Abstract
The progress made over the past years in the field of cancer therapy has led to a significant decrease in cancer mortality, but these therapies have many adverse effects, cardiovascular effects being among the most frequent ones. For increasing lifelong expectancy of surviving cancer patients, cardiac monitoring represents an important task. Current studies and practice recommend echocardiography using strain analysis for monitoring the cardio toxic effects of cancer therapy. The potential of combining imaging techniques with biomarkers for the early detection and diagnosis seems a promising path for future research.
Introduction
The progress made over the past years in the field of cancer therapy has led to a significant decrease in cancer mortality. However, these therapies have many adverse effects, cardiovascular effects being among the most frequent ones. In addition to the fact that they decrease the quality of life, they can lead to a reduction in the overall survival rate, independently of the prognosis of cancer disease [1]. In fact, the term cardiotoxicity refers to myocardial dysfunction and heart failure (HF), the most redoubtable complications of these therapies, which occur in variable proportions, being estimated at 1.7-20.1% for trastuzumab and 1.6-4% for bevacizumab [2].
Currently, efforts are aimed at the early detection of left ventricular dysfunction (LVD) for the initiation of prompt treatment, without compromising cancer therapy. Many studies investigate the role of biomarkers in the early detection of LVD, before this becomes severe enough to induce a decrease in the left ventricular ejection fraction (LVEF). Among the most studied markers, there are cardiac troponins and natriuretic peptides [3].
Discussions
Troponins
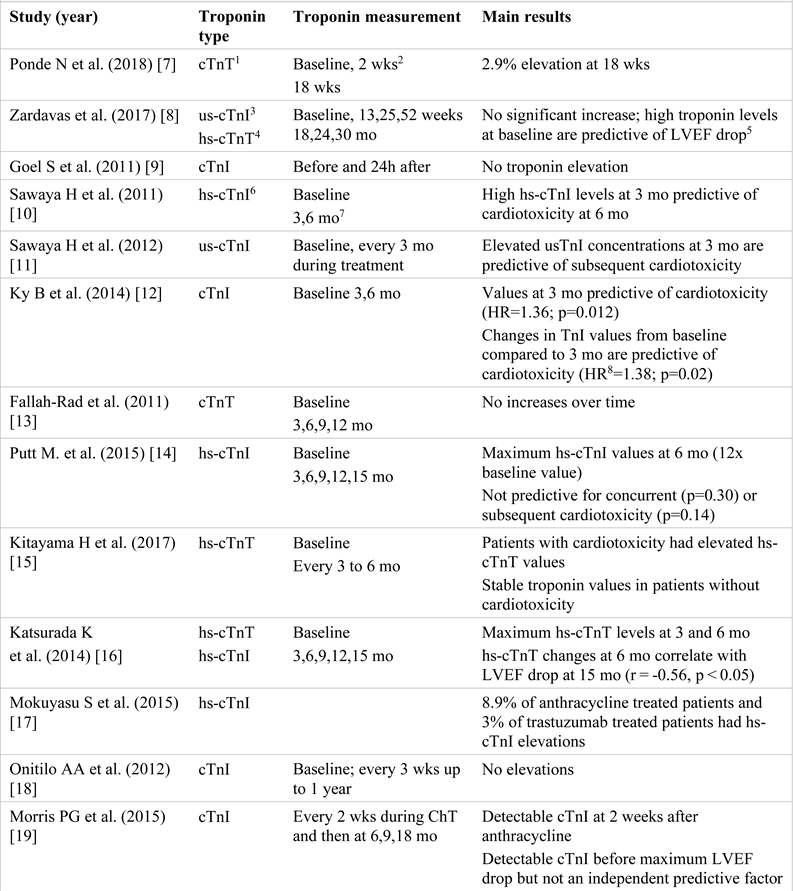
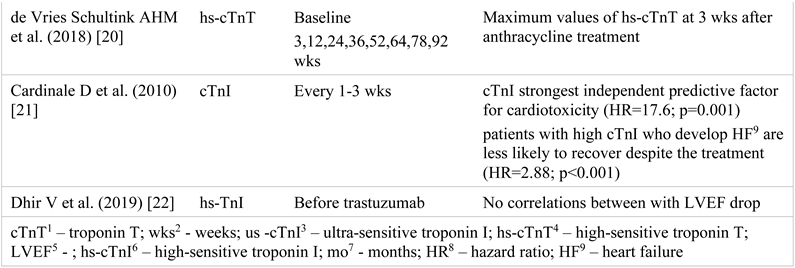
Troponins represent a complex formed by 3 subunits: troponin T (cTnT), troponin I (cTnI), and troponin C (cTnC). Isoforms T and I have cardiac specificity, while cTnC is present in the myocardium as well as in striated muscles [4,5]. Most frequently, their measurement is performed in case of acute coronary syndrome, being essential for the diagnosis and guidance of the therapeutic approach [6]. However, any myocardial lesion, regardless of its mechanism of occurrence, which induces myocyte membrane damage, also induces intracellular depletion of troponins and its subsequent release into the blood [7,8,9,10,11]. Drugs that induce progressive/chronic myocardial injury cause a small but persistent increase in troponins, which is directly proportional to the duration of the therapy [12,13,14,15,16]. Many studies investigate their utility in LVD screening in cancer patients. For this review, 16 studies have been analyzed. Their characteristics and the main results obtained are summarized below (Table 1) [17,18,19,20,21,22].

Table 1.
The characteristics and the main results of the studies investigating cardiac troponin modifications in HER2+ breast cancer patients treated with trastuzumab.
All studies included samples of patients with HER- positive breast carcinoma. One study [7] included patients who did not receive anthracycline prior to trastuzumab therapy.
Regarding the patients sequentially treated with anthracyclines and trastuzumab, studies report divergent results. Some authors consider that increased or detectable troponin values observed during the treatment with trastuzumab might be the result of anthracycline toxicity because they occur shortly after the initiation of trastuzumab therapy and subsequently tend to normalize [8,11,12,21]. A possible explanation could be the different cardiotoxicity mechanisms of the two groups of drugs [23].
For example, Mokuyasu et al. [17] compared cTnI values in patients treated with anthracyclines and patients treated with trastuzumab. The results show that 8.9% of the patients who were also treated with anthracyclines had an increase in cTnI values compared to 3% of the patients treated with trastuzumab. The patients who received no anthracyclines did not have increased hs-cTnI values.
Zardavas et al. [8] demonstrated that in patients with high cTn values, these were already present before the initiation of the therapy with trastuzumab and did not increase significantly afterwards, and that these patients had a 2.4-4.5 times higher risk of a decrease in LVEF. Similarly, Sawaya et al. [11] showed that the highest us- cTnI values were recorded shortly after the completion of the treatment with anthracyclines, followed by their decrease during trastuzumab therapy. According to this study, us-cTnI measurement after the completion of anthracycline treatment has a sensitivity of 48% and a specificity of 73% as a predictor of subsequent cardiotoxicity. The usefulness of troponins I as a prognostic marker of cardiac dysfunction was also demonstrated in the study conducted by Ky B et al. [12].
The study carried out by Cardinale et al. [21] also reported increased troponin values in the majority of the patients (23 of the 36 patients with increased values) at the beginning of the treatment with trastuzumab, with their normalization over the following 3 months. In addition, the authors showed that cTnI values represent the strongest independent predictor of cardiotoxicity. Furthermore, the authors indicated that patients with high troponin levels who develop cardiotoxicity have lower chances of LVEF recovery compared to patients with normal cTnI values (36% vs. 100%) under optimal medical treatment.
The study performed by Katsurada K et al. [16] showed that hs-cTnT values at 6 months represent a predictive factor of the 10% LVEF reduction at 15 months, suggesting that this would be a more useful marker than hs-cTnI. A more recent study [20, 23] also demonstrated the predictive value of hs-cTnT at 3 weeks after the treatment with anthracyclines regarding the trastuzumab-induced decrease in LVEF.
In some studies, [14,19], despite an increase in the troponin values recorded during treatment, the authors could not demonstrate their predictive value in the early detection of cardiotoxicity. On the other hand, there are studies in which the increase in troponins during therapy was not demonstrated [9,13,18,22].
The only study comprising patients treated exclusively with trastuzumab [7] suggests that high troponin values occur in a small number of patients and their measurement is not useful for monitoring trastuzumab-induced cardiotoxicity, but it should be taken into consideration that the small sample and the reduced number of cardiac events did not allow statistical analysis.
In conclusion, although there are many studies that show the usefulness of troponin measurement for the early detection of cardiac toxicity or for the identification of patients at risk of developing HF during the therapy with trastuzumab after anthracycline treatment, further studies with larger samples and standardized measurement methods are required for the implementation of this method in clinical practice. Comparing the results is difficult since different cut-off values for troponin as a marker of cardiotoxicity have been chosen. Moreover, troponin measurement presents a high variability because of the different assays that are used. The imprecision of a typical troponin assay that was used in the past was between 10- 20% at the 99th percentile. The new hs-assays managed to lower this imprecision below 10% [24].
Brain Natriuretic Peptide
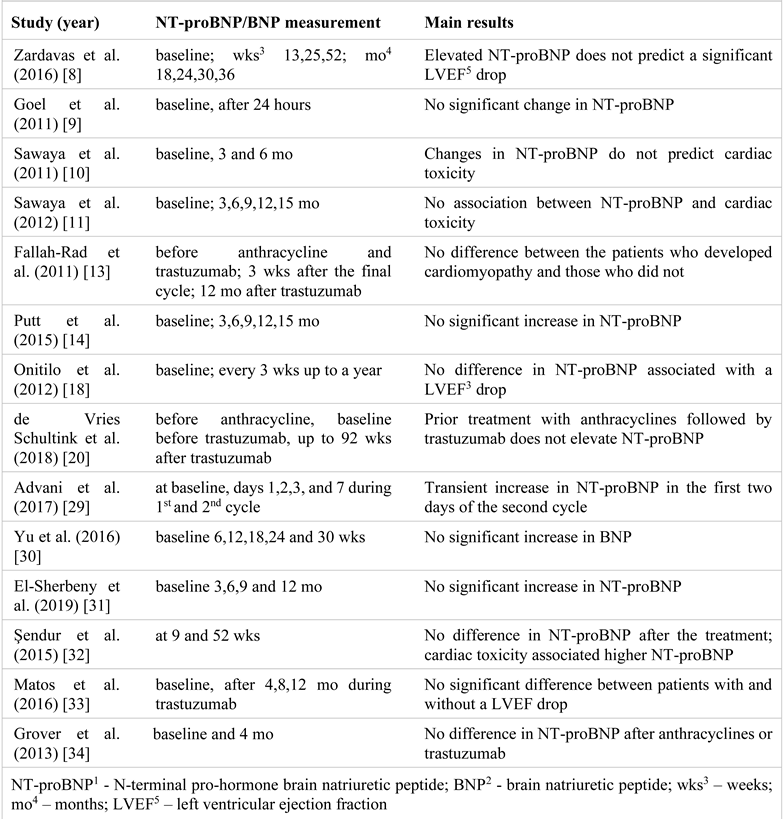
The family of natriuretic peptides includes the atrial natriuretic peptide, brain natriuretic peptide (BNP), and C- type natriuretic peptide (a peptide secreted by the vascular endothelium). BNP is released mainly by atrial myocytes under normal conditions, and by ventricular myocytes in the presence of congestive HF. Thus, increased BNP values are the hallmark of ventricular dysfunction. The precursor pre-proBNP is cleaved into the biologically active BNP molecule and the inactive N-terminal proBNP (NT- proBNP) fragment. In patients with HF, BNP is more increased than the atrial natriuretic peptide and has a half higher half-life (20 min vs. 2 min), while NT-proBNP has the highest half-life (approximately 120 min) and thus persists more in circulation [25]. BNP values are under the influence of several factors. Higher BNP values are seen in older patients (the cut-off point for HF jumps from 450 to 900 pg/ml in patients over 50 years) and in females, but obesity leads to significantly lower BNP. Cut-off points for diagnosing HF need to be higher if the estimated glomerular filtration rate is lower than 60 mL/min/1.73 m2 [25,26].
The widespread use of BNP in clinical practice has led to its study in various chemotherapy regimens. In anthracycline-based therapy, LVD, symptomatic HF, or acute coronary syndrome were associated with BNP values above 100 pg/mL. NT-proBNP elevations correlate with diastolic and systolic dysfunction after high dose chemotherapy [27,28].
So far, studies have not shown a useful role for BNP/NT-proBNP monitoring during trastuzumab treatment. This might be explained by the low cardiotoxicity of trastuzumab compared to anthracyclines and the difference in assays and cut-off values used in various studies [28]. Table 2 provides a summary of the results [8,9,10,11,13,14,18,20,29,30,31,32,33,34]. Studies have not looked specifically for the influence of bevacizumab therapy on BNP in breast cancer patients. Two to four percent of the subjects on bevacizumab treatment develop LVD that associates increased BNP levels [35].

Table 2.
NT-proBNP1/BNP2 changes during the trastuzumab treatment.
Galectin-3
Galectins are β-galactoside-binding lectins with at least one carbohydrate recognition domain. There are 15 types of galectins, distinguished from one another by the type of carbohydrate recognition domain. Galectin-3 is involved in numerous cellular processes such as cellular differentiation, inflammation, fibrosis, and angiogenesis. Cardiovascular diseases such as HF, ischemic heart disease, atrial fibrillation, and renal dysfunction are associated with increased serum galectin-3 values. At the same time, breast tumor tissue has an increased expression of galectin-3, and triple negative breast cancer expresses more galectin-3 than other types of breast cancer [36].
Patients with breast cancer have higher serum galectin- 3 levels compared to healthy subjects (18.41 ng/mL in cancer patients and 6.73 ng/mL in controls (p<0.0001). After 3 and 6 months of trastuzumab, anthracycline and taxane treatment, galectin-3 levels were increased compared to baseline. At the same time, HER2- patients had higher galectin-3 values than HER2+ patients [37]. In contrast, in another study, galectin-3 levels did not increase from baseline after 15 months of trastuzumab, doxorubicin, and paclitaxel treatment [14].
Echocardiographic markers of cardiac dysfunction
Advances in cancer treatment over the last decades have remarkably improved the survival rates of patients diagnosed with solid and hematologic malignancies.
Unfortunately, several chemotherapeutic agents (e.g., trastuzumab, bevacizumab) are known to have cardio toxic effects [37]. Ventricular dysfunction and heart failure, as a complication of cancer therapy, have become major public health concerns, as they are associated with poor prognosis and long-term morbidity and mortality [38]. The development of a subclinical left ventricular dysfunction (cancer therapy–related cardiac dysfunction [CTRCD]), defined by a threshold change in LV ejection fraction (LVEF), may be seen in up to 42% of the patients with cancer in selected treatment groups [39].
Therefore, the early diagnosis and control of cancer therapy-related cardiac dysfunction CTRCD is of crucial importance in patients undergoing therapies. In the absence of robust risk prediction models, cardiologists are very interested in the use of more sensitive markers to detect early myocardial dysfunction [40], for CTRCD prevention. The most frequent echocardiographic markers used in cancer patients are as follows: LVEF by 2D TTE; VTI: lateral S wave in both LV and RV; DTI: the detection of strain (GLS versus GCS versus GRS) and free wall RV strain.
One of the conventional methods for monitoring cardiac function in patients receiving cancer therapy is the determination of 2D left ventricular ejection fraction (LVEF) with serial transthoracic echocardiography (TTE). A value < 53% of LVEF or a decrease > 10% in LVEF during cancer treatment is consistent with cardiac systolic dysfunction [41].
However, this strategy fails to detect early subtle alterations in LV systolic function, as LVEF is dependent on hemodynamic conditions. Tissue velocity imaging (TVI) and strain imaging are novel, sensitive, non-invasive echocardiographic techniques that allow the early detection of LV systolic dysfunction, before a decrease in conventional LVEF [42]. Tissue velocity imaging has been validated in murine models of chemotherapy-induced cardiac dysfunction [43] and more recently, evaluated in the clinical setting of trastuzumab-induced cardiac dysfunction [13]. Neilan et al. demonstrated that a decrease in LVEF, detected by conventional echocardiographic measures (2D LVEF), was observed on day 5 in mice treated with chemotherapy, whereas DTI indices, such as S wave maximal velocity, dropped significantly earlier, on day 2. These findings were corroborated by Jassal et al. [44] in a study conducted in 2009, in which DTI parameters decreased within 24 hours in mice treated with doxorubicin plus trastuzumab, while a decreased LVEF was observed only on day 4.
In the echocardiographic assessment of both left and right ventricular inotropy, tissue Doppler measures, such as S or E wave maximal velocities, can be used to quantify regional systolic and diastolic function with greater accuracy than conventional measures such as LVEF or RV FAC [45]. However, we should acknowledge that systolic velocities, being Doppler derived measures, are angle- dependent. Thus, a good alignment of the Doppler interrogation line with the region of interest is mandatory in order to obtain the correct peak velocities. Nonetheless, peak systolic tissue Doppler velocities can be affected by the overall motion of the heart and the afterload pressures, particularly in the RV [46]. Several investigators have demonstrated an early reduction in lateral E velocity of the mitral annulus in patients receiving anthracyclines [47] but not trastuzumab, which remained reduced during the treatment and for several years thereafter. In a study by Negishi et al. [48] a 10% reduction in E velocity was observed in patients who developed CTRCD. Nevertheless, the reduction was neither statistically significant nor predictive of subsequent reduction in LVEF. In a recent study concerning the RV dysfunction, Keramida et al. [49] showed a global and uniform effect of trastuzumab on myocardial function, affecting both the left and right ventricles. The RV dysfunction was demonstrated by a decrease in both TAPSE and S wave, criteria of an abnormal longitudinal function. Both techniques (M-mode echography and Doppler tissue imaging) are angle-dependent, but also load-dependent.
Among the measures of myocardial function, echocardiography-measured peak systolic global longitudinal strain (GLS) is the most extensively studied marker and it provides an easy, inexpensive, and quantitative assessment of the global long-axis systolic function [50]. As a general idea, strain is defined as a percentage change in myocardial deformation and strain rate as the speed of deformation of myocardial segments. Neither the strain nor the strain rate derived parameters represent pure myocardial contractility, and none of them is load-independent [51,52]. Several studies have linked threshold changes in GLS or an absolute GLS value during cancer treatment with the subsequent development of CTRCD [11]. However, the greatest limitation is that these studies differ in the GLS cut-off values, depending on the vendors used and the populations studied. The cardio- oncology expert consensus document of the American Society of Echocardiography (ASE) and the European Association for Cardiovascular Imaging (EACVI) recommends the routine use of GLS in monitoring patients during cancer therapy whenever possible. In patients with available baseline strain measurements, a relative percentage reduction in GLS of less than 8% is not meaningful, whereas a change greater than 15% is likely to indicate subclinical LV dysfunction (2,52). GLS measurements based on 3 apical views is the preferred technique for the detection of cardiotoxicity, according to Thavendiranathan et al., even though single view LS is less time consuming [53].
Longitudinal strain seems to be affected when cardiotoxicity occurs. Bergamini et al. demonstrated that 2D GLS was the only parameter significantly modified across the studies after 12 months, while, overall, 2D GCS was not significantly reduced. Concerning the 2D GRS, the analysis demonstrated a significant reduction of this parameter, but not all the involved studies showed a coherent result after the same follow-up period [54].
Given the established prognostic significance of the RV dysfunction [55] for the outcome of the patients with heart failure, the finding of concurrent RV impairment at the time of diagnosis of LV cardiotoxicity makes RV GLS a useful measurement during trastuzumab therapy. In the recent study performed by Keramida et al., in patients treated with trastuzumab, the RV FWLS (free wall longitudinal strain) changes were modest. On the other hand, the insignificant change of RV FWLS raises the possibility that the detected impairment of RV GLS is attributed mainly to the intraventricular septum that both ventricles share. Also, the optimal cut-off value of the relative percent change in the RV GLS to predict cardiotoxicity was ≥14.8%, almost identical to the established LV GLS cut-off. We should also point out that both RV GLS and RV FWLS are size-independent estimates of contraction, as opposed to peak S velocity and TAPSE. But all the parameters proposed to detect early changes in RV systolic function are load-dependent; therefore, the presence of pulmonary hypertension may affect them [56].
Data concerning bevacizumab-related cardiotoxicity are scarce in the literature, but the same echocardiographic markers as for trastuzumab are proposed in the follow-up of patients, while the myocardial dysfunction induced seems reversible [57].
To conclude, the most sensitive marker of the early myocardial dysfunction in patients during and after the chemotherapy treatment with either trastuzumab or bevacizumab is global longitudinal strain GLS, having an important prognostic value in the development of end- stage HF [58].
Conclusions
To conclude, the most sensitive marker of the early myocardial dysfunction in patients during and after the chemotherapy treatment with either trastuzumab or bevacizumab is global longitudinal strain GLS, having an important prognostic value in the development of end- stage HF [58].
Conflicts of Interest disclosure
There are no known conflicts of interest in the publication of this article. The manuscript was read and approved by all authors.
Compliance with ethical standards
Any aspect of the work covered in this manuscript has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
Abbreviations
References
- Yeh, E.T.; Bickford, C.L. Cardiovascular complications of cancer therapy: Incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009, 53, 2231–2247. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef] [PubMed]
- Henri, C.; Heinonen, T.; Tardif, J.C. The Role of Biomarkers in Decreasing Risk of Cardiac Toxicity after Cancer Therapy. Biomark Cancer. 2016, 8 (Suppl. 2), 39–45. [Google Scholar] [CrossRef] [PubMed]
- Simões, R.; Silva, L.M.; Cruz, A.L.V.M.; Fraga, V.G.; de Paula Sabino, A.; Gomes, K.B. Troponin as a cardiotoxicity marker in breast cancer patients receiving anthracycline-based chemotherapy: A narrative review. Biomed Pharmacother. 2018, 107, 989–996. [Google Scholar] [CrossRef]
- Witteles, R.M. Biomarkers as Predictors of Cardiac Toxicity From Targeted Cancer Therapies. J Card Fail. 2016, 22, 459–464. [Google Scholar] [CrossRef]
- Henriksen, P.A. Anthracycline cardiotoxicity: An update on mechanisms, monitoring and prevention. Heart. 2018, 104, 971–977. [Google Scholar] [CrossRef]
- Ponde, N.; Bradbury, I.; Lambertini, M.; Ewer, M.; Campbell, C.; Ameels, H.; Zardavas, D.; Di Cosimo, S.; Baselga, J.; Huober, J.; et al. Cardiac biomarkers for early detection and prediction of trastuzumab and/or lapatinib-induced cardiotoxicity in patients with HER2-positive early-stage breast cancer: A NeoALTTO sub-study (BIG 1-06). Breast Cancer Res Treat. 2018, 168, 631–638. [Google Scholar] [CrossRef]
- Zardavas, D.; Suter, T.M.; Van Veldhuisen, D.J.; Steinseifer, J.; Noe, J.; Lauer, S.; Al-Sakaff, N.; Piccart-Gebhart, M.J.; de Azambuja, E. Role of Troponins I and T and N- Terminal Prohormone of Brain Natriuretic Peptide in Monitoring Cardiac Safety of Patients With Early- Stage Human Epidermal Growth Factor Receptor 2- Positive Breast Cancer Receiving Trastuzumab: A Herceptin Adjuvant Study Cardiac Marker Substudy. J Clin Oncol. 2017, 35, 878–884. [Google Scholar] [CrossRef]
- Goel, S.; Simes, R.J.; Beith, J.M. Exploratory analysis of cardiac biomarkers in women with normal cardiac function receiving trastuzumab for breast cancer. Asia Pac J Clin Oncol. 2011, 7, 276–280. [Google Scholar] [CrossRef]
- Sawaya, H.; Sebag, I.A.; Plana, J.C.; Januzzi, J.L.; Ky, B.; Cohen, V.; Gosavi, S.; Carver, J.R.; Wiegers, S.E.; Martin, R.P.; et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol. 2011, 107, 1375–1380. [Google Scholar] [CrossRef]
- Sawaya, H.; Sebag, I.A.; Plana, J.C.; Januzzi, J.L.; Ky, B.; Tan, T.C.; Cohen, V.; Banchs, J.; Carver, J.R.; Wiegers, S.E.; et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012, 5, 596–603. [Google Scholar] [CrossRef]
- Ky, B.; Putt, M.; Sawaya, H.; French, B.; Januzzi, J.L., Jr.; Sebag, I.A.; Plana, J.C.; Cohen, V.; Banchs, J.; Carver, J.R.; et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014, 63, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Fallah-Rad, N.; Walker, J.R.; Wassef, A.; Lytwyn, M.; Bohonis, S.; Fang, T.; Tian, G.; Kirkpatrick, I.D.; Singal, P.K.; Krahn, M.; et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol. 2011, 57, 2263–2270. [Google Scholar] [CrossRef] [PubMed]
- Putt, M.; Hahn, V.S.; Januzzi, J.L.; Sawaya, H.; Sebag, I.A.; Plana, J.C.; Picard, M.H.; Carver, J.R.; Halpern, E.F.; Kuter, I.; et al. Longitudinal Changes in Multiple Biomarkers Are Associated with Cardiotoxicity in Breast Cancer Patients Treated with Doxorubicin, Taxanes, and Trastuzumab. Clin Chem. 2015, 61, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Kitayama, H.; Kondo, T.; Sugiyama, J.; Kurimoto, K.; Nishino, Y.; Kawada, M.; Hirayama, M.; Tsuji, Y. High-sensitive troponin T assay can predict anthracycline- and trastuzumab-induced cardiotoxicity in breast cancer patients. Breast Cancer 2017, 24, 774–782. [Google Scholar] [CrossRef]
- Katsurada, K.; Ichida, M.; Sakuragi, M.; Takehara, M.; Hozumi, Y.; Kario, K. High-sensitivity troponin T as a marker to predict cardiotoxicity in breast cancer patients with adjuvant trastuzumab therapy. Springerplus 2014, 3, 620. [Google Scholar] [CrossRef]
- Mokuyasu, S.; Suzuki, Y.; Kawahara, E.; Seto, T.; Tokuda, Y. High-sensitivity cardiac troponin I detection for 2 types of drug-induced cardiotoxicity in patients with breast cancer. Breast Cancer 2015, 22, 563–569. [Google Scholar] [CrossRef][Green Version]
- Onitilo, A.A.; Engel, J.M.; Stankowski, R.V.; Liang, H.; Berg, R.L.; Doi, S.A. High-sensitivity C-reactive protein (hs- CRP) as a biomarker for trastuzumab-induced cardiotoxicity in HER2-positive early-stage breast cancer: A pilot study. Breast Cancer Res Treat. 2012, 134, 198–291. [Google Scholar] [CrossRef]
- Morris, P.G.; Chen, C.; Steingart, R.; Fleisher, M.; Lin, N.; Moy, B.; Come, S.; Sugarman, S.; Abbruzzi, A.; Lehman, R.; et al. Troponin I and C-reactive protein are commonly detected in patients with breast cancer treated with dose-dense chemotherapy incorporating trastuzumab and lapatinib. Clin Cancer Res. 2011, 17, 3490–3499. [Google Scholar] [CrossRef]
- de Vries Schultink, A.H.M.; Boekhout, A.H.; Gietema, J.A.; Burylo, A.M.; Dorlo, T.P.C.; van Hasselt, J.G.C.; Schellens, J.H.M.; Huitema, A.D.R. Pharmacodynamic modeling of cardiac biomarkers in breast cancer patients treated with anthracycline and trastuzumab regimens. J Pharmacokinet Pharmacodyn. 2018, 45, 431–442. [Google Scholar] [CrossRef]
- Cardinale, D.; Colombo, A.; Torrisi, R.; Sandri, M.T.; Civelli, M.; Salvatici, M.; Lamantia, G.; Colombo, N.; Cortinovis, S.; Dessanai, M.A.; et al. Trastuzumab-induced cardiotoxicity: Clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010, 28, 3910–3916. [Google Scholar] [CrossRef] [PubMed]
- Dhir, V.; Yan, A.T.; Nisenbaum, R.; Sloninko, J.; Connelly, K.A.; Barfett, J.; Haq, R.; Kirpalani, A.; Chan, K.K.W.; Petrella, T.M.; et al. Assessment of left ventricular function by CMR versus MUGA scans in breast cancer patients receiving trastuzumab: A prospective observational study. Int J Cardiovasc Imaging. 2019, 35, 2085–2093. [Google Scholar] [CrossRef] [PubMed]
- Ewer, M.S.; Vooletich, M.T.; Durand, J.B.; Woods, M.L.; Davis, J.R.; Valero, V.; Lenihan, D.J. Reversibility of trastuzumab-related cardiotoxicity: New insights based on clinical course and response to medical treatment. J Clin Oncol. 2005, 23, 7820–7826. [Google Scholar] [CrossRef] [PubMed]
- Apple, F.S.; Sandoval, Y.; Jaffe, A.S.; Ordonez-Llanos, J.; IFCC Task Force on Clinical Applications of Cardiac Bio-Markers. Cardiac Troponin Assays: Guide to Understanding Analytical Characteristics and Their Impact on Clinical Care. Clin Chem. 2017, 63, 73–81. [Google Scholar] [CrossRef]
- Baba, M.; Yoshida, K.; Ieda, M. Clinical Applications of Natriuretic Peptides in Heart Failure and Atrial Fibrillation. Int J Mol Sci. 2019, 20, 2824. [Google Scholar] [CrossRef]
- Januzzi, J.L.; van Kimmenade, R.; Lainchbury, J.; Bayes-Genis, A.; Ordonez-Llanos, J.; Santalo-Bel, M.; Pinto, Y.M.; Richards, M. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: An international pooled analysis of 1256 patients: The International Collaborative of NT-proBNP Study. Eur Heart J. 2006, 27, 330–337. [Google Scholar] [CrossRef]
- Shah, K.S.; Yang, E.H.; Maisel, A.S.; Fonarow, G.C. The Role of Biomarkers in Detection of Cardio-toxicity. Curr Oncol Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Stevens, P.L.; Lenihan, D.J. Cardiotoxicity due to Chemotherapy: The Role of Biomarkers. Curr Cardiol Rep. 2015, 17, 603. [Google Scholar] [CrossRef]
- Advani, P.; Hoyne, J.; Moreno-Aspita, A.; Dubin, M.; Brock, S.; Harlow, C.; Chumsri, S.; Suter, T.; Blackshear, J.L. High- Sensitivity Troponin T and NT-proBNP Kinetics in Breast Cancer Chemotherapy. Chemotherapy. 2017, 62, 334–338. [Google Scholar] [CrossRef]
- Yu, A.F.; Manrique, C.; Pun, S.; Liu, J.E.; Mara, E.; Fleisher, M.; Patil, S.; Jones, L.W.; Steingart, R.M.; Hudis, C.A.; et al. Cardiac Safety of Paclitaxel Plus Trastuzumab and Pertuzumab in Patients With HER2-Positive Metastatic Breast Cancer. Oncologist 2016, 21, 418–424. [Google Scholar] [CrossRef]
- El-Sherbeny, W.S.; Sabry, N.M.; Sharbay, R.M. Prediction of trastuzumab-induced cardiotoxicity in breast cancer patients receiving anthracycline-based chemotherapy. J Echocardiogr. 2019, 17, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Şendur, M.A.; Aksoy, S.; Yorgun, H.; Ozdemir, N.; Yilmaz, F.M.; Yazıcı, O.; Zungun, C.; Aytemir, K.; Zengin, N.; Altundag, K. Comparison of the long term cardiac effects associated with 9 and 52 weeks of trastuzumab in HER2-positive early breast cancer. Curr Med Res Opin. 2015, 31, 547–556. [Google Scholar] [CrossRef]
- Matos, E.; Jug, B.; Blagus, R.; Zakotnik, B. A Prospective Cohort Study on Cardiotoxicity of Adjuvant Trastuzumab Therapy in Breast Cancer Patients. Arq Bras Cardiol. 2016, 107, 40–47. [Google Scholar] [CrossRef]
- Grover, S.; Leong, D.P.; Chakrabarty, A.; Joerg, L.; Kotasek, D.; Cheong, K.; Joshi, R.; Joseph, M.X.; DePasquale, C.; Koczwara, B.; et al. Left and right ventricular effects of anthracycline and trastuzumab chemotherapy: A prospective study using novel cardiac imaging and biochemical markers. Int J Cardiol. 2013, 168, 5465–5467. [Google Scholar] [CrossRef]
- Oladiran, O.; Nazir, S. Bevacizumab: A Rare Cause of Nonischemic Cardiomyopathy. Case Rep Cardiol. 2018, 2018, 1361326. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (Review). Int J Mol Med. 2018, 41, 599–614. [Google Scholar] [CrossRef]
- De Iuliis, F.; Salerno, G.; Taglieri, L.; Lanza, R.; Cardelli, P.; Scarpa, S. Circulating neuregulin-1 and galectin-3 can be prognostic markers in breast cancer. Int J Biol Markers. 2017, 32, e333–e336. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef]
- Armenian, S.H.; Armstrong, G.T.; Aune, G.; Chow, E.J.; Ehrhardt, M.J.; Ky, B.; Moslehi, J.; Mulrooney, D.A.; Nathan, P.C.; Ryan, T.D.; et al. Cardiovascular Disease in Survivors of Childhood Cancer: Insights Into Epidemiology, Pathophysiology, and Prevention. J Clin Oncol. 2018, 36, 2135–2144. [Google Scholar] [CrossRef]
- Seidman, A.; Hudis, C.; Pierri, M.K.; Shak, S.; Paton, V.; Ashby, M.; Murphy, M.; Stewart, S.J.; Keefe, D. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002, 20, 1215–1221. [Google Scholar] [CrossRef]
- Hare, J.L.; Brown, J.K.; Leano, R.; Jenkins, C.; Woodward, N.; Marwick, T.H. Use of myocardial deformation imaging to detect preclinical myocardial dysfunction before conventional measures in patients undergoing breast cancer treatment with trastuzumab. Am Heart J. 2009, 158, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014, 27, 911–939. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.M.; Sanderson, J.E.; Marwick, T.H.; Oh, J.K. Tissue Doppler imaging a new prognosticator for cardiovascular diseases. J Am Coll Cardiol. 2007, 49, 1903–1914. [Google Scholar] [CrossRef]
- Neilan, T.G.; Jassal, D.S.; Perez-Sanz, T.M.; Raher, M.J.; Pradhan, A.D.; Buys, E.S.; Ichinose, F.; Bayne, D.B.; Halpern, E.F.; Weyman, A.E.; et al. Tissue Doppler imaging predicts left ventricular dysfunction and mortality in a murine model of cardiac injury. Eur Heart J. 2006, 27, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
- Jassal, D.S.; Han, S.Y.; Hans, C.; Sharma, A.; Fang, T.; Ahmadie, R.; Lytwyn, M.; Walker, J.R.; Bhalla, R.S.; Czarnecki, A.; et al. Utility of tissue Doppler and strain rate imaging in the early detection of trastuzumab and anthracycline mediated cardiomyopathy. J Am Soc Echocardiogr. 2009, 22, 418–424. [Google Scholar] [CrossRef]
- Kukulski, T.; Hübbert, L.; Arnold, M.; Wranne, B.; Hatle, L.; Sutherland, G.R. Normal regional right ventricular function and its change with age: A Doppler myocardial imaging study. J Am Soc Echocardiogr. 2000, 13, 194–204. [Google Scholar] [CrossRef]
- Ho, E.; Brown, A.; Barrett, P.; Morgan, R.B.; King, G.; Kennedy, M.J.; Murphy, R.T. Subclinical anthracycline- and trastuzumab-induced cardiotoxicity in the long- term follow-up of asymptomatic breast cancer survivors: A speckle tracking echocardiographic study. Heart 2010, 96, 701–707. [Google Scholar] [CrossRef]
- Negishi, K.; Negishi, T.; Hare, J.L.; Haluska, B.A.; Plana, J.C.; Marwick, T.H. Independent and incremental value of deformation indices for prediction of trastuzumab- induced cardiotoxicity. J Am Soc Echocardiogr. 2013, 26, 493–498. [Google Scholar] [CrossRef]
- Keramida, K.; Farmakis, D.; Bingcang, J.; Sulemane, S.; Sutherland, S.; Bingcang, R.A.; Ramachandran, K.; Tzavara, C.; Charalampopoulos, G.; Filippiadis, D. Longitudinal changes of right ventricular deformation mechanics during trastuzumab therapy in breast cancer patients. Eur J Heart Fail. 2019, 21, 529–535. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Poulin, F.; Lim, K.D.; Plana, J.C.; Woo, A.; Marwick, T.H. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: A systematic review. J Am Coll Cardiol. 2014, 63, 2751–2768. [Google Scholar] [CrossRef]
- Sinan, G.; Mehmet, K.; Süleyman, Y.; Cemalettin, A. Use of native Y-saphenous vein graft in multi-vessel coronary bypass surgery. J Clin Invest Surg. 2020, 5, 96–99. [Google Scholar] [CrossRef]
- Oreto, L.; Todaro, M.C.; Umland, M.M.; Kramer, C.; Qamar, R.; Carerj, S.; Khandheria, B.K.; Paterick, T.E. Use of echocardiography to evaluate the cardiac effects of therapies used in cancer treatment: What do we know? J Am Soc Echocardiogr. 2012, 25, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Thavendiranathan, P.; Negishi, T.; Coté, M.A.; Penicka, M.; Massey, R.; Cho, G.Y.; Hristova, K.; Vinereanu, D.; Popescu, B.A.; Izumo, M.; et al. Single Versus Standard Multiview Assessment of Global Longitudinal Strain for the Diagnosis of Cardiotoxicity During Cancer Therapy. JACC Cardiovasc Imaging 2018, 11, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Bergamini, C.; Dolci, G.; Truong, S.; Zanolla, L.; Benfari, G.; Fiorio, E.; Rossi, A.; Ribichini, F.L. Role of Speckle Tracking Echocardiography in the Evaluation of Breast Cancer Patients Undergoing Chemotherapy: Review and Meta-analysis of the Literature. Cardiovasc Toxicol. 2019, 19, 485–492. [Google Scholar] [CrossRef]
- Charbonnel, C.; Convers-Domart, R.; Rigaudeau, S.; Taksin, A.L.; Baron, N.; Lambert, J.; Ghez, S.; Georges, J.L.; Farhat, H.; Lambert, J.; et al. Assessment of global longitudinal strain at low-dose anthracycline-based chemotherapy, for the prediction of subsequent cardiotoxicity. Eur Heart J Cardiovasc Imaging. 2017, 18, 392–401. [Google Scholar] [CrossRef]
- Puwanant, S.; Park, M.; Popović, Z.B.; Tang, W.H.; Farha, S.; George, D.; Sharp, J.; Puntawangkoon, J.; Loyd, J.E.; Erzurum, S.C.; et al. Ventricular geometry, strain, and rotational mechanics in pulmonary hypertension. Circulation. 2010, 121, 259–266. [Google Scholar] [CrossRef]
- Hawkes, E.A.; Okines, A.F.; Plummer, C.; Cunningham, D. Cardiotoxicity in patients treated with bevacizumab is potentially reversible. J Clin Oncol. 2011, 29, e560–e562. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Kokkinidis, D.G.; Kampaktsis, P.N.; Amir, E.A.; Marwick, T.H.; Gupta, D.; Thavendiranathan, P. Assessment of Prognostic Value of Left Ventricular Global Longitudinal Strain for Early Prediction of Chemotherapy-Induced Cardiotoxicity: A Systematic Review and Meta-analysis. JAMA Cardiol. 2019, 4, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
© 2021 by the author. 2021 Ionela-Emilia Popovici, Angela Cozma, Olga Hilda Orasan, George Ciulei, Ana Maria Poenar, Cristina Buchman, Simina Tarmure, Sorina Secara, Adela Sitar-Taut, Teodora Alexescu, Andrada Lazar, Vasile Negrean, Lucia Maria Procopciuc