An Investigation of Acute Effects at Various Doses of Malathion on Glucose Homeostasis and Insulin Resistance in Rat Liver, Pancreas and Serum
Abstract
:Introduction
Materials and Methods
Data Analyses
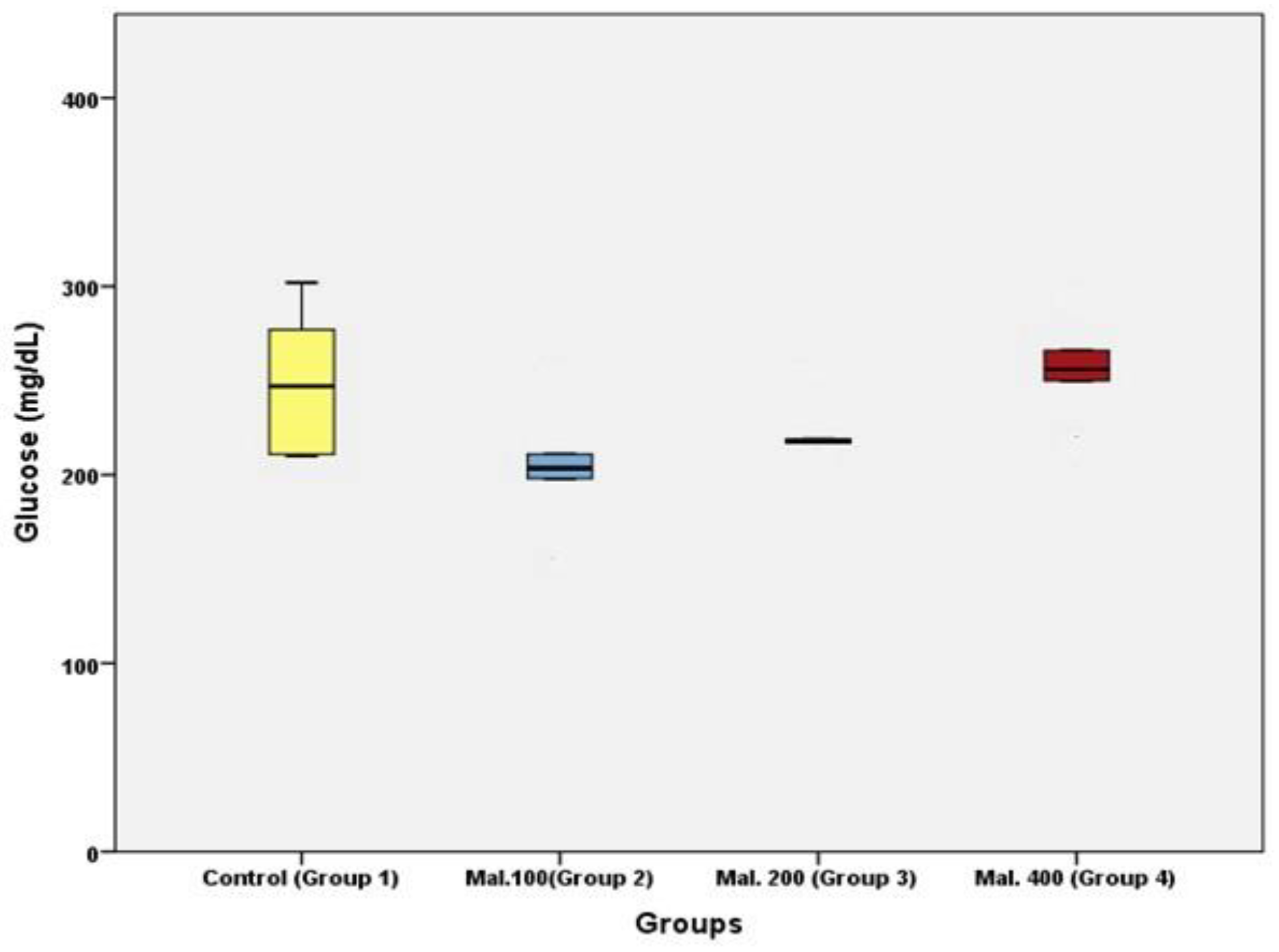
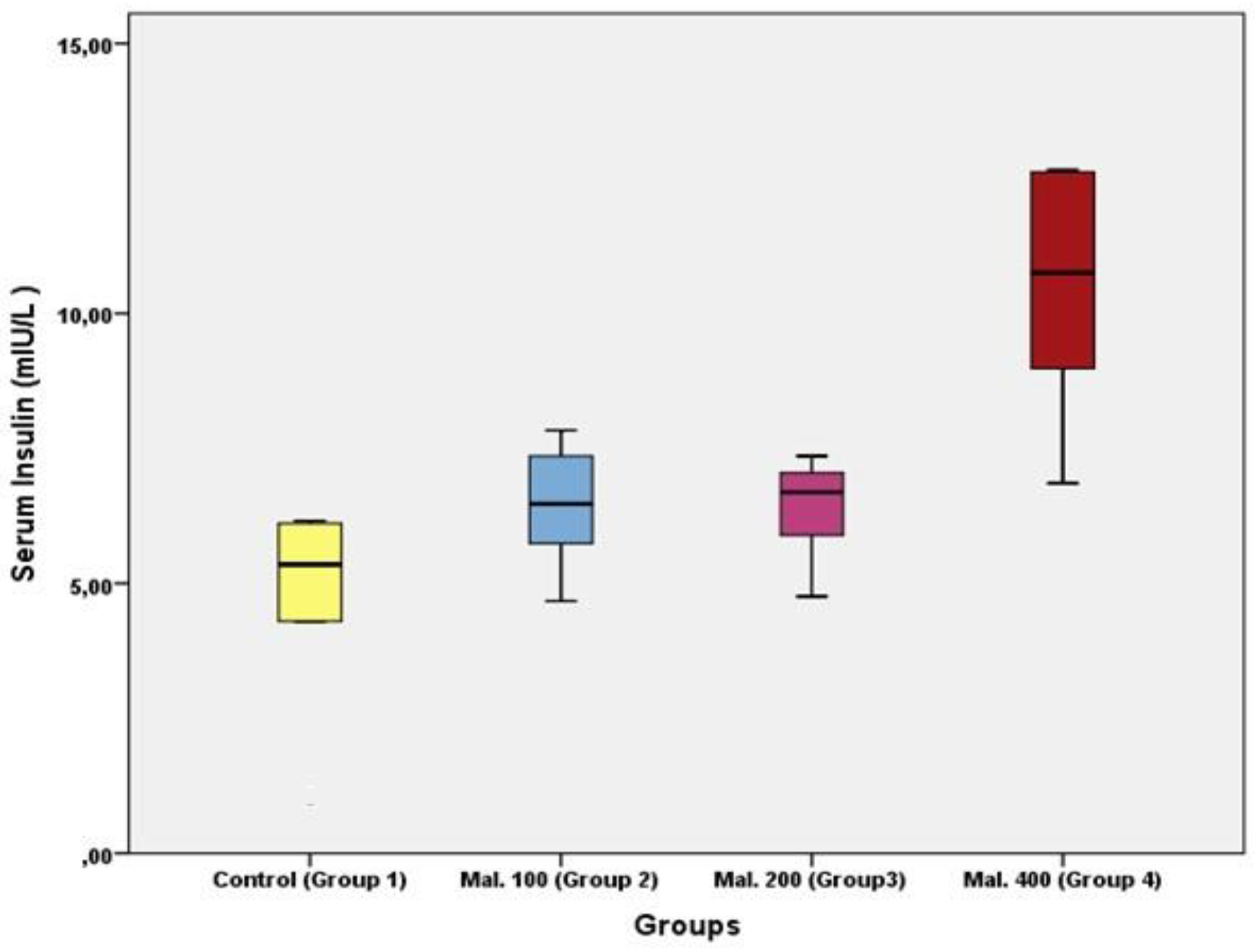
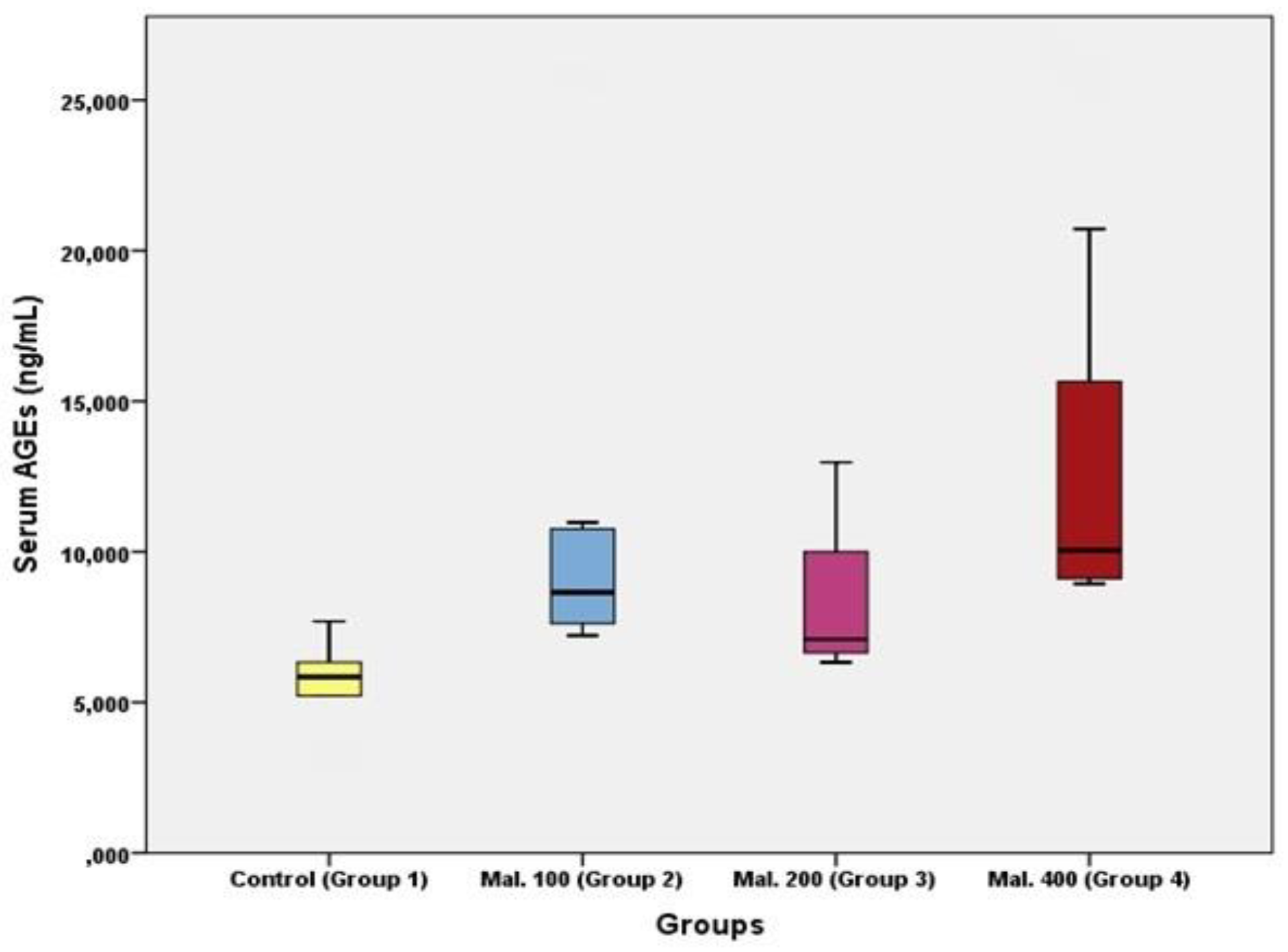
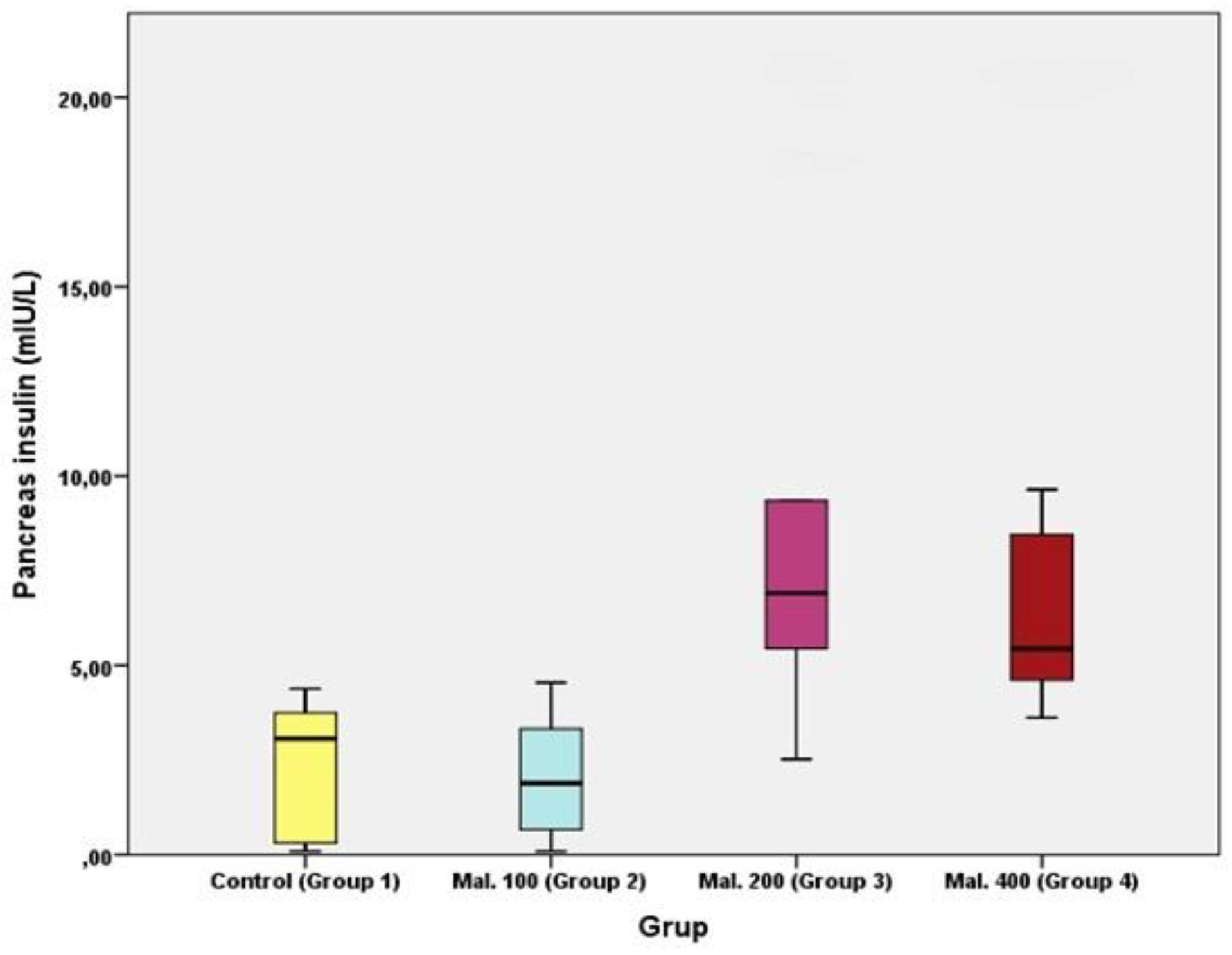
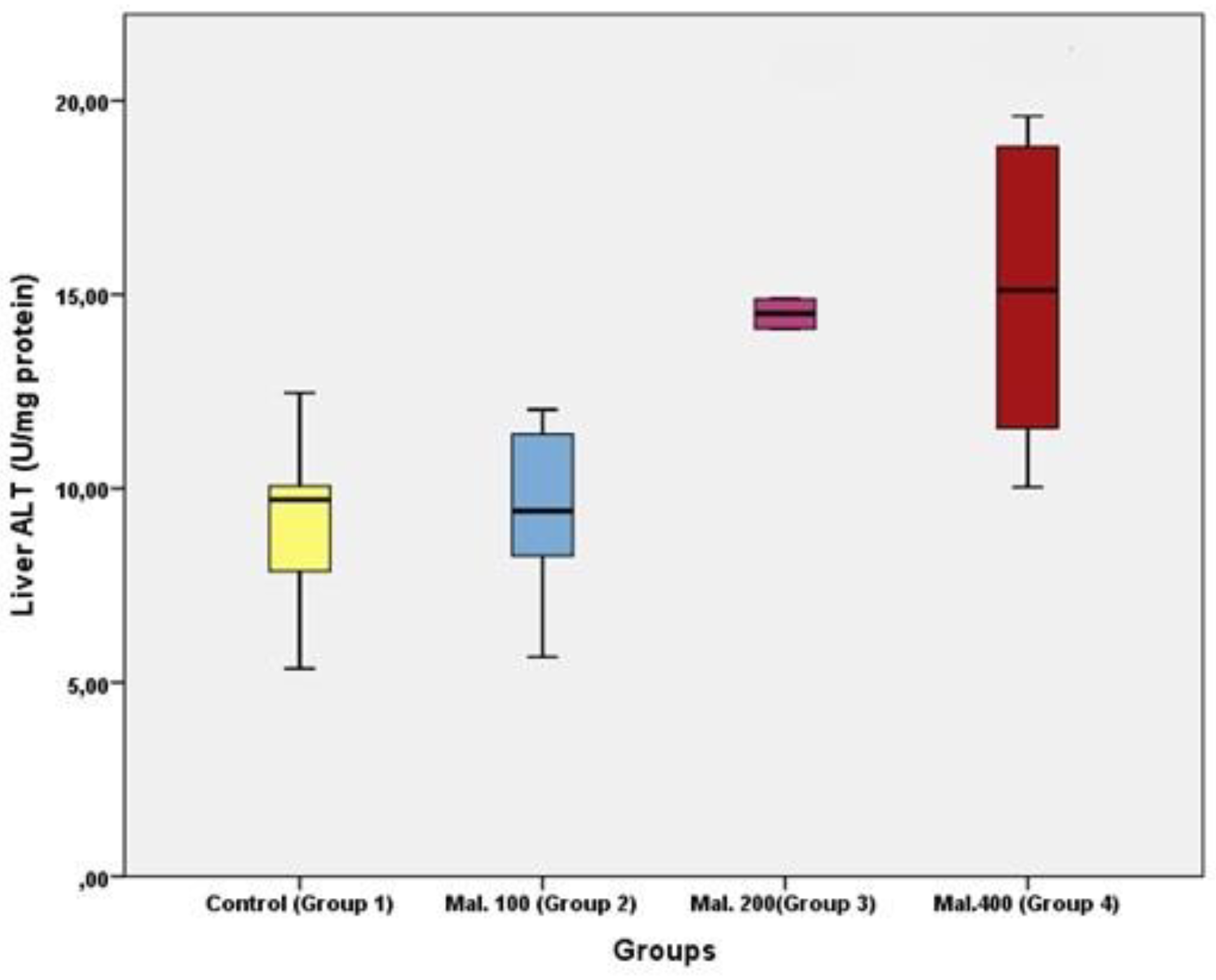
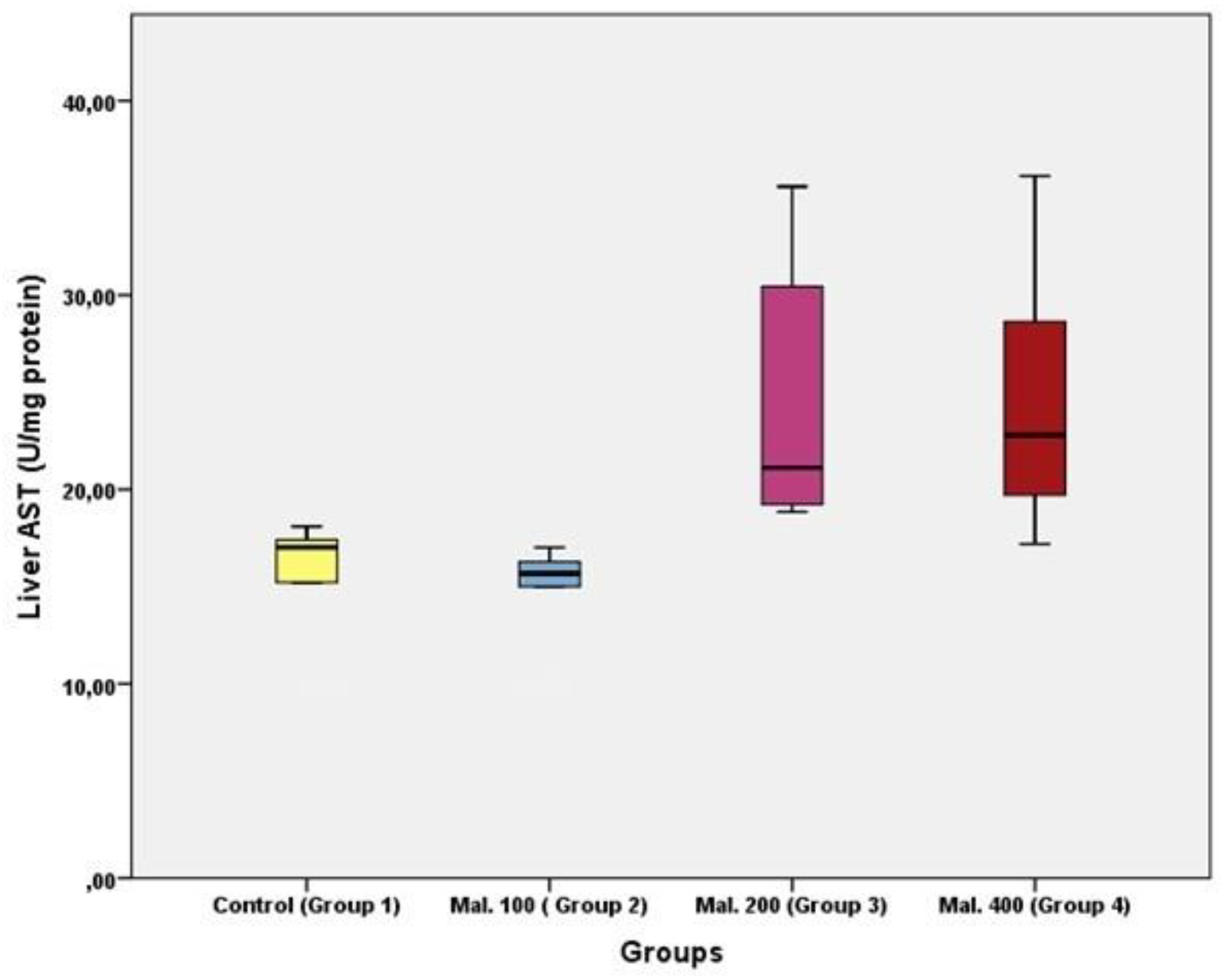
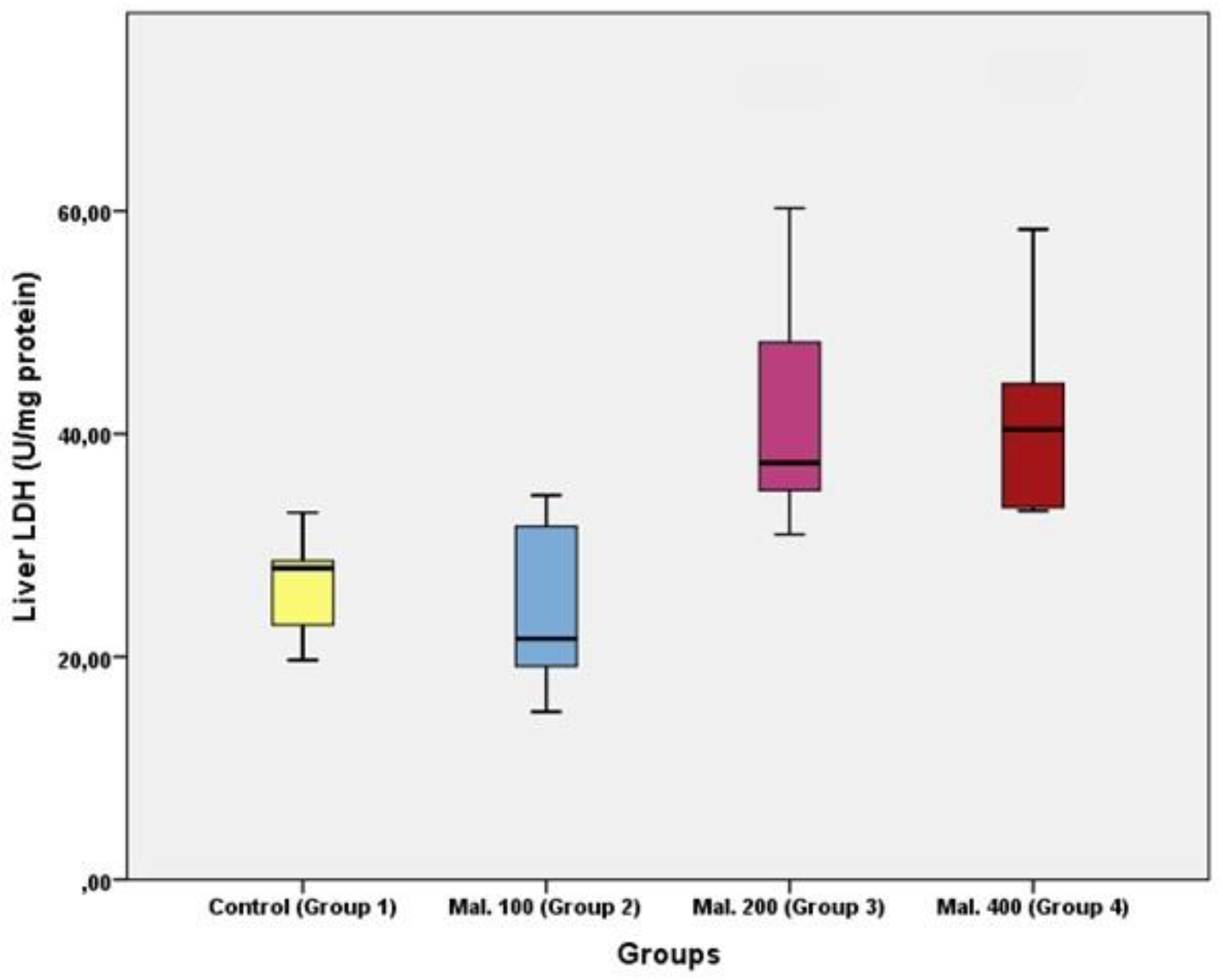
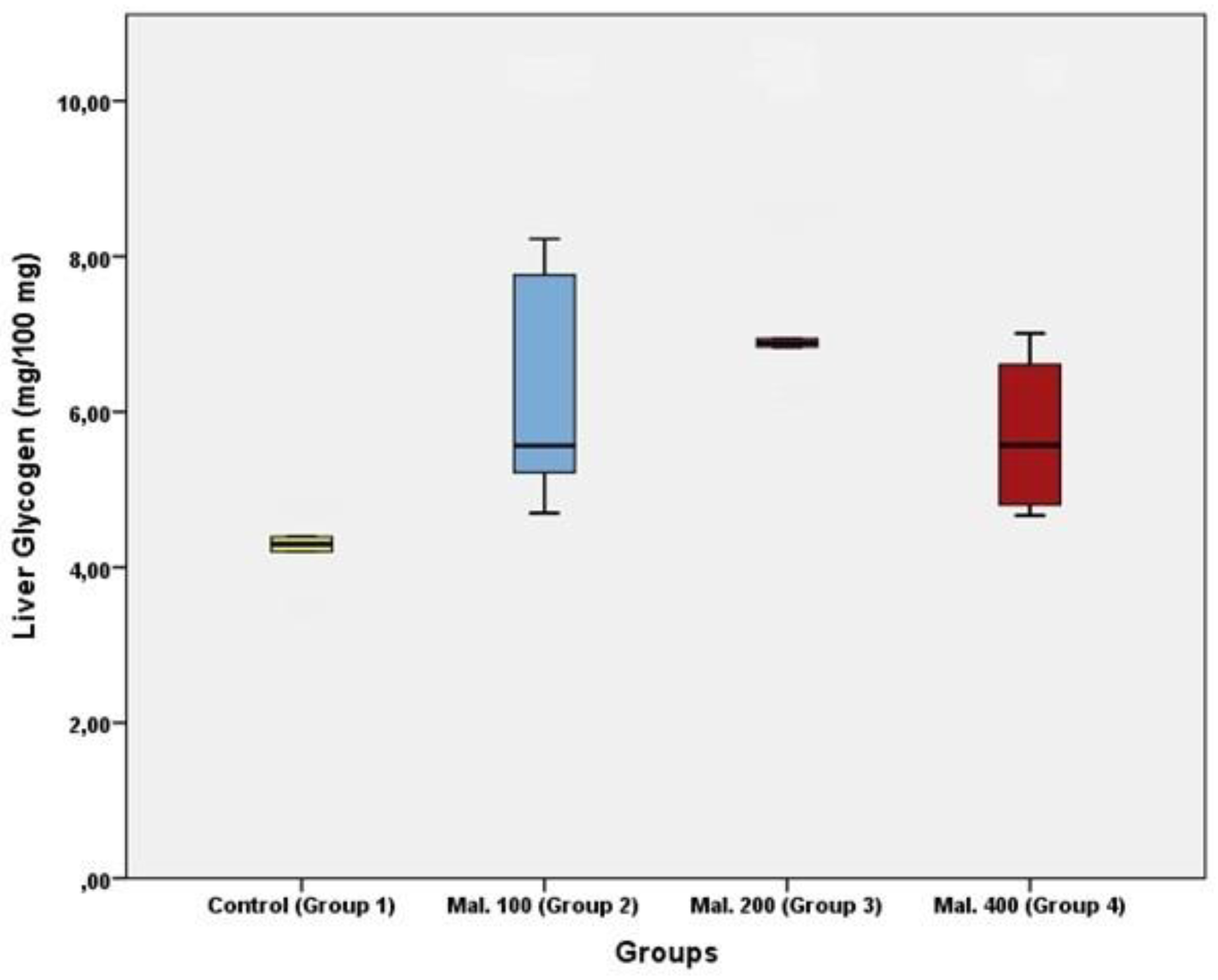
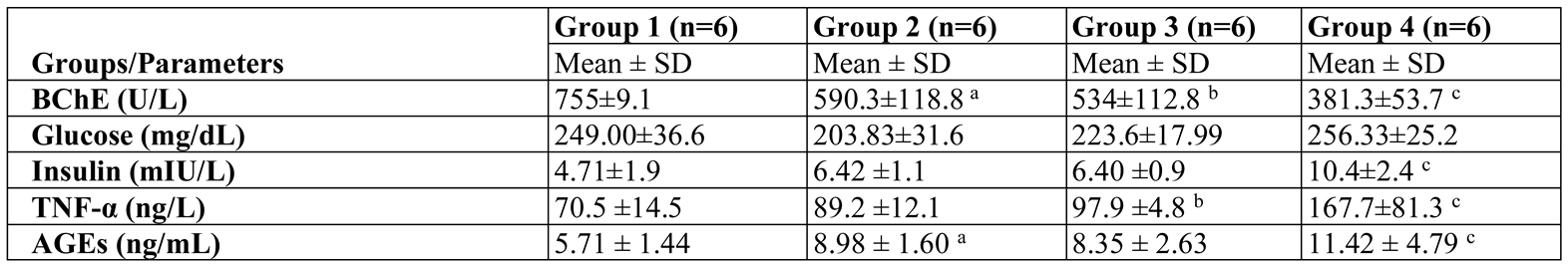
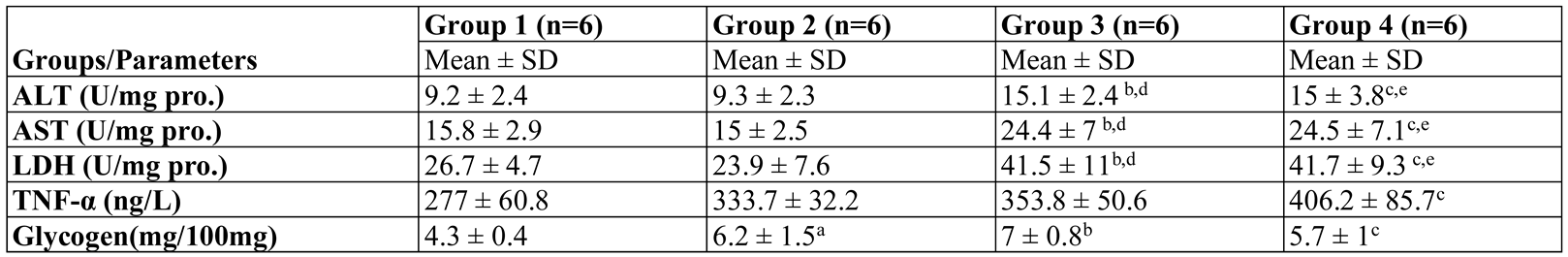
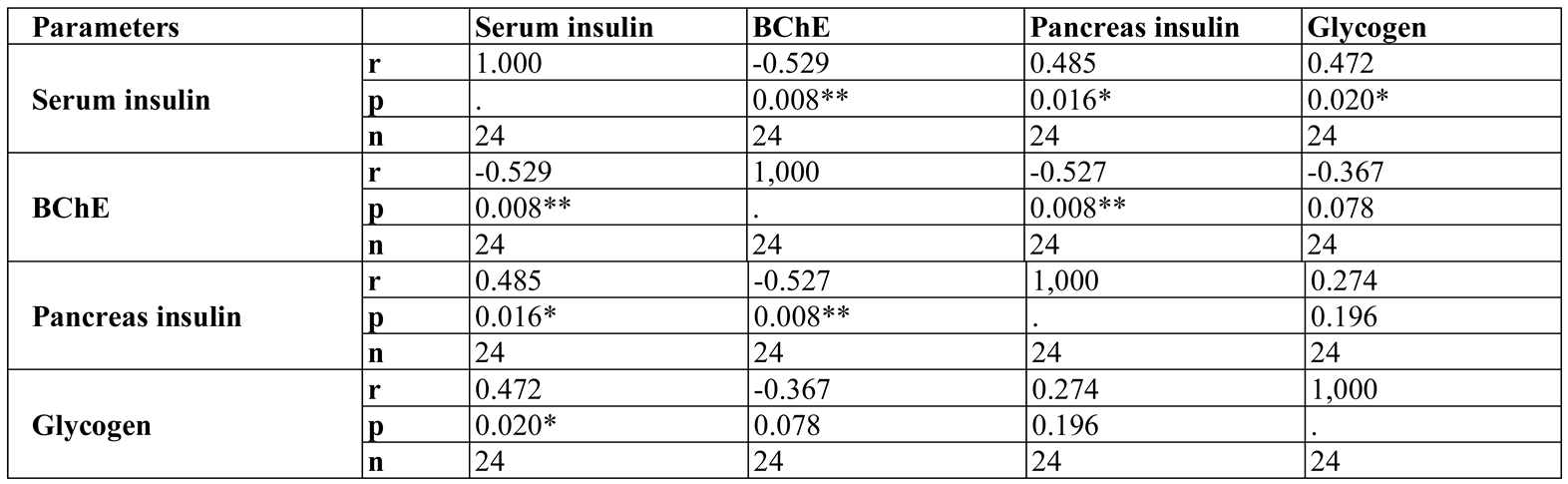
Results
Discussions
Conclusions
References
- Karami-Mohajeri, S.; Abdollahi, M. Toxic influence of organophosphate, carbamate, and organochlorine pesticides on cellular metabolism of lipids, proteins, and carbohydrates: A systematic review. Hum Exp Toxicol. 2011, 30, 1119–1140. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, R.E.; Smith, R.P.; Hodge, H.C. Clinical Toxicology of Commercial Products; Williams and Wilkins: USA, 1984; Volume 5, pp. 1120–1125. [Google Scholar]
- Available online: http://pmep.cce.cornell.edu/profiles/extoxnet/haloxyfop-methylparathion/malathion-ext.
- Abdollahi, M.; Donyavi, M.; Pournourmohammadi, S.; Saadat, M. Hyperglycemia associated with increased hepatic glycogen phosphorylase and phosphoenolpyruvate carboxykinase activities in rats following subchronic exposure to malathion. Comp Biochem Physiol C Toxicol Pharmacol. 2004, 137, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K. Malathion induced biochemical changes in rat. Acta Pharmacol Toxicol (Copenh). 1974, 35, 191–194. [Google Scholar] [CrossRef]
- Burg, M.B. Molecular basis of osmotic regulation. Am J Physiol. 1995, 268 Pt 2, F983–F996. [Google Scholar] [CrossRef]
- Flores, D.; Souza, V.; Betancourt, M.; Teteltitla, M.; González-Márquez, H.; Casas, E.; Bonilla, E.; Ramírez-Noguera, P.; Gutiérrez-Ruíz, M.C.; Ducolomb, Y. Oxidative stress as a damage mechanism in porcine cumulus-oocyte complexes exposed to malathion during in vitro maturation. Environ Toxicol. 2017, 32, 1669–1678. [Google Scholar] [CrossRef]
- Palimeri, S.; Palioura, E.; Kandarakis, E.D. Current perspectives on the health risks associated with the consumption of advanced glycation end products: Recommendations for dietary management. Metab Syndr Obes. 2015, 8, 415–426. [Google Scholar]
- Krebs, M.; Roden, M. Molecular mechanisms of lipid-induced insulin resistance in muscle, liver and vasculature. Diabetes Obes Metab. 2005, 7, 621–632. [Google Scholar] [CrossRef]
- Pournourmohammadi, S.; Farzami, B.; Ostad, S.N.; Azizi, E.; Abdollahi, M. Effects of malathion subchronic exposure on rat skeletal muscle glucose metabolism. Environ Toxicol Pharmacol. 2005, 19, 191–196. [Google Scholar] [CrossRef]
- Panahi, P.; Vosough-Ghanbari, S.; Pournourmohammadi, S.; Ostad, S.N.; Nikfar, S.; Minaie, B.; Abdollahi, M. Stimulatory effects of malathion on the key enzymes activities of insulin secretion in Langerhans islets, glutamate dehydrogenase and glucokinase. Toxicol Mech Methods. 2006, 16, 67–161. [Google Scholar] [CrossRef]
- Rezg, R.; Mornagui, B.; El-Arbi, M.; Kamoun, A.; El-Fazaa, S.; Gharbi, N. Effect of subchronic exposure to malathion on glycogen phosphorylase and hexokinase activities in rat liver using native PAGE. Toxicology 2006, 223, 9–14. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurements with the folin phenol reagent. J Biol Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Seifter, S.; Dayton, S.; Novic, B.; Muntwyler, E. The estimation of glycogen with the anthrone reagent. Archives of Biochemistry 1950, 25, 191–200. [Google Scholar]
- Naughton, S.X.; Terry, A.V. Neurotoxicity in acute and repeated organophosphate exposure. Toxicology 2018, 408, 101–112. [Google Scholar] [CrossRef]
- Ilie, M.A.; Caruntu, C.; Tampa, M.; Georgescu, S.R.; Matei, C.; Negrei, C.; Ion, R.M.; Constantin, C.; Neagu, M.; Boda, D. Capsaicin: Physicochemical properties, cutaneous reactions and potential applications in painful and inflammatory conditions. Exp Ther Med. 2019, 18, 916–925. [Google Scholar] [CrossRef]
- Hazarika, A.; Sarkar, S.N.; Hajare, S.; Kataria, M.; Malik, J.K. Influence of malathion pretreatment on the toxicity of anilofos in male rats: A biochemical interaction study. Toxicology 2003, 185, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gashi, M.; Gashi, S.; Berisha, M.; Mekaj, A.; Gashi, G. Cases of poisoning with organophosphates treated at the University Clinical Centre of Kosova. Med Arh. 2010, 64, 48–50. [Google Scholar] [PubMed]
- Kwesiga, B.; Ario, A.R.; Bulage, L.; Kataria, M.; Malik, J.K. Fatal cases associated with eating chapatti contaminated with organophosphate in Tororo District, Eastern Uganda, 2015: Case series. BMC Public Health. 2019, 19, 767. [Google Scholar] [CrossRef]
- Larsen, K.O.; Hanel, H.K. Effect of exposure to organophosphorus compounds on S-cholinesterase in workers removing poisonous depots. Scand J Work Environ Health. 1982, 8, 222–226. [Google Scholar] [CrossRef]
- Karami-Mohajeri, S.; Hadian, M.R.; Fouladdel, S.; Azizi, E.; Ghahramani, M.H.; Hosseini, R.; Abdollahi, M. Mechanism of muscular electrophysiological and mitochondrial dysfunction following exposure to malathion, an organophosphorus pesticide. Hum Exp Toxicol. 2014, 33, 251–263. [Google Scholar] [CrossRef]
- Mostafalou, S.; Eghbal, M.A.; Nili-Ahmadabadi, A.; Baeeri, M.; Abdollahi, M. Biochemical evidence on the potential role of organophosphates in hepatic glucose metabolism toward insulin resistance through inflammatory signaling and free radical pathways. Toxicol Ind Health. 2012, 28, 840–851. [Google Scholar] [CrossRef]
- Bratoeva, K.Z.; Radanova, M.A.; Merdzhanova, A.V.; Donev, I.S. Protective role of s-adenosylmethionine against fructose-induced oxidative damage in obesity. J Mind Med Sci. 2017, 4, 163–171. [Google Scholar] [CrossRef]
- Sharmin, S.; Salam, A.; Haque, F.; Islam, S.; Shahjahan, M. Changes in hematological parameters and gill morphology in common carp exposed to sub-lethal concentrations of Malathion. Asian J Med Biol Res. 2016, 2, 370–378. [Google Scholar] [CrossRef]
- Ghafour-Rashidi, Z.; Dermenaki-Farahani, E.; Aliahmadi, A.; Esmaily, H.; Mohammadirad, A.; Ostad, N.S.; Abdollahi, M. Protection by cAMP and cGMP phosphodiesterase inhibitors of diazinon-induced hyperglycemia and oxidative/nitrosative stress in rat Langerhans islets cells: Molecular evidence for involvement of non-cholinergic mechanisms. Pest Biochem Physiol. 2007, 87, 261–270. [Google Scholar] [CrossRef]
- Mahadev, K.; Motoshima, H.; Wu, X.; Ruddy, J.M.; Arnold, R.S.; Cheng, G.; Lambeth, J.D.; Goldstein, B.J. The NAD(P)H oxidase homolog Nox4 modulates insulin stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004, 24, 1844–1854. [Google Scholar] [CrossRef]
- Henriksen, E.J.; Diamond-Stanic, M.K.; Marchionne, E.M. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic Biol Med. 2011, 51, 993–999. [Google Scholar] [CrossRef]
- Kandalaft, K.; Liu, S.; Manivel, C.; Borner, J.W.; Dressel, T.D.; Sutherland, D.E.; Goodale, R.L. Organophosphate increases the sensitivity of human exocrine pancreas to acetylcholine. Pancreas. 1991, 6, 398–403. [Google Scholar] [CrossRef]
- Ayub, S.; Verma, J.; Das, N. Effect of endosulfan and malathion on lipid per-oxidation nitrite and TNF-alpha release by rat peritoneal macrophages. Int Immunopharmacol. 2003, 3, 1819–1828. [Google Scholar] [CrossRef]
- Hariri, A.T.; Moallem, S.A.; Mahmoudi, M.; Memar, B.; Hosseinzadeh, H. Sub-acute effects of diazinon on biochemical indices and specific biomarkers in rats: Protective effects of crocin and safranal. Food Chem Toxicol. 2010, 48, 2803–2808. [Google Scholar] [CrossRef]
- Vosough-Ghanbari, S.; Sayyar, P.; Pournourmohammadi, S.; Aliahmadia, A.; Ostad, S.N.; Abdollahi, M. Stimulation of insulin and glucagon synthesis in rat Langerhans islets by malathion in vitro Evidence for mitochondrial interaction andinvolvement of subcellular noncholinergic mechanisms. Pest Biochem Physiol. 2007, 89, 130–136. [Google Scholar] [CrossRef]
- Nili-Ahmadabadi, A.; Pourkhalili, N.; Fouladdel, S.; Pakzad, M.; Mostafalou, S.; Hassani, S.; Baeeri, M.; Azizi, E.; Ostad, S.N.; Sharifzadeh, R.H.M.; Abdollahi, M. On the biochemical and molecular mechanisms by which malathion induces dysfunction in pancreatic islets in vivo and in vitro. Pest Biochem Physiol. 2013, 106, 51–60. [Google Scholar] [CrossRef]
- Lasram, M.M.; Lamine, A.J.; Dhouib, I.B.; Bouzid, K.; Annabi, A.; Belhadjhmida, N.; Ahmed, M.B.; El Fazaa, S.; Abdelmoula, J.; Gharbi, N. Antioxidant and anti-inflamatory effects of N-acetylcystein against malathion-induced liver damages and immuntoxicity in rats. Life Sci. 2014, 107, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Lasram, M.M.; Annabi, A.B.; Rezg, R.; Elj, N.; Slimen, S.; Kamoun, A.; El-Fazaa, S.; Gharbi, N. Effect of short-time malathion administration on glucose homeostasis in Wistar rat. Pest Biochem Physiol. 2008, 92, 114–119. [Google Scholar] [CrossRef]
- Cohen, P.; Nimmo, H.G.; Proud, C.G. How does insulin stimulate glycogen synthesis? Biochem Soc Symp 1978, 43, 69–95. [Google Scholar]
- El-Shenawy, N.S.; El-Salmy, F.; Al-Eisa, R.; El-Ahmary, B. Amelioratory effect of vitamin E on organophosphorus insecticide diazinon-induced oxidative stress in mice liver. Pest Biochem Physiol. 2010, 96, 101–107. [Google Scholar] [CrossRef]
- Acker, C.I.; Nogueira, C.W. Chlorpyrifos acute exposure induces hyperglycemia and hyperlipidemia in rats. Chemosphere. 2012, 89, 602–608. [Google Scholar] [CrossRef]



 |
 |
 |
 |
 |
© 2020 by the author. 2020 Murat Ekremoğlu, Çınar Severcan, Özge Tuğce Pasaoğlu, Bayram Şen, Hatice Pasaoğlu.
Share and Cite
Ekremoğlu, M.; Severcan, Ç.; Pasaoğlu, Ö.T.; Şen, B.; Pasaoğlu, H. An Investigation of Acute Effects at Various Doses of Malathion on Glucose Homeostasis and Insulin Resistance in Rat Liver, Pancreas and Serum. J. Mind Med. Sci. 2020, 7, 85-93. https://doi.org/10.22543/7674.71.P8593
Ekremoğlu M, Severcan Ç, Pasaoğlu ÖT, Şen B, Pasaoğlu H. An Investigation of Acute Effects at Various Doses of Malathion on Glucose Homeostasis and Insulin Resistance in Rat Liver, Pancreas and Serum. Journal of Mind and Medical Sciences. 2020; 7(1):85-93. https://doi.org/10.22543/7674.71.P8593
Chicago/Turabian StyleEkremoğlu, Murat, Çınar Severcan, Özge Tuğce Pasaoğlu, Bayram Şen, and Hatice Pasaoğlu. 2020. "An Investigation of Acute Effects at Various Doses of Malathion on Glucose Homeostasis and Insulin Resistance in Rat Liver, Pancreas and Serum" Journal of Mind and Medical Sciences 7, no. 1: 85-93. https://doi.org/10.22543/7674.71.P8593
APA StyleEkremoğlu, M., Severcan, Ç., Pasaoğlu, Ö. T., Şen, B., & Pasaoğlu, H. (2020). An Investigation of Acute Effects at Various Doses of Malathion on Glucose Homeostasis and Insulin Resistance in Rat Liver, Pancreas and Serum. Journal of Mind and Medical Sciences, 7(1), 85-93. https://doi.org/10.22543/7674.71.P8593



