Abstract
Objectives. Basal cell carcinomas (BCCs) are malignant tumors which rarely metastasize, are slow-growing, and extensively locally destructive. BCCs more than 5 cm in diameter are defined as giant. Most often they arise as a result of neglect, as the patient avoids, delays, or refuses to see a doctor. The large tumor diameter and consequently of the post-excisional defect make these lesions difficult to treat surgically with respect to selecting the surgical reconstruction technique. Method. We studied a group of 9 patients, aged 60 to 85 years, diagnosed with giant basal cell carcinomas (GBCCs) with periocular location in which surgery was indicated. Results. In all cases, complete excision with histologically clear margins was performed and for the coverage of the remaining defect various, complex, and sometimes two- stage reconstructive techniques were used. Conclusions. Giant cell carcinoma of the periocular region requires extensive and risky surgery, especially when performed on the elderly. Early referral to a doctor avoids all these risks, in all cases the pathological diagnosis was nodular BCCs. The aesthetic and functional outcomes were good to very good, and the patients reported being highly satisfied.
Introduction
Although it represents only a small percentage of the body surface area, approximately 5 to 10 percent of all skin cancers occur in the periocular region [1]. Basal cell carcinomas (BCCs) account for 90% of malignant eyelid tumors, being the most common type of skin cancer in Europe, Africa, and North America [2]. BCCs are most commonly located on areas with prolonged exposure to solar ultraviolet radiation, which is the main risk factor, and confirmation of BCCs diagnosis is a negative prognostic factor for the development of other associated skin lesions [3]. Usually, these malignant tumors are slow- growing, rarely metastasize, but have a significant local destructive potential [4]. However, BCCs may present a rare but aggressive biological variant, named giant basal cell carcinomas (GBCCs) defined as BCCs with a diameter of 5 cm or more characterized by deep tissue invasion, rapid growth and high risk of metastasis, increased rate of local complications, and poor prognosis [5,6]. GBCCs account for 0.4–1% of all BCCs [7]. The pathogenesis of GBCCs is sometimes linked to a spontaneous mutation in the human patch gene (PTCH), mapped to the q22.33 locus of chromosome 9; however, over 30% of GBCCs cases are due to the delay in seeking medical attention, most often associated to mental deficiency, low socioeconomic status, poor hygiene, advanced age, or the fact that BCCs are painless [8]. Although mortality is low, the morbidity caused by this tumor is significant due to its locally invasive and relapse potential [9,10]. The metastatic potential of BCCs is estimated to be less than 0.03%, while GBCCs have a higher rate of metastasis [11]. It is reported that systemic metastases predominantly occur in giant GBCCs exceeding 100 cm2 in surface area or 25 cm in diameter [12]. Surgical excision is highly effective, being the preferred treatment option over time. BCCs should be excised with 3–4 mm safety margins from the adjacent macroscopically healthy tissue to exclude microscopic invasion of neoplastic cells. Recurrences at the operative site, especially periorbital, perinasal, and periauricular, should be treated with great caution; thus, re-excision should be carried out early as it has been found that a high number of BCCs relapse to become much more aggressive [13]. The current study includes a group of 9 patients with nodular BCCsover 5 cm in diameter and different periocular locations. In all cases, excision with adequate margins was performed. Given the large size of the tumors as well as of the soft-tissue defects after tumor excision, different reconstruction techniques were used, most often of great complexity, in some cases even two-stage.
Materials and Methods
The current study includes a group of 9 patients, 8 men and one woman, aged 60 to 85 years, who presented to the Clinic of Plastic Surgery and Reconstructive Microsurgery, St. Spiridon Emergency Hospital Iasi, between January 2017 and July 2018. All patients presented on admission ulcerative lesions, bleeding in 3 cases in the periocular region. In 4 cases the tumor masses were located at the external angle of the right eye, in 2 cases on the lower eyelid, in 2 cases at the external angle of the left eye, and in one case in the glabellar region with extension to the inner angles of both eyes (Figure 1). These lesions ranged from 5 to 7.5 cm in diameter, which defines them as "giant", and were present for 5 to 12 years.
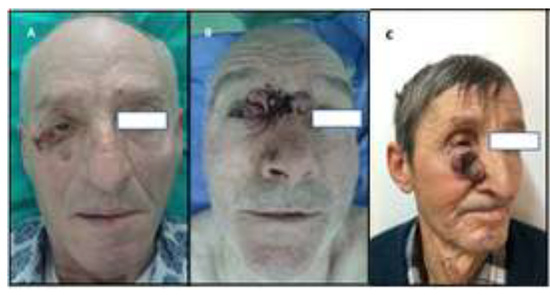
Figure 1.
(A) the external angle of the right eye. (B) the glabellar region with extension to the inner angles of both eyes. (C) the lower eyelid.
Physical examination on admission made a presumptive diagnosis of ulcerated GBCCs carcinoma based on the macroscopic appearance, location, and size of the lesions, highly suggestive for this type of neoplasm, as well as on case history that revealed slow growth over a long period of time. Patients were informed about the procedures to be undergone and informed consent was obtained from all study patients. Preoperative imaging explorations consisted of computer tomography (CT) examination to determine the degree of tumor invasion of the eyeball and its appendages. In all cases, tumor invasion of the eyeball was absent, the reason why the indication for exenteration was excluded with the agreement and in collaboration with the eye surgeon. In all cases, surgery was performed under general anesthesia with endotracheal intubation and consisted of the surgical removal of the masses with adequate surgical margins (Figure 2).
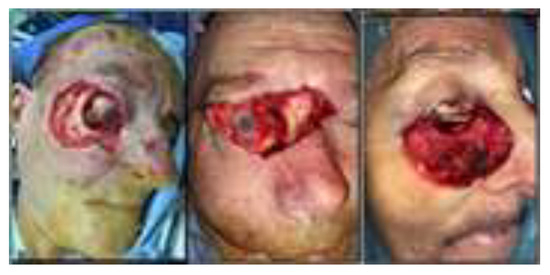
Figure 2.
(A) surgical removal of the tumoral masses with adequate surgical margins with bone extension, including periocular fat. (B) wide excision of the giant cell carcinoma of the glabellar region. (C) wide excision of the giant cell carcinoma of the lower eyelid.
As all patients were aged over 60, the preoperative preparation was done in collaboration with the cardiologist and anesthesiologist. Coverage of post-excisional soft- tissue defects required complex and varied reconstruction techniques. Three of the cases required two-stage reconstruction. The Mustard-type flap, the forehead and genian flap techniques (Fricke flap in one case), and advancement genian flap, of which one anchored to the zygomatic bone, were used in 3 cases each. In three cases, eyeball protection required its coverage with a conjunctival flap that was sectioned three months postoperatively, and the eyes reopened (Figure 3).
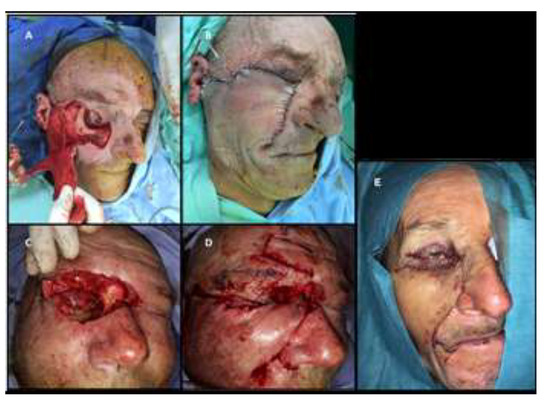
Figure 3.
(A) Mustarde- flap. (B) the coverage of the tissue defect with Mustarde flap. (C) eyeball protection with a conjunctival flapwith eye closed. (D) association between forhead and genian flap. (E) coverage of the soft tissue postexcisional defect with a Mustarde flap.
Results
The immediate postoperative course was favorable in all study cases. In only one case did we find distal venous congestion of the flap with a necrotic zone. To cover the lower eyelid soft tissue defect resulting from necrectomy, a split-thickness skin graft was performed 14 days after surgery. The postoperative edema, ecchymoses, and erythema disappeared completely after 4–6 weeks, and the end result (functional and esthetic) was assessed approximately 3–6 months postoperatively (Figure 4).
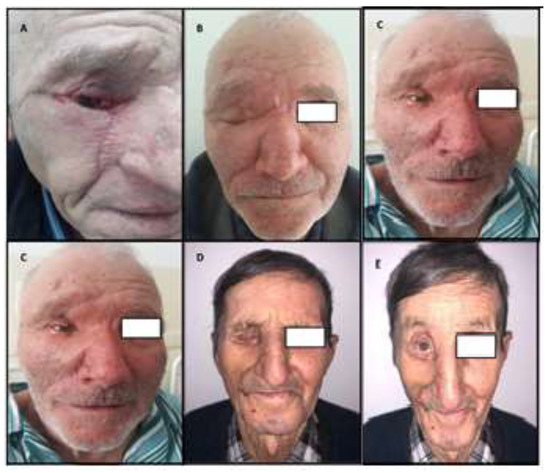
Figure 4.
(A–E): functional and aesthetic good results in all cases.
The diagnosis of certainty was made by the histopathological examination which revealed in all casesand ulcerated nodular BCCs (Figure 5).
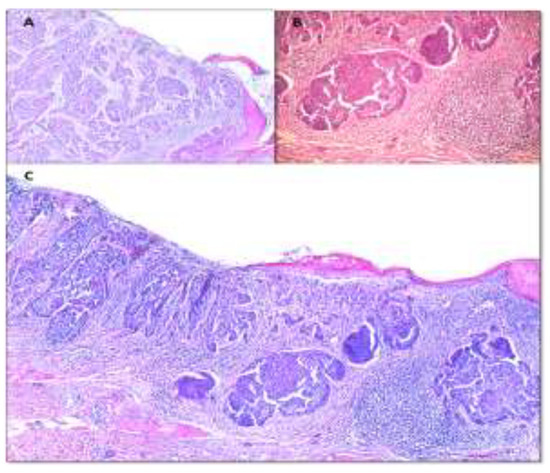
Figure 5.
(A) Nodular basal cell carcinoma ulcerated with fibrin-leukocyte exudate on the surface. Tumor cell nests with microcystic dilatations, associating reduced desmoplasticstroma (×5 H&E staining). (B) Nodular basal cell carcinoma tumor islands of various dimensions, having artefactual cleavage space with adjacent tumor stroma (×10 H&E staining). (C) Nodular basal cell carcinoma with tumor nodules of different sizes and shapes. Area of ulceration on the surface with fibrin- leukocyte debris (×5 H&E staining).
The indication for adjuvant radio-chemotherapy was subsequently established by the oncologist. In all cases the excision was performed with histologically clear margins (safety margins), re-excision not being needed in any of these cases.
Long-term course was good, with complete flap re-integration and healing of donor area, without pathological scars. The results were assessed from a functional point of view (eyelids), color, and texture of flaps, as well as the appearance and esthetic impact of the scars, taking into account the degree of patient satisfaction. All patients were satisfied with the functional as well as the esthetic outcomes, with no eyelid occlusion disturbances that could possibly affect the visual function. No less important was the psychological improvement and the rapid social reintegration of these patients, although most of them were retired. The histopathological examinations confirmed the excision of the masses within the oncological limits in all the plans, and clinically no recurrences were found 18 months postoperatively.
Discussions
GBCCs are rarely reported in the literature, being a rare oncologic entity. In the TNM classification, they are designated T3 [14]. In a study of 17 cases diagnosed with GBCCs, Maimaiti et al. reported an 11/6 male predominance, while another study by Shuo Fang et al. from the Plastic and Reconstructive Surgery Clinic of Shanghai Hospital, China showed a 9/6 male predominance. In our study, we found a higher male predominance, namely 8/1. In the literature, no concrete causes for this higher incidence in men are reported. It is believed that a possible explanation may be the involvement of men in jobs with prolonged exposure to ultra violet radiation, associated with a possible higher degree of neglect or alcohol consumption. Mean age at the time of GBCCs diagnosis is about 67 years [15,16]. In our study group the mean age was 72.2 years.
The socio-economic status of the patients is considered to be a major risk factor. As all the patients included in this study resided in rural areas, living in precarious conditions and having difficulties in accessing healthcare could be considered factors that may have contributed to their late presentation (after 5 to 12 years) to a specialist, findings supported by other reports in the literature [8]. Old age is another important risk factor demonstrated by the current study, the youngest patient in our study group being aged 60 and the oldest 85 [9]. Given the patients’ age, possible mental deficiencies should not be excluded. All 9 patients included in this study reported that they did not experience pain throughout tumor development. However, Randle et al. reported that GBCCs have a greater propensity to metastasize, especially when they reach critical dimensions of over 10 cm, and Archontaki et al. found a 17.6% incidence of metastases at the time of presentation based on the review of the published literature until that time (30 articles, 51 patients); fortunately, in our study group no metastases were identified at the time of presentation or during postoperative follow-up [6,14]. GBCCs show a predilection for lymph node metastasis, but also possible is the hematogenous spread with metastases to the parenchymal internal organs or bone metastases, which causes anemia by replacement of the hematopoietic marrow or secondary amyloidosis of the kidney, spleen, or intestines, which would considerably worsen the prognosis [17,18,19].
Key to successful treatment of periocular GBCCs appears to be radical surgical excision, even if it requires the removal of important eyelid anatomic elements and causes large soft-tissue defects that require laborious reconstruction methods [20]. In most small BCCs, the 2- 4mm safety margins provide adequate excision, but GBCCs have a higher incidence of subclinical extension requiring excisions with 3-5mm safety margins. The periocular area is of major functional and esthetic importance, which is why it requires special attention, especially when it comes to the defects post-excision of a GCBCs, where direct suture would give unsatisfactory results due to the resulting tension on ocular adnexa [21]. The methods for the reconstruction of post-excisional defects in the periocular area require the knowledge and respect of some principles related to the use of flaps and skin grafts that offer safer and more efficient solutions, the flaps being preferred over the grafts in this anatomical region because their homogeneity in color and texture will lead to a better esthetic, functional, and morphological unification with the surrounding tissues. In addition, free skin plasty cannot be used when the excision reveals slightly vascularized anatomical elements, blood vessels, or nerves [22]. Often, negative pressure therapy cannot be used to obtained a real granular bed for split skin grafting. In order to use negative pressure therapy, we must have the certainty of a complete excision of the tumor [23]. Loco- regional flaps had been used for the reconstruction of defects in all 9 study patients, the obtained being reported above.
Radiation therapy may have an adjuvant or palliative role in GBCCs, especially in patients with extensive facial lesions or altered general conditions that would not tolerate such an extensive surgery, but the response to this type of treatment is heterogeneous and difficult to predict. There appears to be no correlation between tumor location and the type of response to radiation therapy, but the nodular subtype of BCCs is considered to respond well to radiation therapy, and the sclerosing BCCs is described as having a high recurrence rate after radiation therapy [18,24]. It should be mentioned there is high operative risk for these patients who are often elderly and in which no other form of local anesthesia can be used [25]. The localization of these large tumors of the face is a real challenge in choosing the surgical technique, being much more difficult than in other anatomical regions of the body [26,27].
Vismodegib is a new treatment option, the only medication approved by the US Food and Drug Administration in 2012 for the treatment of locally advanced or metastatic BCCs. In 54% of the patients receiving Vismodegib treatment, the absence of residual disease in control biopsies was confirmed. The average duration of treatment is 7.6 months, and the common side effects are muscle spasms, dysgeusia, alopecia, and weight loss. This therapeutic option may be a good alternative in patients with contraindications for surgery or metastases [28].
Conclusions
Early detection of BCCs and radical surgical treatment are probably the most effective weapon against the development of a GBCCs. This could be achieved by improving the primary health care services and encouraging patients to undergo surgery at earlier stages of the disease, as well as educating them with regard to the course of this disease. Regarding the surgical treatment of periocular GBCCs, tumor resection should be done with safety margins. Reconstruction of post-excisional defects can be performed with local flaps, which is a safe method that provides good functional and esthetic outcomes, as confirmed by the results of this study. Absence of tumor invasion in the resection margins must be confirmed by histopathological examination. Given the high relapse rate, a long-term follow-up of these patients is required.
Conflict of interest disclosure
There are no known conflicts of interest in the publication of this article. The manuscript was read and approved by all authors.
Compliance with ethical standards
Any aspect of the work covered in this manuscript has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
Abbreviations
Basal cell carcinomas—BCCs, giant basal cell carcinomas—GBCCs, human patch gene—PTCH, computer tomography—CT.
References
- Kleinstein, R.N.; Lehman, H.F. Incidence and prevalence of eye cancer. Am J Optom Physiol Opt. 1977, 54, 49–51. [Google Scholar] [CrossRef]
- Bath-Hextall, F.; Leonardi-Bee, J.; Smith, C.; Meal, A.; Hubbard, R. Trends in incidence of skin basal cell carcinoma. Additional evidence from a UK primary care database study. Int J Cancer. 2007, 121, 2105–2108. [Google Scholar] [CrossRef]
- Furdova, A.; Lukacko, P. Periocular Basal Cell Carcinoma Predictors for Recurrence and Infiltration of the Orbit. J Craniofac Surg. 2017, 28, e84–e87. [Google Scholar] [CrossRef]
- Bowyer, J.D.; Sullivan, T.J.; Whitehead, K.J.; Kelly, L.E.; Allison, R.W. The management of perineural spread of squamous cell carcinoma to the ocular adnexae. Ophthalmic Plast Reconstr Surg. 2003, 19, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Page, D.L.; Fleming, I.D.; Fritz, A. AJCC Cancer Staging Manual, 6th ed.; New York, NY, USA, 2002. [Google Scholar]
- Archontaki, M.; Stavrianos, S.D.; Korkolis, D.P.; et al. Giant Basal cell carcinoma: Clinicopathological analysis of 51 cases and review of the literature. Anticancer Res. 2009, 29, 2655–2663. [Google Scholar] [PubMed]
- Zoccali, G.; Pajand, R.; Papa, P.; Orsini, G.; Lomartire, N.; Giuliani, M. Giant basal cell carcinoma of the skin: Literature review and personal experience. J Eur Acad Dermatol Venereol. 2012, 26, 942–952. [Google Scholar] [CrossRef]
- Rusiñol, J.S.; Llorca, V.; Mezzardi, N.A.; Casas, J.G.; Larralde, M. Carcinoma basocelulargigante: Comunicación de dos lesiones en un paciente. Dermatol Argent. 2011, 17, 67–69. [Google Scholar]
- Alexa, O.; Pertea, M.; Malancea, R.I.; Puha, B.; Veliceasa, B. Our experience in the surgical treatment of acetabular fractures using „spring plate” technique. Med Surg J. 2019, 123, 275–81. [Google Scholar]
- Wysong, A.; Aasi, S.Z.; Tang, J.Y. Update on metastatic basal cell carcinoma: A summary of published cases from 1981 through 2011. JAMA Dermatol. 2013, 149, 615–616. [Google Scholar] [PubMed]
- Snow, S.N.; Sahl, W.; Lo, J.S.; et al. Metastatic basal cell carcinoma. Report of five cases. Cancer 1994, 73, 328–335. [Google Scholar]
- Sahl, W.J., Jr.; Snow, S.N.; Levine, N.S. Giant basal cell carcinoma. Report of two cases and review of the literature. Report of two cases and review of the literature. J Am Acad Dermatol. 1994, 30 Pt 2, 856–859. [Google Scholar]
- Boulinguez, S.; Grison-Tabone, C.; Lamant, L.; et al. Histological evolution of recurrent basal cell carcinoma and therapeutic implications for incompletely excised lesions. Br J Dermatol. 2004, 151, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Randle, H.W.; Roenigk, R.K.; Brodland, D.G. Giant basal cell carcinoma (T3). Who is at risk? Cancer 1993, 72, 1624–1630. [Google Scholar] [CrossRef] [PubMed]
- Maimaiti, A.; Mijiti, A.; Yarbag, A.; Moming, A. Giant basal cell carcinoma of the face: Surgical management and challenges for reconstruction. J Laryngol Otol. 2016, 130, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Yang, C.; Zhang, Y.; et al. The Use of Composite Flaps in the Management of Large Full-Thickness Defects of the Lower Eyelid. Medicine 2016, 95, e2505. [Google Scholar] [CrossRef]
- Robinson, J.K.; Dahiya, M. Basal cell carcinoma with pulmonary and lymph node metastasis causing death. Arch Dermatol. 2003, 139, 643–648. [Google Scholar] [CrossRef]
- Schwartz, R.A.; De Jager, R.L.; Janniger, C.K.; Lambert, W.C. Giant basal cell carcinoma with metastases and myelophthisic anemia. J Surg Oncol. 1986, 33, 223–226. [Google Scholar] [CrossRef]
- Pertea, M.; Poroch, V.; Grosu, O.M.; Manole, A.; Velenciuc, N.; Lunca, S. Use of WALLANT technique in hand surgery, safe and advantageous. Personal experience. J Clin Invest Surg. 2018, 3, 26–31. [Google Scholar] [CrossRef]
- Elshamma, N.A.; Al Qabbani, A.; Alkatan, H.M.; Al-Qattan, M.M. The use of forehead flaps in the management of large basal cell carcinomas of the medial canthus/medial lower eyelid in Saudi patients. Saudi J Ophthalmol. 2013, 27, 223–225. [Google Scholar] [CrossRef]
- Marchac, D.; Papadopoulos, O.; Duport, G. Curative and aesthetic results of surgical treatment of 138 basal-cell carcinomas. J Dermatol Surg Oncol. 1982, 8, 379–387. [Google Scholar] [CrossRef]
- Fogagnolo, P.; Colletti, G.; Valassina, D.; Allevi, F.; Rossetti, L. Partial and total lower lid reconstruction: Our experience with 41 cases. Ophthalmologica 2012, 228, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Velenciuc, N.; Poroch, V.; et al. Efficacy of negative pressure therapy (NPWT) in the management of wounds of different etiologies. Rev Chim. 2018, 69, 1980–86. [Google Scholar] [CrossRef]
- Barnes, E.A.; Breen, D.; Culleton, S.; et al. Palliative radiotherapy for non-melanoma skin cancer. Clin Oncol (R Coll Radiol). 2010, 22, 844–849. [Google Scholar] [CrossRef]
- Pertea, M.; Grosu, O.M.; Veliceasa, B.; et al. Effectiveness and safety of wide awake local anesthesia no tourniquet (Walant) technique in hand surgery. Rev Chim. 2019, 70, 3587–91. [Google Scholar]
- Pertea, M.; Grosu, O.M.; Terinte, C.; Poroch, V.; Velenciuc, N.; Lunca, S. Nail bed solitary neurofibroma: A case report and literature review. Medicine 2019, 98, e14111. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Velenciuc, N.; Grosu, O.M.; Veliceasa, B.; et al. Reconstruction of heel soft tissue defects using sensate medial plantar flap. J Mind Med Sci. 2018, 5, 250–54. [Google Scholar] [CrossRef]
- Sekulic, A.; Migden, M.R.; Oro, A.E.; et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012, 366, 2171–2179. [Google Scholar] [CrossRef]
© 2020 by the author. 2020 Oxana Madalina Grosu, Vladimir Poroch, Natalia Velenciuc, Sorinel Lunca