Periodontitis as a Potential Risk Factor for Premature Delivery
Abstract
:Introduction
Discussions
The Conjecture of Pregnancy and the Periodontal Disease
Oral Microorganisms and Their Feasible Extra-Oral Effect
The Vaginal Microbiota, Periodontal Disease and Preterm Labor
Periodontal Treatment and the Result on Preterm Birth Incidence
Conclusions
Conflicts of Interest disclosure
Compliance with ethical standards
References
- WHO: Preterm Birth Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/preterm-birth (accessed on 23 January 2021).
- Liu, L.; Oza, S.; Hogan, D.; Chu, Y.; Perin, J.; Zhu, J.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of under-5 mortality in 2000-15: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016, 388, 3027–3035. [Google Scholar] [CrossRef] [PubMed]
- Black, R.E.; Cousens, S.; Johnson, H.L.; Lawn, J.E.; Rudan, I.; Bassani, D.G.; Jha, P.; Campbell, H.; Walker, C.F.; Cibulskis, R.; et al. Global, regional, and national causes of child mortality in 2008: A systematic analysis. Lancet 2010, 375, 1969–1987. [Google Scholar] [CrossRef] [PubMed]
- Unicef. Available online: https://data.unicef.org/country/rou/ (accessed on 7 January 2021).
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.; Wojdyla, D.; Say, L.; Betran, A.P.; Merialdi, M.; Requejo, J.H.; Rubens, C.; Menon, R.; Van Look, P.F. The worldwide incidence of preterm birth: A systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010, 88, 31–38. [Google Scholar] [CrossRef]
- Rogers, E.E.; Hintz, S.R. Early neurodevelopmental outcomes of extremely preterm infants. Semin Perinatol. 2016, 40, 497–509. [Google Scholar] [CrossRef]
- Barfield, W.D. Public Health Implications of Very Preterm Birth. Clin Perinatol. 2018, 45, 565–577. [Google Scholar] [CrossRef]
- Hodnett, E.D.; Fredericks, S.; Weston, J. Support during pregnancy for women at increased risk of low birthweight babies. Cochrane Database Syst Rev. 2010, CD000198. [Google Scholar] [CrossRef]
- Iams, J.D.; Romero, R.; Culhane, J.F.; Goldenberg, R.L. Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet 2008, 371, 164–175. [Google Scholar] [CrossRef]
- Cobb, C.M.; Kelly, P.J.; Williams, K.B.; Babbar, S.; Angolkar, M.; Derman, R.J. The oral microbiome and adverse pregnancy outcomes. Int J Womens Health 2017, 9, 551–559. [Google Scholar] [CrossRef]
- Katz, J.; Lee, A.C.; Kozuki, N.; Lawn, J.E.; Cousens, S.; Blencowe, H.; Ezzati, M.; Bhutta, Z.A.; Marchant, T.; Willey, B.A.; et al. Mortality risk in preterm and small- for-gestational-age infants in low-income and middle- income countries: A pooled country analysis. Lancet 2013, 382, 417–425. [Google Scholar] [CrossRef]
- Raghupathy, R. Cytokines as key players in the pathophysiology of preeclampsia. Med Princ Pract. 2013, 22 (Suppl. 1), 8–19. [Google Scholar] [CrossRef] [PubMed]
- Zenclussen, A.C. Adaptive immune responses during pregnancy. Am J Reprod Immunol. 2013, 69, 291–303. [Google Scholar] [CrossRef]
- Du, M.; Piao, H.; Li, D. The 3rd international conference on reproductive immunology in Shanghai: September 27-29, 2013. Shanghai, China. Am J Reprod Immunol. 2014, 71, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Laird, S.M.; Tuckerman, E.M.; Cork, B.A.; Linjawi, S.; Blakemore, A.I.; Li, T.C. A review of immune cells and molecules in women with recurrent miscarriage. Hum Reprod Update 2003, 9, 163–174. [Google Scholar] [CrossRef]
- Adams Waldorf, K.M.; McAdams, R.M. Influence of infection during pregnancy on fetal development. Reproduction 2013, 146, R151–R162. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci Transl Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Buekens, P.; Fraser, W.D.; Beck, J.; Offenbacher, S. Periodontal disease and adverse pregnancy outcomes: A systematic review. BJOG 2006, 113, 135–143. [Google Scholar] [CrossRef]
- Fischer, L.A.; Demerath, E.; Bittner-Eddy, P.; Costalonga, M. Placental colonization with periodontal pathogens: The potential missing link. Am J Obstet Gynecol. 2019, 221, 383–392.e3. [Google Scholar] [CrossRef]
- Armitage, G.C. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999, 4, 1–6. [Google Scholar] [CrossRef]
- Darveau, R.P. Periodontitis: A polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010, 8, 481–490. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Palmer, R.J., Jr.; Periasamy, S.; Jakubovics, N.S. Oral multispecies biofilm development and the key role of cell-cell distance. Nat Rev Microbiol. 2010, 8, 471–480. [Google Scholar] [CrossRef]
- Nibali, L.; Farias, B.C.; Vajgel, A.; Tu, Y.K.; Donos, N. Tooth loss in aggressive periodontitis: A systematic review. J Dent Res. 2013, 92, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Mombelli, A. Early-onset periodontitis. Ann Periodontol. 1999, 4, 39–53. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Faveri, M.; Figueiredo, L.C.; Duarte, P.M.; Mestnik, M.J.; Mayer, M.P.; Feres, M. Microbiological profile of untreated subjects with localized aggressive periodontitis. J Clin Periodontol. 2009, 36, 739–749. [Google Scholar] [CrossRef]
- Cairo, F.; Nieri, M.; Gori, A.M.; Tonelli, P.; Branchi, R.; Castellani, S.; Abbate, R.; Pini-Prato, G.P. Markers of systemic inflammation in periodontal patients: Chronic versus aggressive periodontitis. An explorative cross- sectional study. Eur J Oral Implantol. 2010, 3, 147–153. [Google Scholar] [PubMed]
- Garlet, G.P.; Martins, W., Jr.; Ferreira, B.R.; Milanezi, C.M.; Silva, J.S. Patterns of chemokines and chemokine receptors expression in different forms of human periodontal disease. J Periodontal Res. 2003, 38, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Bohiltea, R.; Turcan, N.; Cavinder, C.M.; Ducu, I.; Paunica, I.; Andronache, L.F.; Cirstoiu, M.M. Risk factors, predictive markers and prevention strategies for intrauterine fetal death. An integrative review. J Mind Med Sci. 2020, 7, 52–60. [Google Scholar] [CrossRef]
- Lubberts, E.; van den Bersselaar, L.; Oppers-Walgreen, B.; Schwarzenberger, P.; Coenen-de Roo, C.J.; Kolls, J.K.; Joosten, L.A.; van den Berg, W.B. IL-17 promotes bone erosion in murine collagen-induced arthritis through loss of the receptor activator of NF-kappa B ligand/osteoprotegerin balance. J Immunol. 2003, 170, 2655–2662. [Google Scholar] [CrossRef]
- Suga, K.; Saitoh, M.; Kokubo, S.; Nozaki, K.; Fukushima, S.; Yasuda, S.; Sasamata, M.; Miyata, K. Synergism between interleukin-11 and bone morphogenetic protein-2 in the healing of segmental bone defects in a rabbit model. J Interferon Cytokine Res. 2004, 24, 343–349. [Google Scholar] [CrossRef]
- Figuero, E.; Carrillo-de-Albornoz, A.; Herrera, D.; Bascones-Martínez, A. Gingival changes during pregnancy: I. Influence of hormonal variations on clinical and immunological parameters. J Clin Periodontol. 2010, 37, 220–229. [Google Scholar] [CrossRef]
- Lin, D.; Moss, K.; Beck, J.D.; Hefti, A.; Offenbacher, S. Persistently high levels of periodontal pathogens associated with preterm pregnancy outcome. J Periodontol. 2007, 78, 833–841. [Google Scholar] [CrossRef]
- Oana-Denisa, B.; Octavian-Gabriel, O.; Adrian VDumitru Ioana, P.; Anca Daniela, S. Maternal infections with an increased risk of transmission to the foetus; a literature review. J Clin Invest Surg. 2020, 5, 66–72. [Google Scholar] [CrossRef]
- Massaro, C.R.; Buratti, M.; de Paula, T.N.P.; Piana, E.A.; Wachter, F.; Hoshi, A.T.; Nassar, C.A.; Nassar, P.O. Maternal periodontal disease as a risk factor for preterm birth and low-birth-weight babies: A case-control study. Gen Dent. 2020, 68, 44–49. [Google Scholar] [PubMed]
- Blanc, V.; O’Valle, F.; Pozo, E.; Puertas, A.; León, R.; Mesa, F. Oral bacteria in placental tissues: Increased molecular detection in pregnant periodontitis patients. Oral Dis. 2015, 21, 905–912. [Google Scholar] [CrossRef]
- Bobetsis, Y.A.; Graziani, F.; Gürsoy, M.; Madianos, P.N. Periodontal disease and adverse pregnancy outcomes. Periodontol 2000. 2020, 83, 154–174. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Marin, C.; Spratt, D.A.; Millar, M.R.; Simmonds, M.; Kempley, S.T.; Allaker, R.P. Identification of bacteria and potential sources in neonates at risk of infection delivered by Caesarean and vaginal birth. J Med Microbiol. 2012, 61 Pt 1, 31–41. [Google Scholar] [CrossRef]
- Katz, J.; Chegini, N.; Shiverick, K.T.; Lamont, R.J. Localization of P. gingivalis in preterm delivery placenta. J Dent Res. 2009, 88, 575–578. [Google Scholar] [CrossRef]
- Vander Haar, E.L.; So, J.; Gyamfi-Bannerman, C.; Han, Y.W. Fusobacterium nucleatum and adverse pregnancy outcomes: Epidemiological and mechanistic evidence. Anaerobe 2018, 50, 55–59. [Google Scholar] [CrossRef]
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Prince, A.L.; Chu, D.M.; Seferovic, M.D.; Antony, K.M.; Ma, J.; Aagaard, K.M. The perinatal microbiome and pregnancy: Moving beyond the vaginal microbiome. Cold Spring Harb Perspect Med. 2015, 5, a023051. [Google Scholar] [CrossRef]
- Pretorius, C.; Jagatt, A.; Lamont, R.F. The relationship between periodontal disease, bacterial vaginosis, and preterm birth. J Perinat Med. 2007, 35, 93–99. [Google Scholar] [CrossRef]
- Seymour, G.J.; Ford, P.J.; Cullinan, M.P.; Leishman, S.; Yamazaki, K. Relationship between periodontal infections and systemic disease. Clin Microbiol Infect. 2007, 13 (Suppl. 4), 3–10. [Google Scholar] [CrossRef] [PubMed]
- Fardini, Y.; Chung, P.; Dumm, R.; Joshi, N.; Han, Y.W. Transmission of diverse oral bacteria to murine placenta: Evidence for the oral microbiome as a potential source of intrauterine infection. Infect Immun. 2010, 78, 1789–1796. [Google Scholar] [CrossRef]
- Dörtbudak, O.; Eberhardt, R.; Ulm, M.; Persson, G.R. Periodontitis, a marker of risk in pregnancy for preterm birth. J Clin Periodontol. 2005, 32, 45–52. [Google Scholar] [CrossRef]
- Boggess, K.A.; Moss, K.; Madianos, P.; Murtha, A.P.; Beck, J.; Offenbacher, S. Fetal immune response to oral pathogens and risk of preterm birth. Am J Obstet Gynecol. 2005, 193(Pt2), 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Bansal, J.; Bansal, A.; Kukreja, N.; Kukreja, U. Periodontal diseases as an emerging potential risk factor for adverse pregnancy outcomes: A review of concepts. J Turk Ger Gynecol Assoc. 2011, 12, 176–180. [Google Scholar] [CrossRef]
- Huck, O.; Tenenbaum, H.; Davideau, J.L. Relationship between periodontal diseases and preterm birth: Recent epidemiological and biological data. J Pregnancy 2011, 2011, 164654. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Geisinger, M.L.; Geurs, N.C.; Griffin, R.; Vassilopoulos, P.J.; Vermeulen, L.; Haigh, S.; Reddy, M.S. Effect of intensive oral hygiene regimen during pregnancy on periodontal health, cytokine levels, and pregnancy outcomes: A pilot study. J Periodontol. 2014, 85, 1684–1692. [Google Scholar] [CrossRef]
- Kim, A.J.; Lo, A.J.; Pullin, D.A.; Thornton-Johnson, D.S.; Karimbux, N.Y. Scaling and root planing treatment for periodontitis to reduce preterm birth and low birth weight: A systematic review and meta-analysis of randomized controlled trials. J Periodontol. 2012, 83, 1508–1519. [Google Scholar] [CrossRef]
- López, N.J.; Smith, P.C.; Gutierrez, J. Periodontal therapy may reduce the risk of preterm low birth weight in women with periodontal disease: A randomized controlled trial. J Periodontol. 2002, 73, 911–924. [Google Scholar] [CrossRef] [PubMed]
- Offenbacher, S.; Lieff, S.; Boggess, K.A.; Murtha, A.P.; Madianos, P.N.; Champagne, C.M.; McKaig, R.G.; Jared, H.L.; Mauriello, S.M.; Auten, R.L., Jr.; et al. Maternal periodontitis and prematurity. Part I: Obstetric outcome of prematurity and growth restriction. Ann Periodontol. 2001, 6, 164–174. [Google Scholar] [CrossRef] [PubMed]
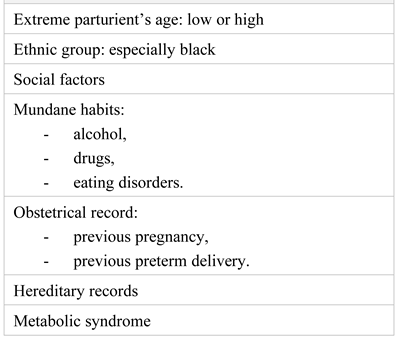 |
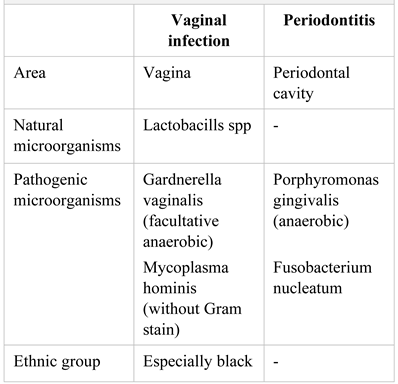 |
© 2021 by the author. 2021 Ana Turcu-Duminică, Anca Silvia Dumitriu, Stana Paunica, Corina Gică, Radu Botezatu, Nicolae Gică, Gheorghe Peltecu, Anca Maria Panaitescu
Share and Cite
Turcu-Duminică, A.; Dumitriu, A.S.; Paunica, S.; Gică, C.; Botezatu, R.; Gică, N.; Peltecu, G.; Panaitescu, A.M. Periodontitis as a Potential Risk Factor for Premature Delivery. J. Mind Med. Sci. 2021, 8, 27-33. https://doi.org/10.22543/7674.81.P2733
Turcu-Duminică A, Dumitriu AS, Paunica S, Gică C, Botezatu R, Gică N, Peltecu G, Panaitescu AM. Periodontitis as a Potential Risk Factor for Premature Delivery. Journal of Mind and Medical Sciences. 2021; 8(1):27-33. https://doi.org/10.22543/7674.81.P2733
Chicago/Turabian StyleTurcu-Duminică, Ana, Anca Silvia Dumitriu, Stana Paunica, Corina Gică, Radu Botezatu, Nicolae Gică, Gheorghe Peltecu, and Anca Maria Panaitescu. 2021. "Periodontitis as a Potential Risk Factor for Premature Delivery" Journal of Mind and Medical Sciences 8, no. 1: 27-33. https://doi.org/10.22543/7674.81.P2733
APA StyleTurcu-Duminică, A., Dumitriu, A. S., Paunica, S., Gică, C., Botezatu, R., Gică, N., Peltecu, G., & Panaitescu, A. M. (2021). Periodontitis as a Potential Risk Factor for Premature Delivery. Journal of Mind and Medical Sciences, 8(1), 27-33. https://doi.org/10.22543/7674.81.P2733



