225. [Google Scholar] [PubMed]
- Crosbie, E.J.; Zwahlen, A.; Creutzberg, C.; et al. Endometrial cancer. Lancet. 2016, 387, 1094–1108. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.M.; Mohan, V. Changing definitions of metabolic syndrome. Indian J Endocrinol Metab. 2012, 16, 7–12. [Google Scholar] [CrossRef]
- Ghanbari Andarieh, M.; Agajani Delavar, M.; Moslemi, D.; Esmaeilzadeh, S. Risk Factors for Endometrial Cancer: Results from a Hospital-Based Case-Control Study. Asian Pac J Cancer Prev. 2016, 17, 4791–4796. [Google Scholar] [CrossRef]
- Al-Zoughool, M.; Dossus, L.; Kaaks, R.; et al. Risk of endometrial cancer in relationship to cigarette smoking: results from the EPIC study. Int J Cancer. 2007, 121, 2741–2747. [Google Scholar] [CrossRef]
- Viswanathan, A.N.; Feskanich, D.; De Vivo, I.; Hunter, D.J.; Barbieri, R.L.; Rosner, B.; Colditz, G.A.; Hankinson, S.E. Smoking and the risk of endometrial cancer: results from the Nurses' Health Study. Int J Cancer. 2005, 114, 996–1001. [Google Scholar] [CrossRef]
- Motofei, I.G. Biology of Cancer; From Cellular Cancerogenesis to Supracellular Evolution of Malignant Phenotype. Cancer Invest. 2018, 36, 309–317. [Google Scholar] [CrossRef]
- Raglan, O.; Kalliala, I.; Markozannes, G.; Cividini, S.; Gunter, M.J.; Nautiyal, J.; Gabra, H.; Paraskevaidis, E.; Martin-Hirsch, P.; Tsilidis, K.K.; Kyrgiou, M. Risk factors for endometrial cancer: An umbrella review of the literature. Int J Cancer. 2019, 145, 1719–1730. [Google Scholar] [CrossRef]
- Hamet, P. Cancer and hypertension: a potential for crosstalk? J Hypertens. 1997, 15 12 Pt 2, 1573–1577. [Google Scholar] [CrossRef]
- Soler, M.; Chatenoud, L.; Negri, E.; Parazzini, F.; Franceschi, S.; la Vecchia, C. Hypertension and hormone-related neoplasms in women. Hypertension. 1999, 34, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Harlan, D.M.; Archer, M.C.; Bergenstal, R.M.; Gapstur, S.M.; Habel, L.A.; Pollak, M.; Regensteiner, J.G.; Yee, D. Diabetes and cancer: a consensus report. CA Cancer J Clin. 2010, 60, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Bjørge, T.; Stocks, T.; Lukanova, A.; Tretli, S.; Selmer, R.; Manjer, J.; Rapp, K.; Ulmer, H.; Almquist, M.; Concin, H.; Hallmans, G.; Jonsson, H.; Stattin, P.; Engeland, A. Metabolic syndrome and endometrial carcinoma. Am J Epidemiol. 2010, 171, 892–902. [Google Scholar] [CrossRef]
- Trabert, B.; Wentzensen, N.; Felix, A.S.; Yang, H.P.; Sherman, M.E.; Brinton, L.A. Metabolic syndrome and risk of endometrial cancer in the United States: a study in the SEER-medicare linked database. Cancer Epidemiol Biomarkers Prev. 2015, 24, 261–267. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J. The Role of Metabolic Syndrome in Endometrial Cancer: A Review. Front Oncol. 2019, 9, 744. [Google Scholar] [CrossRef]
- O'Connor, K.A.; Ferrell, R.J.; Brindle, E.; Shofer, J.; Holman, D.J.; Miller, R.C.; Schechter, D.E.; Singer, B.; Weinstein, M. Total and unopposed estrogen exposure across stages of the transition to menopause. Cancer Epidemiol Biomarkers Prev. 2009, 18, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, E.J.; Zwahlen, M.; Kitchener, H.C.; Egger, M.; Renehan, A.G. Body mass index, hormone replacement therapy, and endometrial cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010, 19, 3119–3130. [Google Scholar] [CrossRef]
- Pintaudi, B.; Di Vieste, G.; Nicolucci, A.; et al. The impact of clinical and psychological characteristics on alexithymia in type 1 diabetes. Mediterranean Journal of Clinical Psychology. 2021, 9. [Google Scholar] [CrossRef]
- Bruchim, I.; Sarfstein, R.; Werner, H. The IGF Hormonal Network in Endometrial Cancer: Functions, Regulation, and Targeting Approaches. Front Endocrinol (Lausanne). 2014, 5, 76. [Google Scholar] [CrossRef]
- Tian, W.; Teng, F.; Gao, J.; Gao, C.; Liu, G.; Zhang, Y.; Yu, S.; Zhang, W.; Wang, Y.; Xue, F. Estrogen and insulin synergistically promote endometrial cancer progression via crosstalk between their receptor signaling pathways. Cancer Biol Med. 2019, 16, 55–70. [Google Scholar] [CrossRef]
- de Luca, C.; Olefsky, J.M. Inflammation and insulin resistance. FEBS Lett. 2008, 582, 97–105. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Tanwar, P.S. VEGF-mTOR signaling links obesity and endometrial cancer. Oncoscience. 2018, 5, 150–151. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Dannenberg, A.J. The obese adipose tissue microenvironment in cancer development and progression. Nat Rev Endocrinol. 2019, 15, 139–154. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Zhao, L.; Zhou, J.; Cheng, Y.; Xu, B.; Wang, J.; Wei, L. Spontaneous formation of tumorigenic hybrids between human omental adipose-derived stromal cells and endometrial cancer cells increased motility and heterogeneity of cancer cells. Cell Cycle. 2019, 18, 320–332. [Google Scholar] [CrossRef]
- Shi, J.; Fan, J.; Su, Q.; Yang, Z. Cytokines and Abnormal Glucose and Lipid Metabolism. Front Endocrinol (Lausanne). 2019, 10, 703. [Google Scholar] [CrossRef] [PubMed]
- Cao, H. Adipocytokines in obesity and metabolic disease. J Endocrinol. 2014, 220, T47–T59. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Shi, J.; Long, Y.; Tian, H.; Li, X.; Zhao, A.Z.; Li, R.F.; Chen, T. Adiponectin and Endometrial Cancer: A Systematic Review and Meta-Analysis. Cell Physiol Biochem. 2015, 36, 1670–1678. [Google Scholar] [CrossRef]
- Gelsomino, L.; Naimo, G.D.; Catalano, S.; Mauro, L.; Andò, S. The Emerging Role of Adiponectin in Female Malignancies. Int J Mol Sci. 2019, 20, 2127. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T. Adiponectin and adiponectin receptors. Endocr Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef]
- Yabushita, H.; Iwasaki, K.; Obayashi, Y.; Wakatsuki, A. Clinicopathological roles of adiponectin and leptin receptors in endometrial carcinoma. Oncol Lett. 2014, 7, 1109–1117. [Google Scholar] [CrossRef][Green Version]
- Peng, J.; Tsang, J.Y.; Ho, D.H.; Zhang, R.; Xiao, H.; Li, D.; Zhu, J.; Wang, F.; Bian, Z.; Lui, V.C.; Xu, A.; Tam, P.K.; Lamb, J.R.; Xia, H.; Chen, Y. Modulatory effects of adiponectin on the polarization of tumor-associated macrophages. Int J Cancer. 2015, 137, 848–858. [Google Scholar] [CrossRef]
- Cai, Z.F.; Deng, L.; Wang, M.M.; Zhang, J.Q.; Li, L. Effect of AMPK/mTOR/S6K1 pathways and the insulin- sensitizing effect for adiponectin in endometrial cancer cells. Zhonghua Fu Chan Ke Za Zhi. 2018, 53, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Wu, H.; Cao, J. Circulating adiponectin and risk of endometrial cancer. PLoS One. 2015, 10, e0129824. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mohammadi, M.; Mianabadi, F.; Mehrad-Majd, H. Circulating visfatin levels and cancers risk: A systematic review and meta-analysis. J Cell Physiol. 2019, 234, 5011–5022. [Google Scholar] [CrossRef]
- Cymbaluk-Płoska, A.; Chudecka-Głaz, A.; Pius- Sadowska, E.; Sompolska-Rzechuła, A.; Machaliński, B.; Menkiszak, J. Circulating Serum Level of Visfatin in Patients with Endometrial Cancer. Biomed Res Int. 2018, 2018, 8576179. [Google Scholar] [CrossRef]
- Ciuhu, A.N.; Pantea-Stoian, A.M.; Nitipir, C.; et al. Assessment of cachexia in cancer patients with advanced disease. International Conference on Interdisciplinary Management of Diabetes Mellitus and its Complications (INTERDIAB) Interdiab 2017: Diabetes mellitus in internal medicine. 2017, 139–147. ISSN: 2393-3488.
- Wang, Z.; Gao, S.; Sun, C.; Li, J.; Gao, W.; Yu, L. Clinical significance of serum adiponectin and visfatin levels in endometrial cancer. Int J Gynaecol Obstet. 2019, 145, 34–39. [Google Scholar] [CrossRef]
- Zahid, H.; Subbaramaiah, K.; Iyengar, N.M.; Zhou, X.K.; Chen, I.C.; Bhardwaj, P.; Gucalp, A.; Morrow, M.; Hudis, C.A.; Dannenberg, A.J.; Brown, K.A. Leptin regulation of the p53-HIF1α/PKM2-aromatase axis in breast adipose stromal cells: a novel mechanism for the obesity-breast cancer link. Int J Obes (Lond). 2018, 42, 711–720. [Google Scholar] [CrossRef]
- Koushiou, M.; Kapatais, A.; Iasonidou, E.; Adonis, M.; Ferreira, N. The moderating role of body image inflexibility in the relation between weight concerns and symptoms of eating disorders in Cypriot University students. Mediterranean Journal of Clinical Psychology. 2021, 9. [Google Scholar] [CrossRef]
- Zhou, X.; Li, H.; Chai, Y.; Liu, Z. Leptin Inhibits the Apoptosis of Endometrial Carcinoma Cells Through Activation of the Nuclear Factor κB-inducing Kinase/IκB Kinase Pathway. Int J Gynecol Cancer. 2015, 25, 770–778. [Google Scholar] [CrossRef]
- Petridou, E.; Belechri, M.; Dessypris, N.; Koukoulomatis, P.; Diakomanolis, E.; Spanos, E.; Trichopoulos, D. Leptin and body mass index in relation to endometrial cancer risk. Ann Nutr Metab. 2002, 46, 147–51. [Google Scholar] [CrossRef]
- Gao, J.; Tian, J.; Lv, Y.; Shi, F.; Kong, F.; Shi, H.; Zhao, L. Leptin induces functional activation of cyclooxygenase-2 through JAK2/STAT3, MAPK/ERK, and PI3K/AKT pathways in human endometrial cancer cells. Cancer Sci. 2009, 100, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lv, L.; Xiao, W.; Gong, C.; Yin, J.; Wang, D.; Sheng, H. Leptin activates STAT3 and ERK1/2 pathways and induces endometrial cancer cell proliferation. J Huazhong Univ Sci Technolog Med Sci. 2011, 31, 365. [Google Scholar] [CrossRef]
- Ekremoğlu, M.; Severcan, Ç.; Pasaoğlu, Ö.T.; Şen, B.; Pasaoğlu, H. An investigation of acute effects at various doses of malathion on glucose homeostasis and insulin resistance in rat liver, pancreas and serum. J Mind Med Sci. 2020, 7, 85–93. [Google Scholar] [CrossRef]
- Lippitz, B.E.; Harris, R.A. Cytokine patterns in cancer patients: A review of the correlation between interleukin 6 and prognosis. Oncoimmunology. 2016, 5, e1093722. [Google Scholar] [CrossRef]
- He, Y.Y.; Cai, B.; Yang, Y.X.; Liu, X.L.; Wan, X.P. Estrogenic G protein-coupled receptor 30 signaling is involved in regulation of endometrial carcinoma by promoting proliferation, invasion potential, and interleukin-6 secretion via the MEK/ERK mitogen-activated protein kinase pathway. Cancer Sci. 2009, 100, 1051–1061. [Google Scholar] [CrossRef]
- Che, Q.; Liu, B.Y.; Liao, Y.; et al. Activation of a positive feedback loop involving IL-6 and aromatase promotes intratumoral 17β-estradiol biosynthesis in endometrial carcinoma microenvironment. Int J Cancer. 2014, 135, 282–94. [Google Scholar] [CrossRef]
- Johnson, D.E.; O'Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Rahnea-Nita, G.; Badiu, D.C.; Popescu, C.G.; Grigorean, V.T.; Serban, D.; Smarandache, C.G.; Rahnea-Nita, R.A.; Ciuhu, A.N.; Mandu, M.; Andronache, L.F.; Stoian, A.R. The impact of Coronavirus disease 2019 pandemic on the treatment of cancer patients: the first steps in this fight. Mediterranean Journal of Clinical Psychology. 2021, 9. [Google Scholar] [CrossRef]
- Nair, S.; Nguyen, H.; Salama, S.; Al-Hendy, A. Obesity and the Endometrium: Adipocyte-Secreted Proinflammatory TNF α Cytokine Enhances the Proliferation of Human Endometrial Glandular Cells. Obstet Gynecol Int. 2013, 2013, 368543. [Google Scholar] [CrossRef]
- Vousden, K.A.; Lundqvist, T.; Popovic, B.; et al. Discovery and characterisation of an antibody that selectively modulates the inhibitory activity of plasminogen activator inhibitor-1. Sci Rep. 2019, 9, 1605. [Google Scholar] [CrossRef]
- Abbink, K.; Zusterzeel, P.L.M.; Geurts-Moespot, A.; van der Steen, R.; Span, P.N.; Sweep, F.C.G.J. Prognostic significance of VEGF and components of the plasminogen activator system in endometrial cancer. J Cancer Res Clin Oncol. 2020, 146, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Suceveanu, A.I.; Stoian, A.P.; Parepa, I.; et al. Gut Microbiota Patterns in Obese and Type 2 Diabetes (T2D) Patients from Romanian Black Sea Coast Region. Revista de Chimie. 2018, 69, 2260–2267. [Google Scholar] [CrossRef]
- Folsom, A.R.; Anderson, K.E.; Sweeney, C.; Jacobs, D.R., Jr. Diabetes as a risk factor for death following endometrial cancer. Gynecol Oncol. 2004, 94, 740–5. [Google Scholar] [CrossRef] [PubMed]
- Byrne, F.L.; Martin, A.R.; Kosasih, M.; Caruana, B.T.; Farrell, R. The Role of Hyperglycemia in Endometrial Cancer Pathogenesis. Cancers (Basel). 2020, 12, 1191. [Google Scholar] [CrossRef]
- Gu, C.J.; Xie, F.; Zhang, B.; Yang, H.L.; Cheng, J.; He, Y.Y.; Zhu, X.Y.; Li, D.J.; Li, M.Q. High Glucose Promotes Epithelial-Mesenchymal Transition of Uterus Endometrial Cancer Cells by Increasing ER/GLUT4- Mediated VEGF Secretion. Cell Physiol Biochem. 2018, 50, 706–720. [Google Scholar] [CrossRef]
- Shah, H.; Shah, R.; Sanghani, H.; Lakhani, N. Health related quality of life (HRQoL) and its associated surgical factors in diabetes foot ulcer patients. J Clin Invest Surg. 2020, 5, 83–90. [Google Scholar] [CrossRef]
- Hsu, M.C.; Hung, W.C. Pyruvate kinase M2 fuels multiple aspects of cancer cells: from cellular metabolism, transcriptional regulation to extracellular signaling. Mol Cancer. 2018, 17, 35. [Google Scholar] [CrossRef]
- Choi, S.Y.; Collins, C.C.; Gout, P.W.; Wang, Y. Cancer- generated lactic acid: a regulatory, immunosuppressive metabolite? J Pathol. 2013, 230, 350–355. [Google Scholar] [CrossRef]
- Colegio, O.R. Lactic acid polarizes macrophages to a tumor-promoting state. Oncoimmunology. 2015, 5, e1014774. [Google Scholar] [CrossRef]
- Latif, A.; Chadwick, A.L.; Kitson, S.J.; Gregson, H.J.; Sivalingam, V.N.; Bolton, J.; McVey, R.J.; Roberts, S.A.; Marshall, K.M.; Williams, K.J.; Stratford, I.J.; Crosbie, E.J. Monocarboxylate Transporter 1 (MCT1) is an independent prognostic biomarker in endometrial cancer. BMC Clin Pathol. 2017, 17, 27. [Google Scholar] [CrossRef]
- Hernandez, A.V.; Pasupuleti, V.; Benites-Zapata, V.A.; Thota, P.; Deshpande, A.; Perez-Lopez, F.R. Insulin resistance and endometrial cancer risk: A systematic review and meta-analysis. Eur J Cancer. 2015, 51, 2747–2758. [Google Scholar] [CrossRef]
- Rodriguez, A.C.; Blanchard, Z.; Maurer, K.A.; Gertz, J. Estrogen Signaling in Endometrial Cancer: a Key Oncogenic Pathway with Several Open Questions. Horm Cancer. 2019, 10, 51–63. [Google Scholar] [CrossRef]
- Joehlin-Price, A.S.; Stephens, J.A.; Zhang, J.; Backes, F.J.; Cohn, D.E.; Suarez, A.A. Endometrial Cancer Insulin- Like Growth Factor 1 Receptor (IGF1R) Expression Increases with Body Mass Index and Is Associated with Pathologic Extent and Prognosis. Cancer Epidemiol Biomarkers Prev. 2016, 25, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Bacalbasa, N.; Balescu, I.; Dimitriu, M.; Balalau, C.; Vilcu, M.; Brezean, I. Does sentinel lymph node detection play a role in patients with vaginal cancer? J Clin Invest Surg. 2019, 4, 1–4. [Google Scholar] [CrossRef]
- Mu, N.; Xu, T.; Gao, M.; Dong, M.; Tang, Q.; Hao, L.; Wang, G.; Li, Z.; Wang, W.; Yang, Y.; Hou, J. Therapeutic effect of metformin in the treatment of endometrial cancer. Oncol Lett. 2020, 20, 156. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Wu, J.; Wang, K.; Zhao, M.; Wang, C.; Li, L.; Guo, R. Effect of metformin use on the risk and prognosis of endometrial cancer: a systematic review and meta- analysis. BMC Cancer. 2018, 18, 438. [Google Scholar] [CrossRef]
- Nevadunsky, N.S.; Van Arsdale, A.; Strickler, H.D.; Moadel, A.; Kaur, G.; Frimer, M.; Conroy, E.; Goldberg, G.L.; Einstein, M.H. Metformin use and endometrial cancer survival. Gynecol Oncol. 2014, 132, 236–240. [Google Scholar] [CrossRef]
- Arthur, R.S.; Kabat, G.C.; Kim, M.Y.; Wild, R.A.; Shadyab, A.H.; Wactawski-Wende, J.; Ho, G.Y.F.; Reeves, K.W.; Kuller, L.H.; Luo, J.; Beebe-Dimmer, J.; Simon, M.S.; Strickler, H.; Wassertheil-Smoller, S.; Rohan, T.E. Metabolic syndrome and risk of endometrial cancer in postmenopausal women: a prospective study. Cancer Causes Control. 2019, 30, 355–363. [Google Scholar] [CrossRef]
- Bruning, P.F.; Bonfrèr, J.M. Free fatty acid concentrations correlated with the available fraction of estradiol in human plasma. Cancer Res. 1986, 46, 2606–2609. [Google Scholar]
- Li, P.; Shan, B.; Jia, K.; Hu, F.; Xiao, Y.; Zheng, J.; Gao, Y.T.; Wang, H.; Gao, Y. Plasma omega-3 polyunsaturated fatty acids and recurrence of endometrial cancer. BMC Cancer. 2020, 20, 576. [Google Scholar] [CrossRef]
- Yao, L.; Chen, S.; Li, W. Fatostatin inhibits the development of endometrial carcinoma in endometrial carcinoma cells and a xenograft model by targeting lipid metabolism. Arch Biochem Biophys. 2020, 684, 108327. [Google Scholar] [CrossRef] [PubMed]
- Mackintosh, M.L.; Crosbie, E.J. Obesity-driven endometrial cancer: is weight loss the answer? BJOG. 2013, 120, 791–794. [Google Scholar] [CrossRef]
- Linkov, F.; Goughnour, S.L.; Ma, T.; Xu, Z.; Edwards, R.P.; Lokshin, A.E.; Ramanathan, R.C.; Hamad, G.G.; McCloskey, C.; Bovbjerg, D.H. Changes in inflammatory endometrial cancer risk biomarkers in individuals undergoing surgical weight loss. Gynecol Oncol. 2017, 147, 133–138. [Google Scholar] [CrossRef]
- Sun, Y.S.; Zhao, Z.; Yang, Z.N.; Xu, F.; Lu, H.J.; Zhu, Z.Y.; Shi, W.; Jiang, J.; Yao, P.P.; Zhu, H.P. Risk Factors and Preventions of Breast Cancer. Int J Biol Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Dibaba, D.T.; Braithwaite, D.; Akinyemiju, T. Metabolic Syndrome and the Risk of Breast Cancer and Subtypes by Race, Menopause and BMI. Cancers (Basel). 2018, 10, 299. [Google Scholar] [CrossRef]
- van Oers, H.; Schlebusch, L. Indicators of psychological distress and body image disorders in female patients with breast cancer. J Mind Med Sci. 2020, 7, 179–187. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, Y.Y.; Li, Z.R.; Luo, D.L.; Zhang, X.H. The molecular mechanisms between metabolic syndrome and breast cancer. Biochem Biophys Res Commun. 2016, 471, 391–395. [Google Scholar] [CrossRef]
- Belardi, V.; Gallagher, E.J.; Novosyadlyy, R.; LeRoith, D. Insulin and IGFs in obesity-related breast cancer. J Mammary Gland Biol Neoplasia. 2013, 18, 277–89. [Google Scholar] [CrossRef] [PubMed]
- Agnoli, C.; Grioni, S.; Sieri, S.; Sacerdote, C.; Ricceri, F.; Tumino, R.; Frasca, G.; Pala, V.; Mattiello, A.; Chiodini, P.; Iacoviello, L.; De Curtis, A.; Panico, S.; Krogh, V. Metabolic syndrome and breast cancer risk: a case- cohort study nested in a multicentre italian cohort. PLoS One. 2015, 10, e0128891. [Google Scholar] [CrossRef]
- Guo, M.; Liu, T.; Li, P.; Wang, T.; Zeng, C.; Yang, M.; Li, G.; Han, J.; Wu, W.; Zhang, R. Association Between Metabolic Syndrome and Breast Cancer Risk: An Updated Meta-Analysis of Follow-Up Studies. Front Oncol. 2019, 9, 1290. [Google Scholar] [CrossRef]
- Maiti, B.; Kundranda, M.N.; Spiro, T.P.; et al. The association of metabolic syndrome with triple-negative breast cancer. Breast Cancer Res Treat. 2010, 121, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Guenancia, C.; Lefebvre, A.; Cardinale, D.; et al. Obesity as a Risk Factor for Anthracyclines and Trastuzumab Cardiotoxicity in Breast Cancer: A Systematic Review and Meta-Analysis. J Clin Oncol. 2016, 34, 3157–3165. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, S.; Shibata, R.; Ohashi, K.; Ohashi, T.; Daida, H.; Walsh, K.; Murohara, T.; Ouchi, N. Adiponectin ameliorates doxorubicin-induced cardiotoxicity through Akt protein-dependent mechanism. J Biol Chem. 2011, 286, 32790–32800. [Google Scholar] [CrossRef]
- Esposito, K.; Chiodini, P.; Capuano, A.; Bellastella, G.; Maiorino, M.I.; Rafaniello, C.; Giugliano, D. Metabolic syndrome and postmenopausal breast cancer: systematic review and meta-analysis. Menopause. 2013, 20, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, T.; Zeng, C.; et al. Association between metabolic syndrome and prognosis of breast cancer: a meta-analysis of follow-up studies. Diabetol Metab Syndr. 2020, 12, 10. [Google Scholar] [CrossRef]
- Berrino, F.; Villarini, A.; Traina, A.; Bonanni, B.; Panico, S.; Mano, M.P.; Mercandino, A.; Galasso, R.; Barbero, M.; Simeoni, M.; Bassi, M.C.; Consolaro, E.; Johansson, H.; Zarcone, M.; Bruno, E.; Gargano, G.; Venturelli, E.; Pasanisi, P. Metabolic syndrome and breast cancer prognosis. Breast Cancer Res Treat. 2014, 147, 159–165. [Google Scholar] [CrossRef]
- Tecalco-Cruz, A.C.; Pérez-Alvarado, I.A.; Ramírez- Jarquín, J.O.; Rocha-Zavaleta, L. Nucleo-cytoplasmic transport of estrogen receptor alpha in breast cancer cells. Cell Signal. 2017, 34, 121–132. [Google Scholar] [CrossRef]
 |
 |
© 2021 by the author. 2021 Andrada-Luciana Lazar, Romana Vulturar, Adriana Fodor, Olga Hilda Orasan, Camil-Horia-Eusebiu Crișan, Cezar Login, Ioana Para, Vasile Negrean, Brandusa Tiperciuc, Angela Cozma
Share and Cite
Lazar, A.-L.; Vulturar, R.; Fodor, A.; Orasan, O.H.; Crișan, C.-H.E.; Login, C.; Para, I.; Negrean, V.; Tiperciuc, B.; Cozma, A. The Molecular Mechanisms Linking Metabolic Syndrome to Endometrial and Breast Cancers. J. Mind Med. Sci. 2021, 8, 167-178. https://doi.org/10.22543/7674.82.P167178
Lazar A-L, Vulturar R, Fodor A, Orasan OH, Crișan C-HE, Login C, Para I, Negrean V, Tiperciuc B, Cozma A. The Molecular Mechanisms Linking Metabolic Syndrome to Endometrial and Breast Cancers. Journal of Mind and Medical Sciences. 2021; 8(2):167-178. https://doi.org/10.22543/7674.82.P167178
Chicago/Turabian StyleLazar, Andrada-Luciana, Romana Vulturar, Adriana Fodor, Olga Hilda Orasan, Camil-Horia Eusebiu Crișan, Cezar Login, Ioana Para, Vasile Negrean, Brandusa Tiperciuc, and Angela Cozma. 2021. "The Molecular Mechanisms Linking Metabolic Syndrome to Endometrial and Breast Cancers" Journal of Mind and Medical Sciences 8, no. 2: 167-178. https://doi.org/10.22543/7674.82.P167178
APA StyleLazar, A.-L., Vulturar, R., Fodor, A., Orasan, O. H., Crișan, C.-H. E., Login, C., Para, I., Negrean, V., Tiperciuc, B., & Cozma, A. (2021). The Molecular Mechanisms Linking Metabolic Syndrome to Endometrial and Breast Cancers. Journal of Mind and Medical Sciences, 8(2), 167-178. https://doi.org/10.22543/7674.82.P167178



