Highlights
- Selegiline plus doxepin double drug therapy increases significantly the antidepressant effect.
- Selegiline plus doxepin double drug therapy prevents the sedative side effect of doxepin administered as a single drug therapy.
Highlights
- Selegiline plus doxepin double drug therapy increases significantly the antidepressant effect.
- Selegiline plus doxepin double drug therapy prevents the sedative side effect of doxepin administered as a single drug therapy.
Abstract
The severity and complexity of depression can vary widely among individuals, thus making single drug therapy ineffective in some cases. Taking this fact into account and using a mouse model, we set on investigating the possibility of obtaining a synergism of action between a classical tricyclic antidepressant that inhibits noradrenalin and serotonin reuptake (doxepin), and a modern antidepressant that inhibits type-B monoamine oxidase (selegiline). We measured the antidepressant effect using the forced swimming test and the tail suspension test. We determined motor activity using the Activity Cage test. Our results have shown that the antidepressant effect intensifies significantly in the animals treated with both antidepressants simultaneously compared to those treated only with doxepin. Furthermore, we observed that selegiline decreases the sedative effect of doxepin in the Activity Cage test.
Introduction
Depression is becoming a global problem due to the stress caused by the adaptation difficulties that our high- speed way of life currently demands [1]. Mood disorders have been studied for decades and many theories have been proposed to explain the cause of depression. The original monoamine theory of depression suggested a direct involvement of the adrenergic system in the onset of this disorder [2]. Later studies demonstrated a more complex relationship between the various endogenous neurotransmissions, suggesting an indirect role of the adrenergic system in depression as it modulates the function of other transmissions [3].
The first significant breakthrough in the treatment of depressive disorders occurred 60 years ago with the approval of imipramine, thus opening a path for an entire class of drugs—tricyclic antidepressants—which act as nonselective noradrenergic and serotoninergic reuptake inhibitors [4]. At the same time, compounds with other mechanisms such as monoamine oxidase inhibitors, enhanced the synaptic concentration of catecholamines and achieved similar positive effects on the symptoms of depression [5].
Selegiline was created by Joseph Knoll almost 60 years ago and since then, it has been widely used for the treatment of Parkinson`s disease in low doses, Alzheimer`s disease, and major depression in higher doses [6]. Selegiline acts in 3 ways: it reduces dopamine biotransformation through the inhibition of type B monoamine oxidase; it inhibits the dopamine reuptake; and it stimulates dopamine synthesis by blocking the presynaptic dopamine receptors [7].
Although many therapeutic options are currently available, there are still cases of antidepressant-resistant depressions that require either new molecules [8,9,10,11] or new approaches in managing these disorders [12]. Given that a new drug requires significant time and financial resources, combining existent therapies may prove to be a viable solution for treating complex atypical depressions.
Materials and Methods
A population of 70 white male NMRI mice having reached maturity and weighing 34 ± 6g was supplied by the rodent farm of “Carol Davila” University of Medicine and Pharmacy. Animals were kept in cages for 24 hours separately from other animals in order to reduce stress and ensure a gradual transition to the new environment. Later, they were housed in ventilated Plexiglas cages containing groups of 10 individuals with free access to food and water. Temperature and humidity were constant [21-23°C; 45- 55%] and monitored with an Eco Solar TFA30.1037 thermo-hygrometer.
All experiments were conducted in accordance with the EU Directive 63/2010, Romanian Law 43/2014 and Good Animal Practice Regulations. The protocol was approved by the Bioethics Committee of “Carol Davila” University.
The 70 mice were initially subjected to the Activity cage test in order to configure the research groups. Horizontal motor activity (Ugo Basile 47,420 Multiple Activity Cage unit) measured in 5-minute intervals was the parameter used to identify individuals fit for this experiment. After excluding individuals with extreme responses, the 60 remaining mice were divided into 5 groups, each containing 12 animals, in such a manner that the average responses and the standard deviations were as similar as possible. Animals were then allowed two days for acclimation in their new groups. On the day of the experiment, each group was brought to the lab, two hours before being treated, in order to allow adaptation to the new environment where they were kept without food.
The 5 groups were treated as follows:
- Group I (control) – distilled water 0.1 ml/10g orally
- Group II – Doxepin 10 mg/kg bw suspension 0.1% orally
- Group III – Doxepin 15 mg/kg bw suspension 0.15% orally
- Group IV – Doxepin 10 mg/kg bw suspension 0.1% + Selegiline 2.5 mg/kg bw suspension 0.25% orally
- Group V – Doxepin 15 mg/kg bw suspension 0.15% + Selegiline 5 mg/kg bw suspension 0.5% orally
The substances used in this experiment were:
- Doxepin hydrochloride D4526 – Sigma Aldrich, USA
- Selegiline hydrochloride S0360000 – Sigma Aldrich, USA
The tests were executed as follows:
- After one administration – the forced swimming test
- After 1 week of daily administrations (including the testing day) – the tail suspension test and the Activity cage test
- After 2 weeks of daily administrations (including the testing day) – the tail suspension test and the Activity cage test
Testing began one hour after the drugs were administered.
All testing followed a specific protocol: in the testing area, animals were kept in artificial light, without food. Each individual was administered the treatment with an 8- minute delay from the next one (5 minutes for the Activity cage test; 6 minutes for the forced swimming and tail suspension tests and 2 additional minutes to clean or prepare the devices before testing another animal) so that all of them could be tested after the same time interval from the moment of receiving the treatment.
Assessment of motor activity was conducted to assess the effect on the central nervous system by recording the horizontal and vertical movements of each mouse in the Ugo Basile – Activity Cage. The animals were placed individually in a corner of the cage and their movements were recorded for 5 minutes by IR photoelectric cell sensors [9,13].
Assessment of immobility time of mice as a marker of antidepressant activity was conducted in two tests:
- The forced swimming test (FST) consists of placing each mouse in a glass cylinder (25 cm height, 30 cm diameter) containing a 20 cm high column of water at a temperature of 21 ± 1°C and recording the number of seconds it rests afloat motionless during a 4-minute interval after a prior 2-minute interval for accommodation [14,15,16].
- The tail suspension test (TST) consists of suspending a mouse by its own tail in such a manner that it cannot touch neighboring surfaces nor escape. The animal is maintained in this position for 6 minutes and in the last 4 minutes, the number of seconds spent motionless is recorded [17,18].
Statistics
For the statistical evaluation, the GraphPad Prism 5.0 software was used. This software analyzes populations with Gaussian distribution using the Student t-test (for 2 groups) and the ANOVA test (for multiple groups). In case of statistical significance in the ANOVA (p < 0.05), a further post-test was conducted (Dunnett). The normality of the distribution was determined using the D`Agostino – Pearson test.
Results and Discussions
- Antidepressant activity
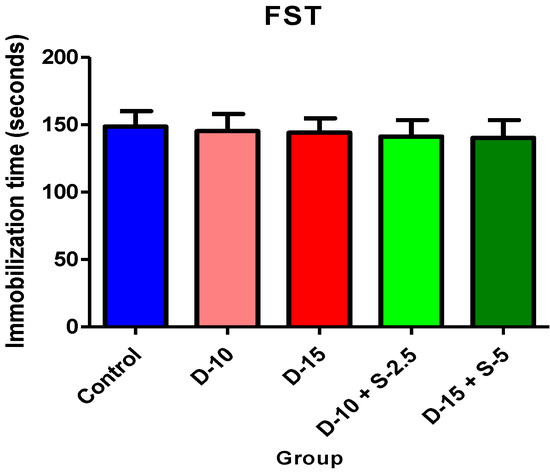
Figure 1.
Immobilization time + SD in FST after a single dose.
Figure 1.
Immobilization time + SD in FST after a single dose.

Table 1.
Immobilization time results in forced swimming test.
Table 1.
Immobilization time results in forced swimming test.
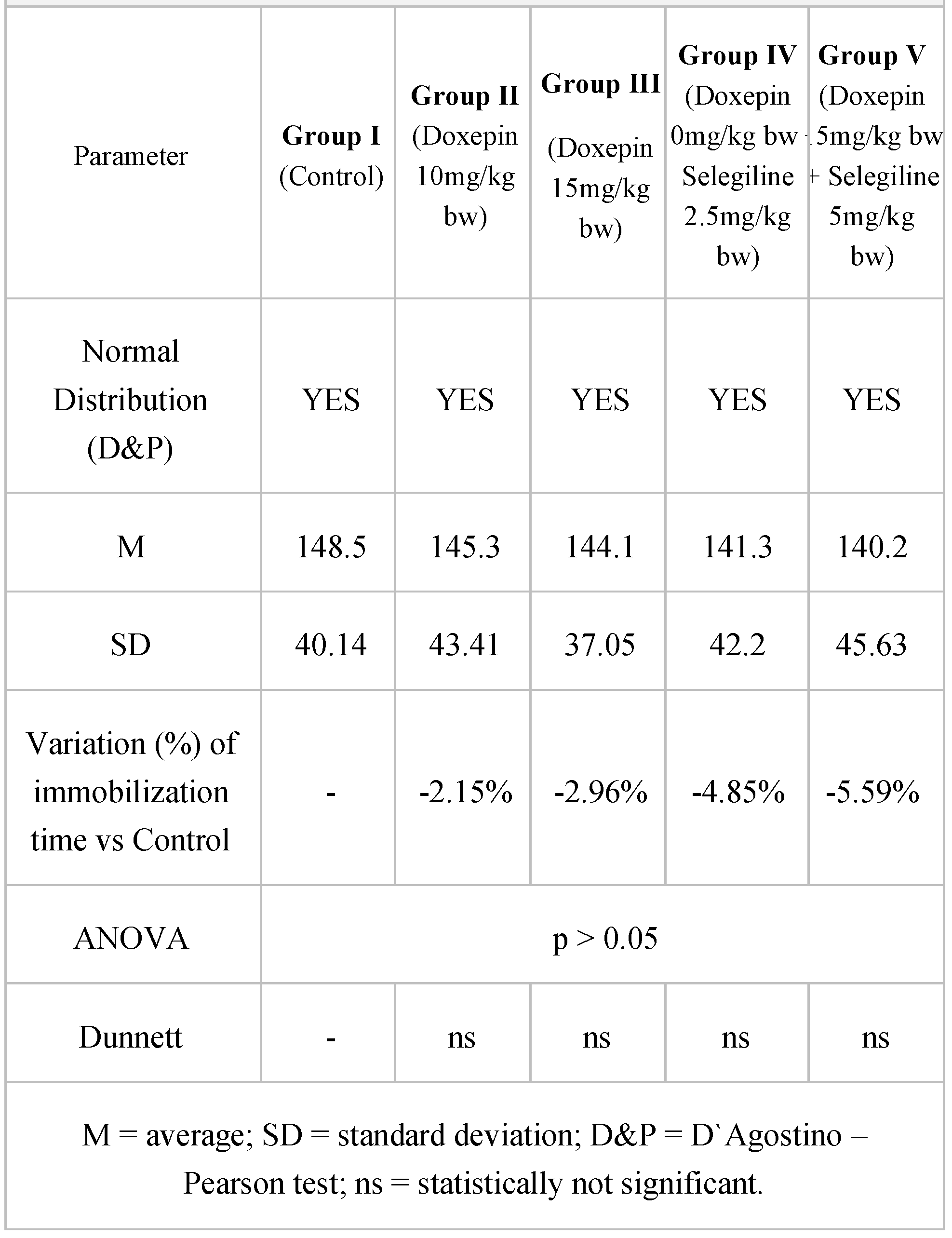 |
The forced swimming test performed after the acute administration revealed unsurprising results in the test groups compared to the control group. Even though statistical significance was not achieved, a higher decrease in the immobilization time was noticed in the groups treated with the combination selegiline + doxepin, compared to control, versus the groups treated only with doxepin.
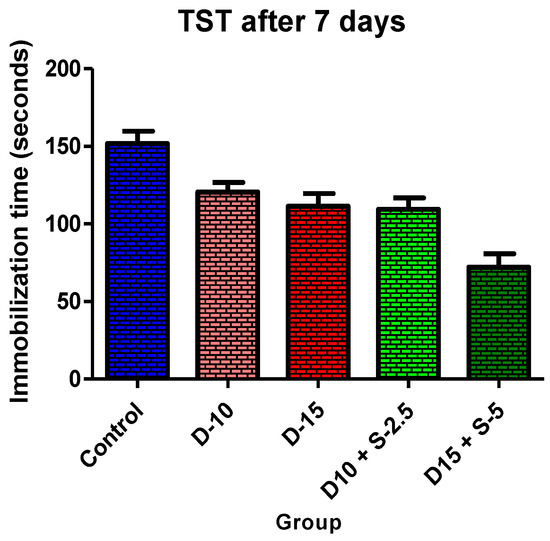
Figure 2.
Immobilization time + SD in TST after 7 days of treatment.
Figure 2.
Immobilization time + SD in TST after 7 days of treatment.
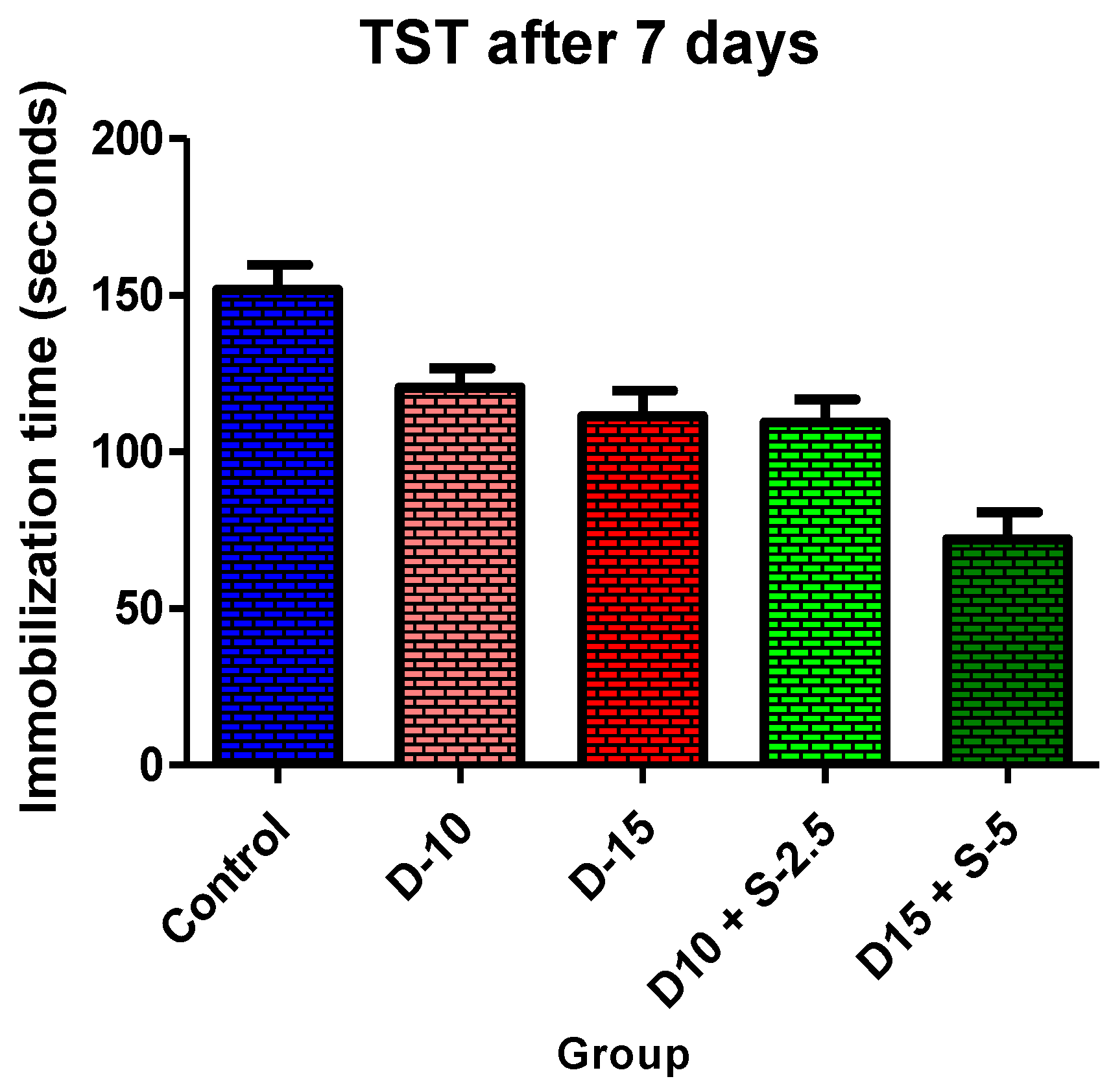

Table 2.
Immobilization time results in tail suspension test after 7 days of treatment.
Table 2.
Immobilization time results in tail suspension test after 7 days of treatment.
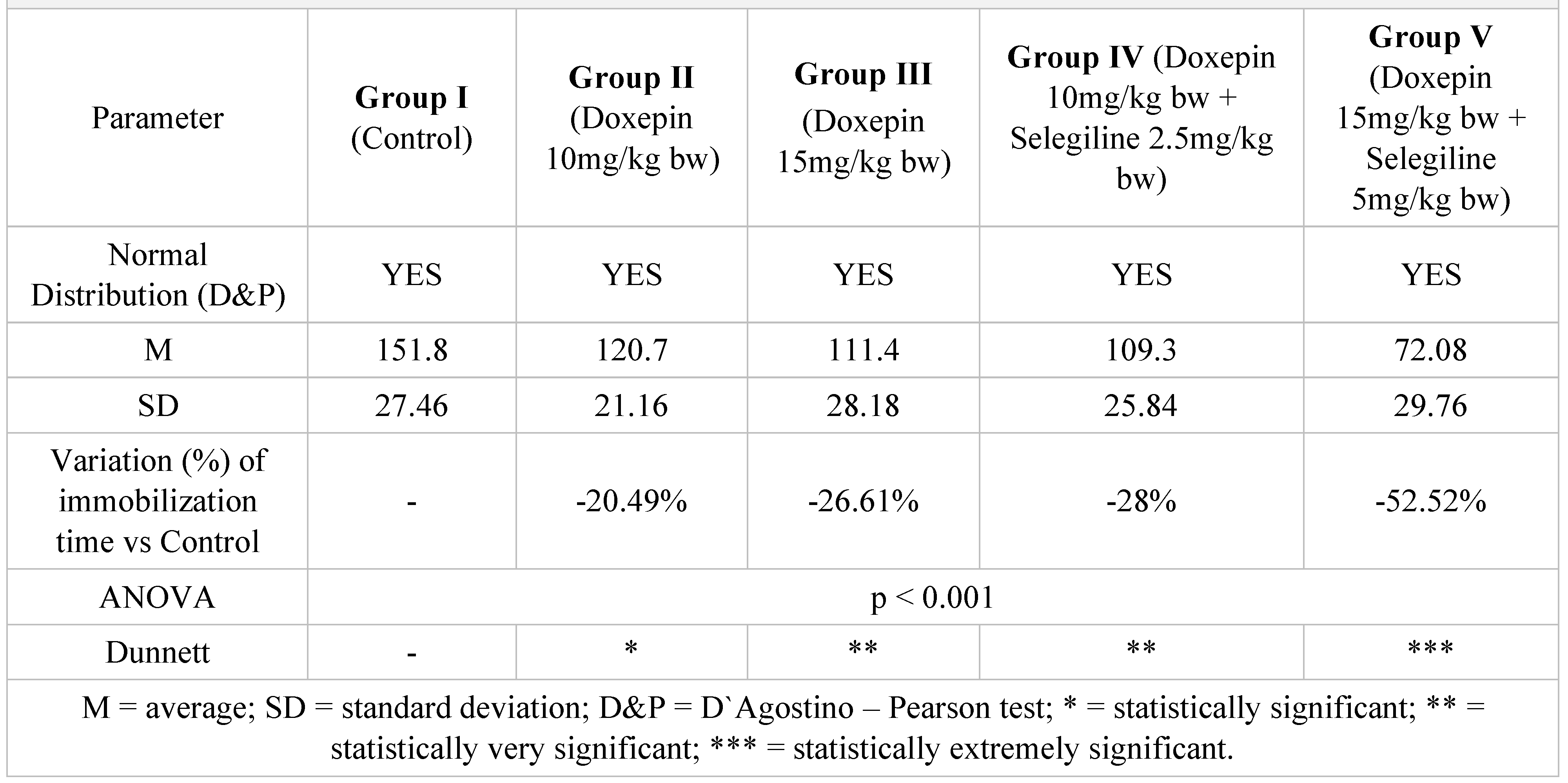 |
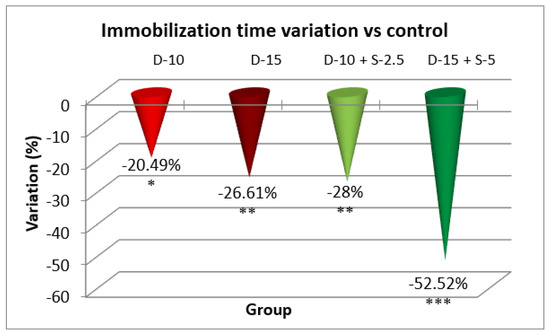
Figure 3.
The evaluation of the antidepressant effect in TST after 7 days of treatment.
Figure 3.
The evaluation of the antidepressant effect in TST after 7 days of treatment.
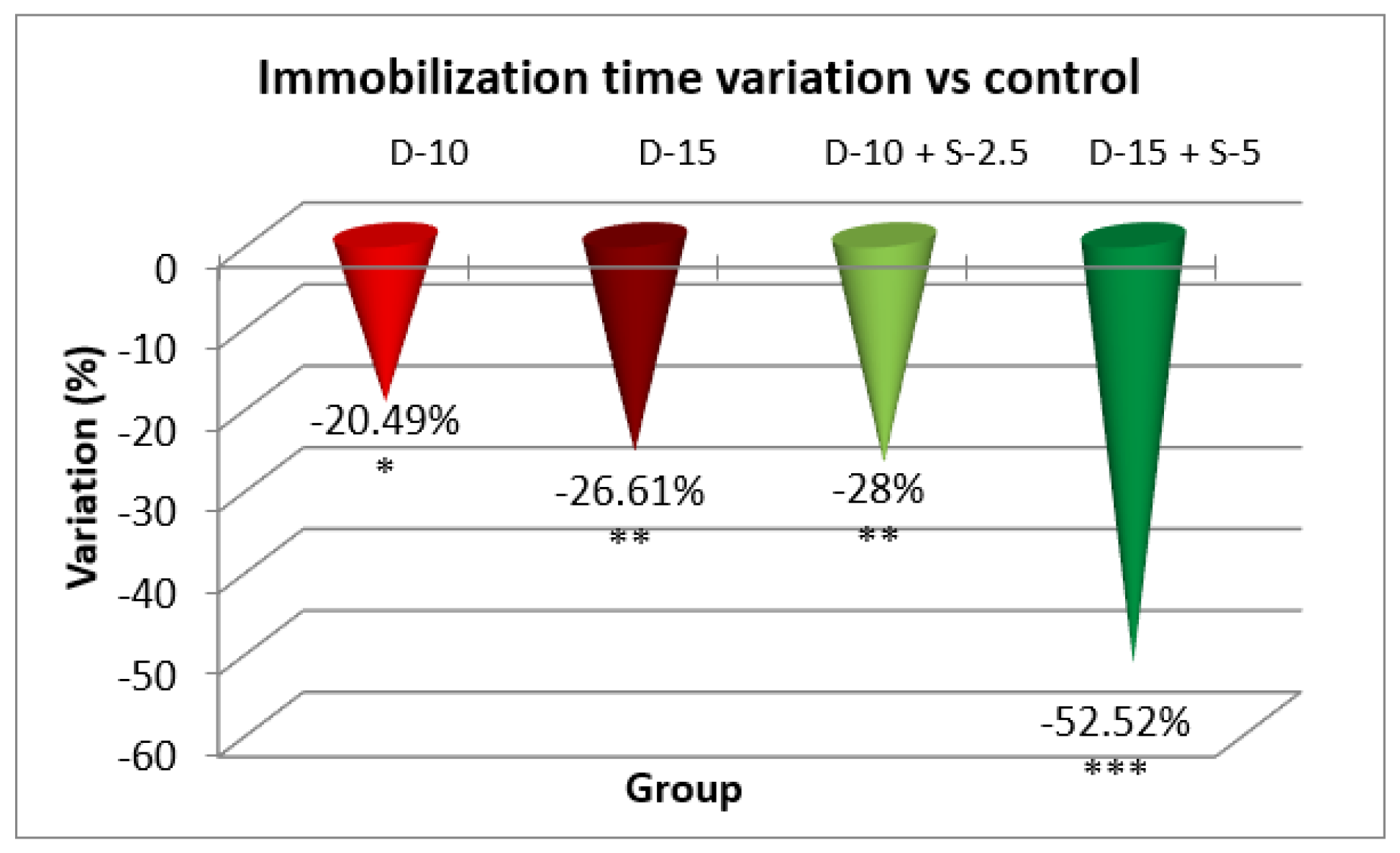
In the immobilization test, after 7 days of treatment, the antidepressant effect of the doxepin + selegiline combination in high doses compared to control is more potent than the single drug doxepin therapy, as is shown by statistical significance.
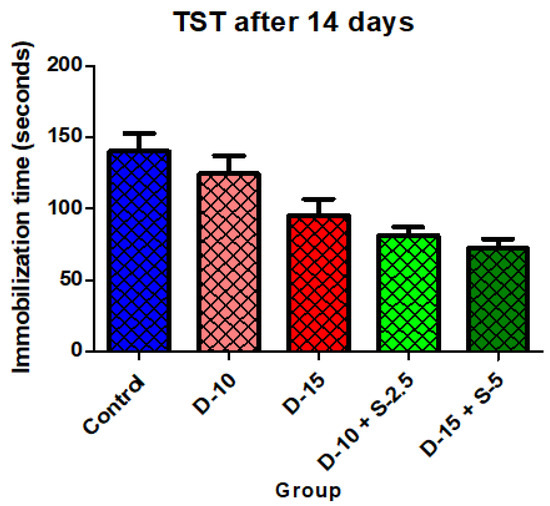
Figure 4.
Immobilization time + SD in TST after 14 days of treatment.
Figure 4.
Immobilization time + SD in TST after 14 days of treatment.
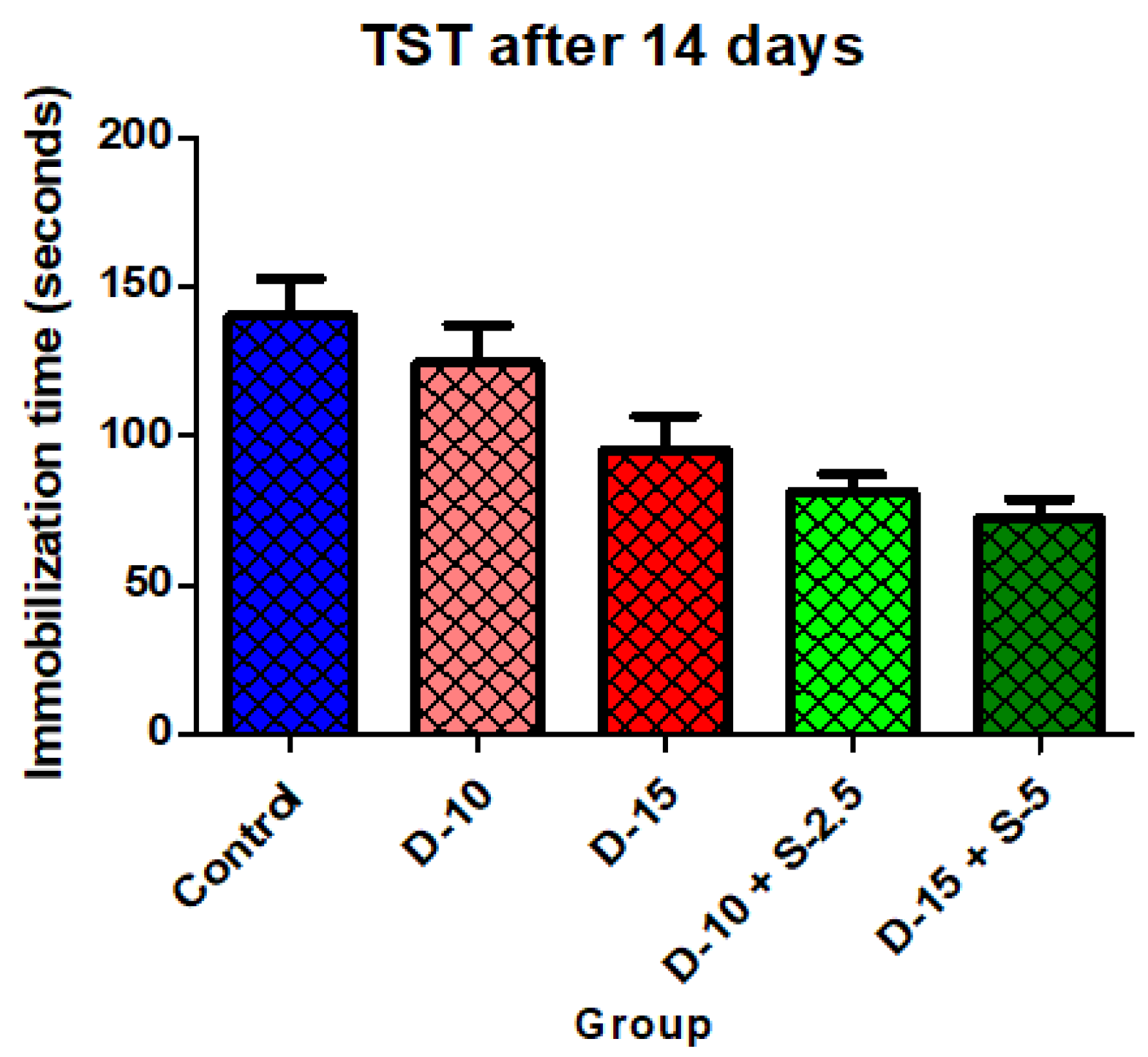

Table 3.
Immobilization time results in tail suspension test after 14 days of treatment.
Table 3.
Immobilization time results in tail suspension test after 14 days of treatment.
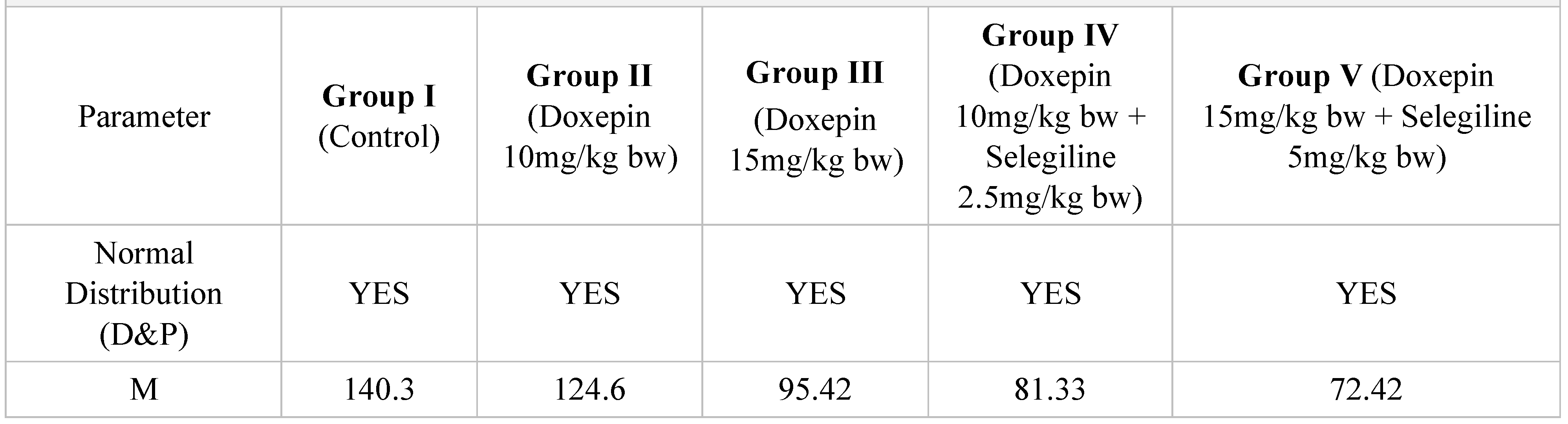 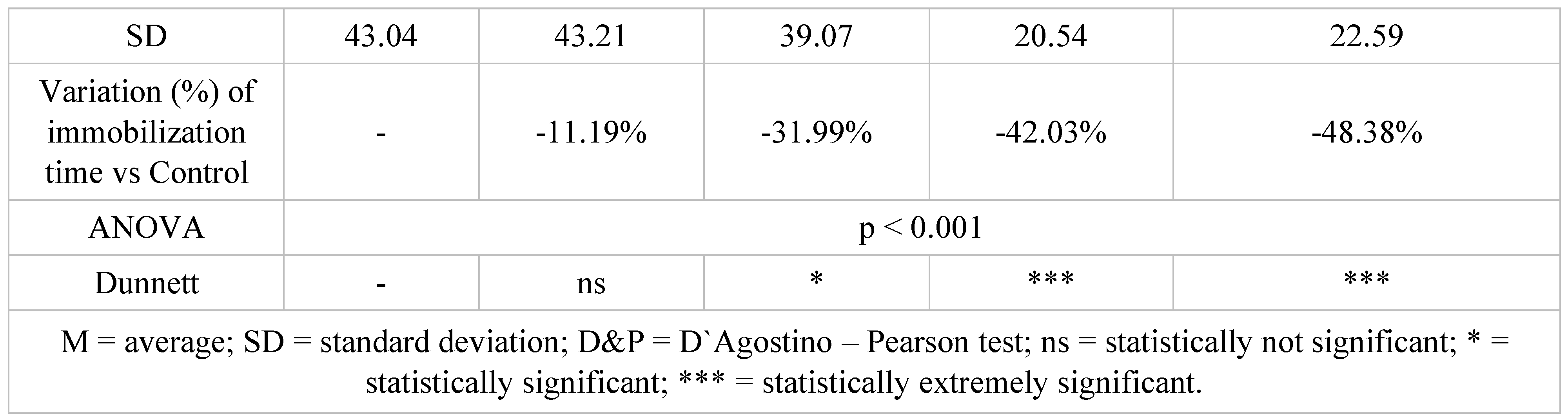 |
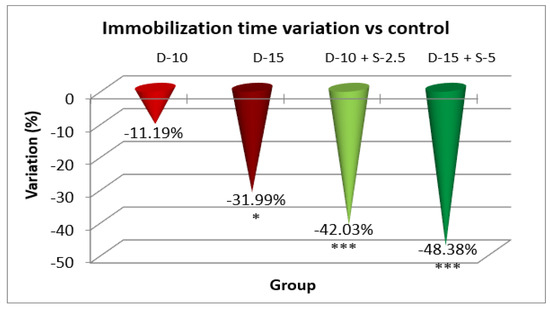
Figure 5.
The evaluation of the antidepressant effect in TST after 14 days of treatment.
Figure 5.
The evaluation of the antidepressant effect in TST after 14 days of treatment.
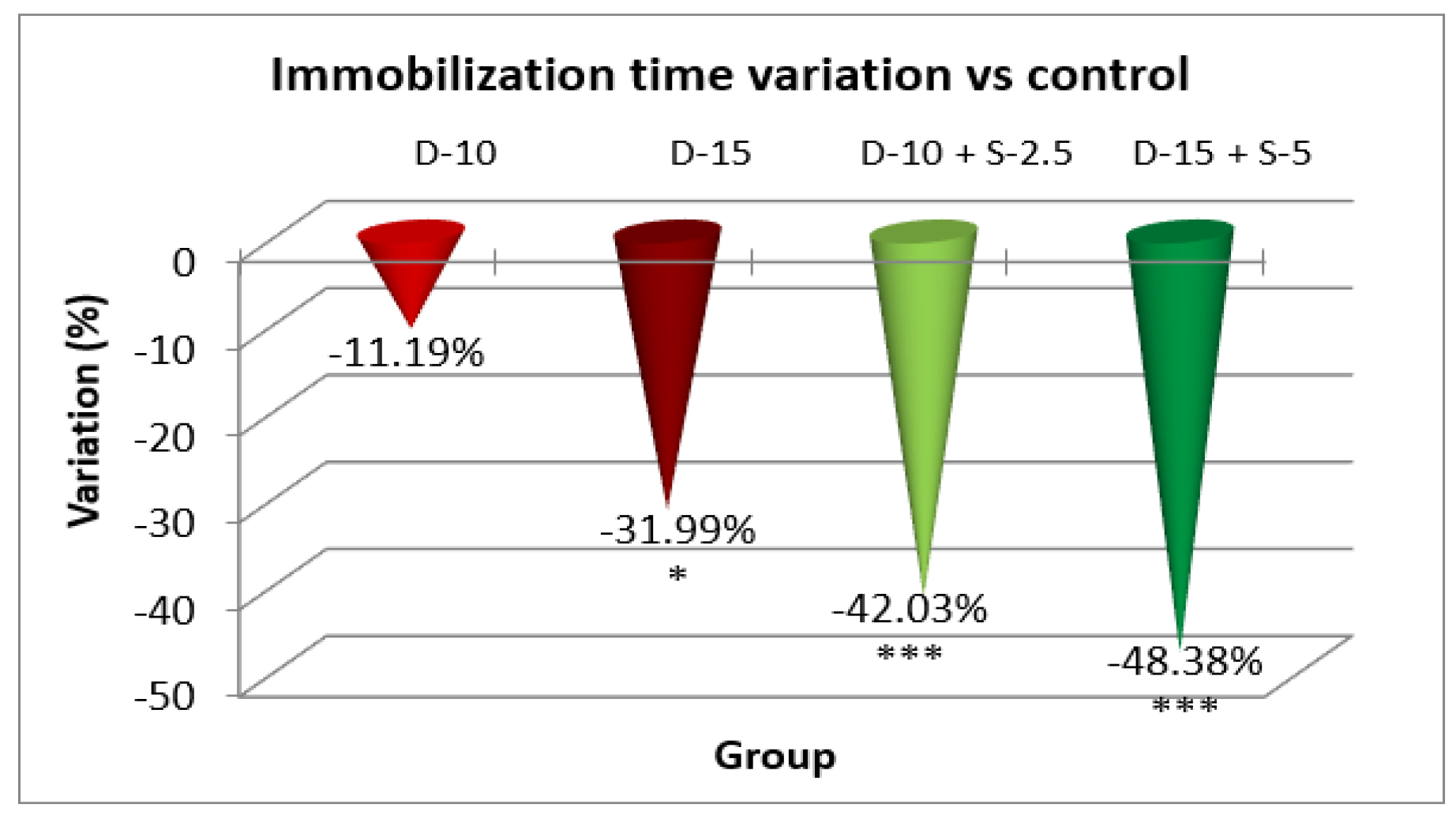
After a two-week treatment period, the effectiveness of combining selegiline with doxepin intensifies in both groups, while the group treated with a low doxepin dose tends to become less effective compared to the control group. The statistical significance of the immobilization time decrease was noticeably stronger in the groups treated with the two combined antidepressants.
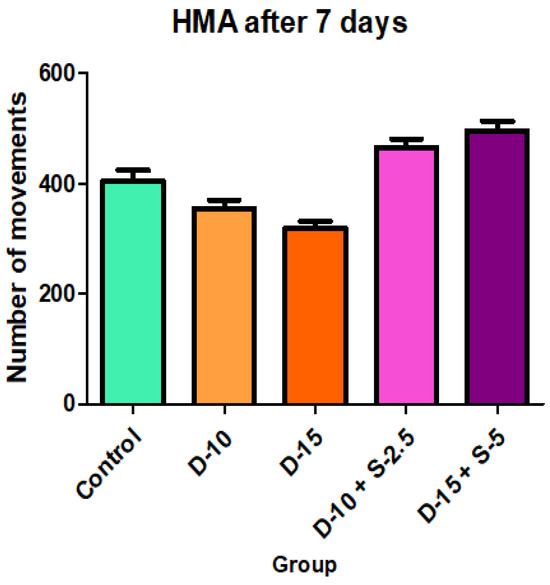
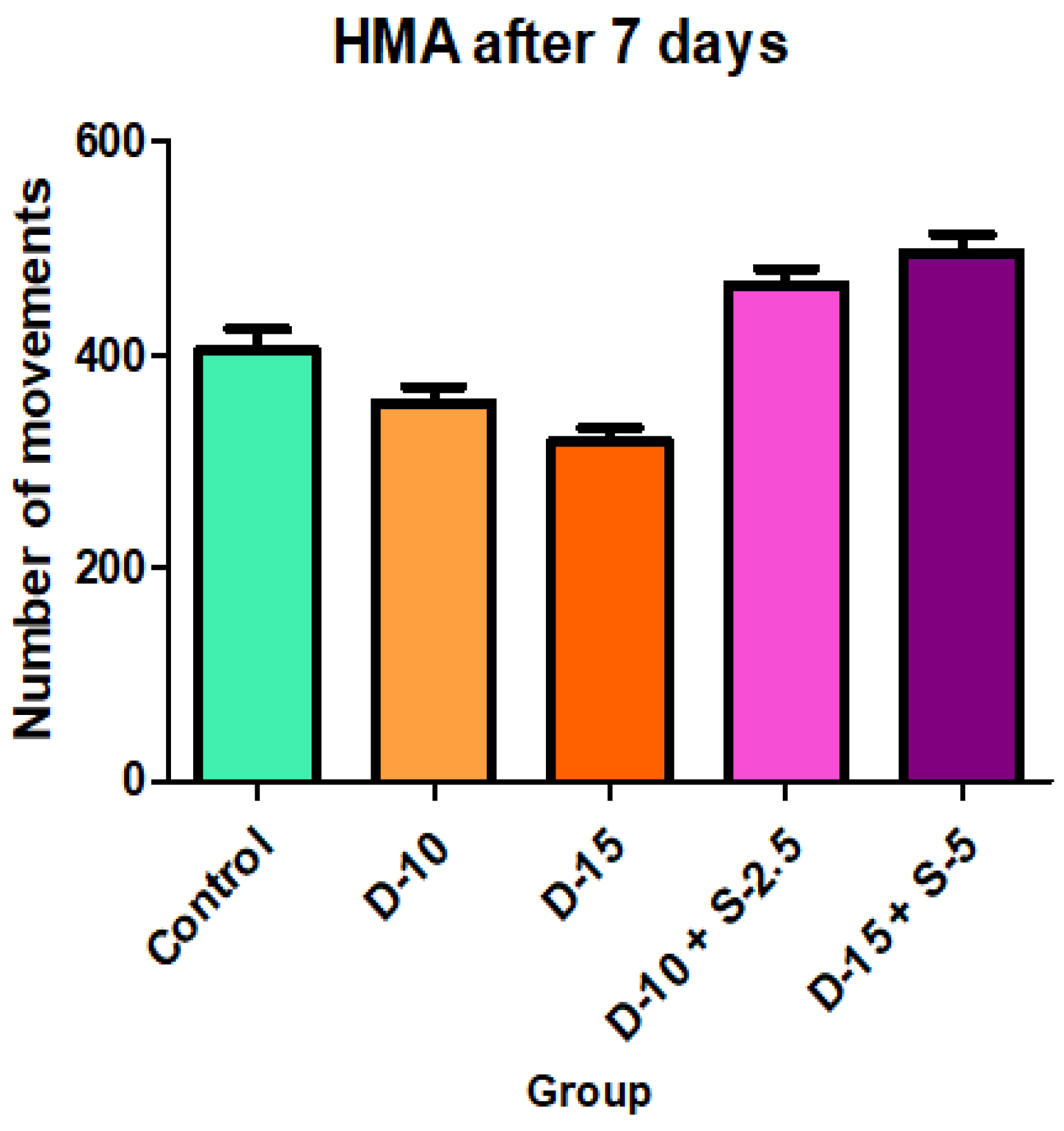
Figure 6.
Horizontal motor activity + SD after 7 days of treatment.
Figure 6.
Horizontal motor activity + SD after 7 days of treatment.
- 2.
- Motor behavior

Table 4.
Horizontal motor activity in 5 minutes after a 7-day treatment.
Table 4.
Horizontal motor activity in 5 minutes after a 7-day treatment.
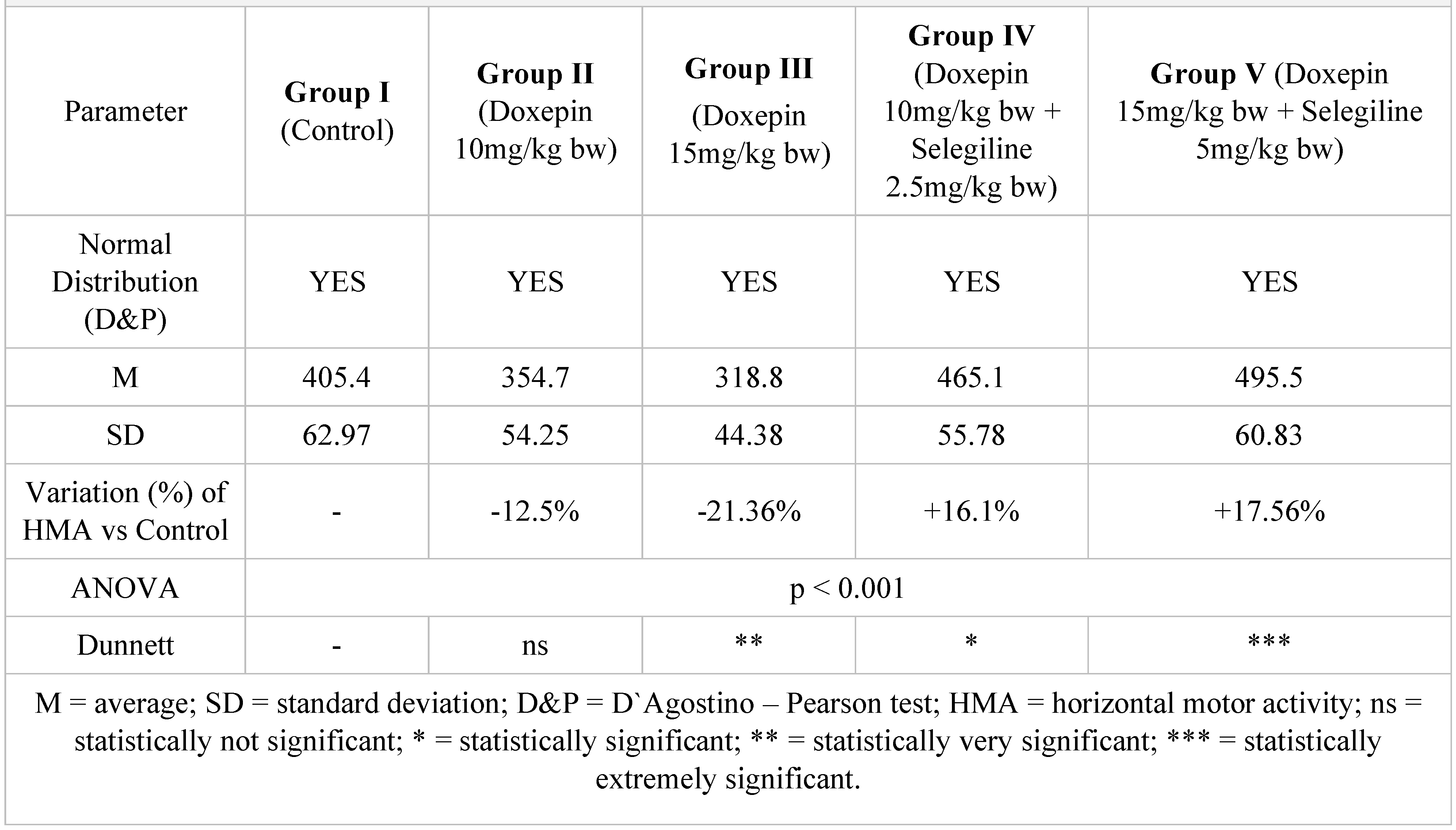 |
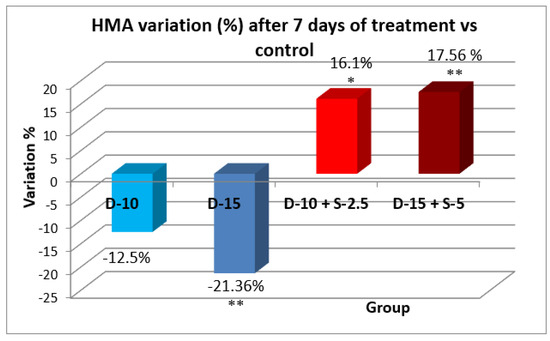
Figure 7.
Evolution of the horizontal motor activity after one week compared to the control group.
Figure 7.
Evolution of the horizontal motor activity after one week compared to the control group.
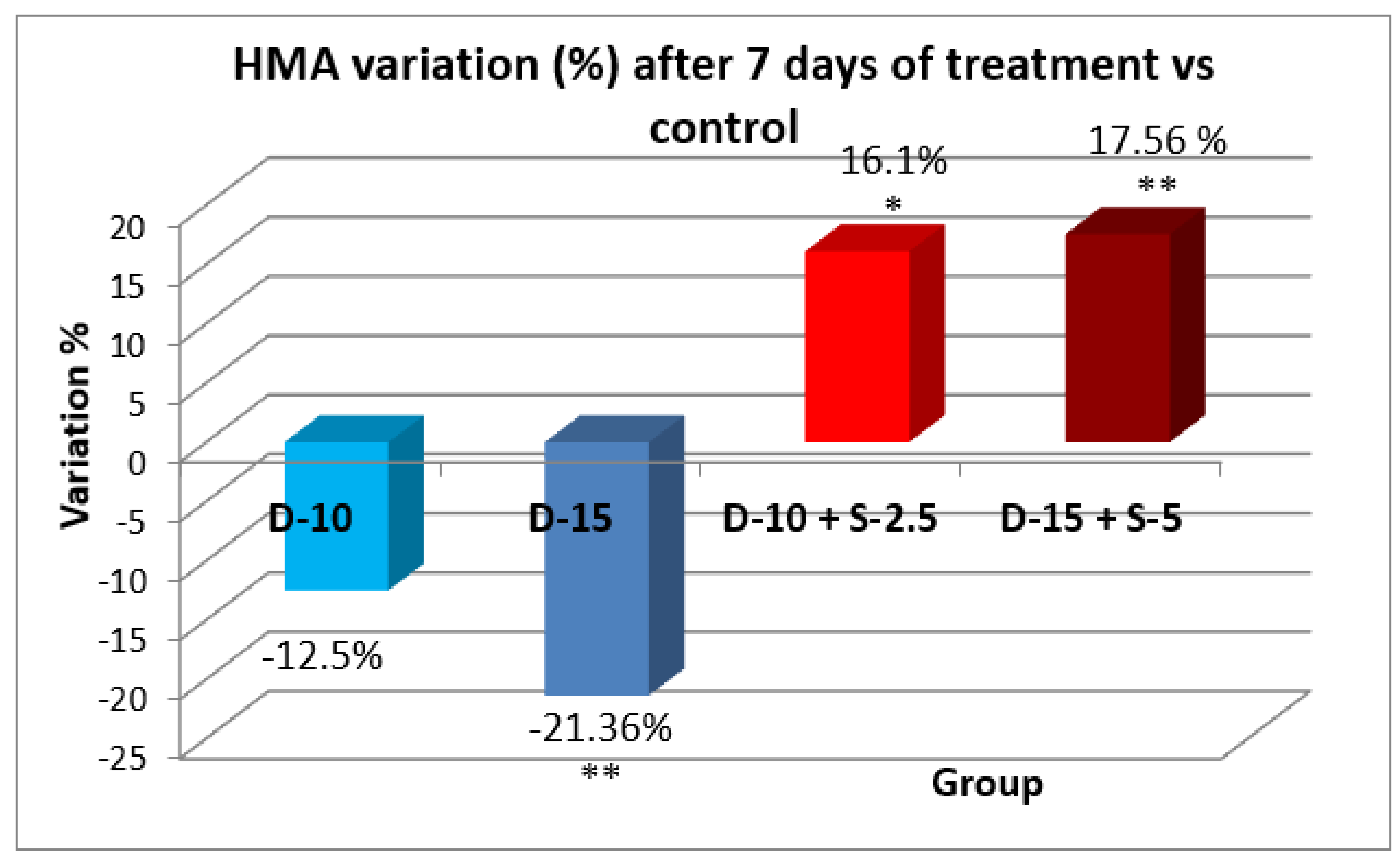
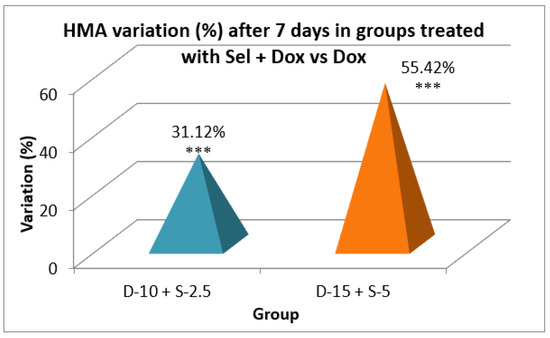
Figure 8.
Evolution of the horizontal motor activity after one week in the groups treated with the combination of selegiline + doxepin versus groups treated only with doxepin (*** - p<0.001 in t-Student test).
Figure 8.
Evolution of the horizontal motor activity after one week in the groups treated with the combination of selegiline + doxepin versus groups treated only with doxepin (*** - p<0.001 in t-Student test).
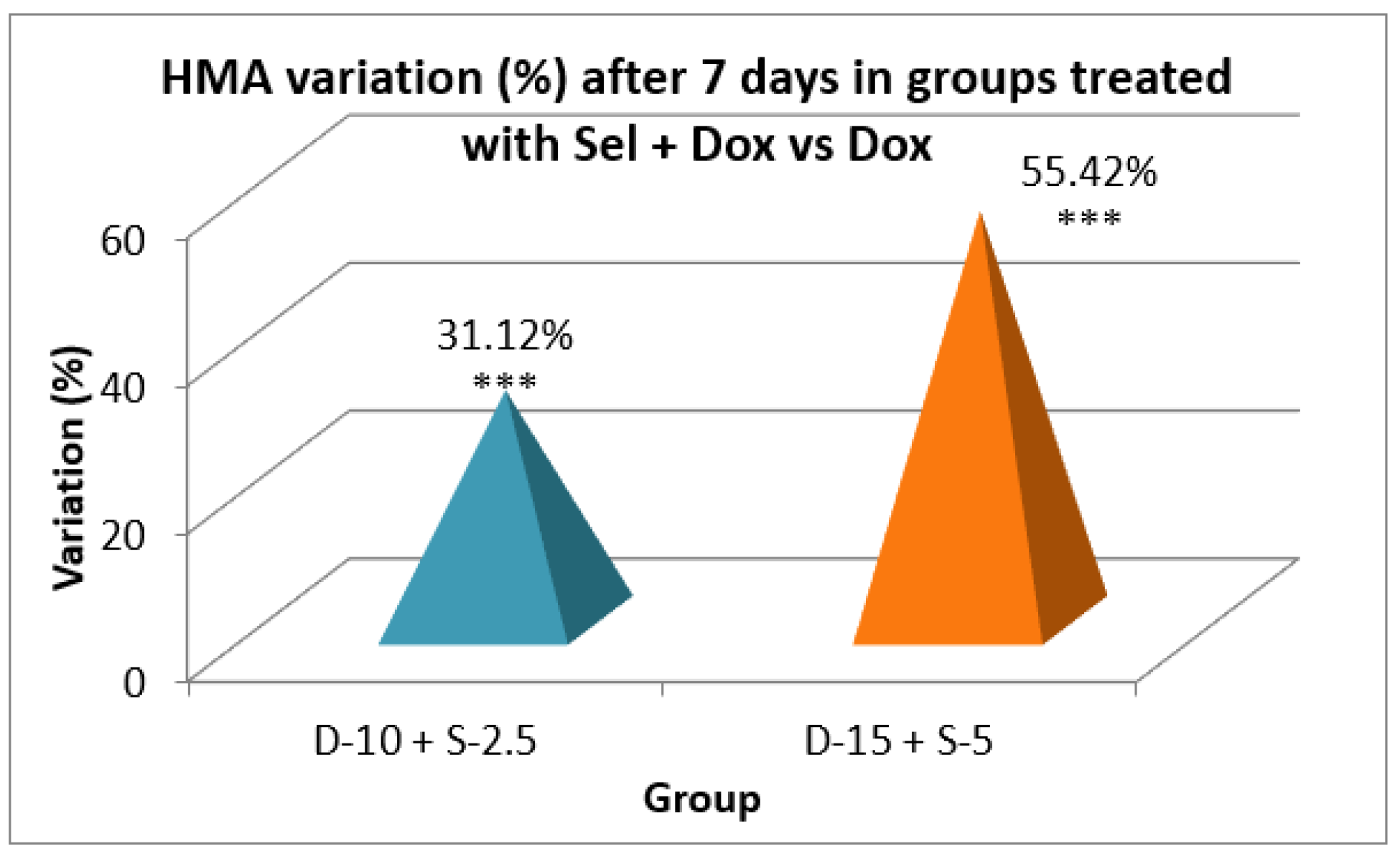

Table 5.
Vertical motor activity in 5 minutes after a 7-day treatment.
Table 5.
Vertical motor activity in 5 minutes after a 7-day treatment.
 |
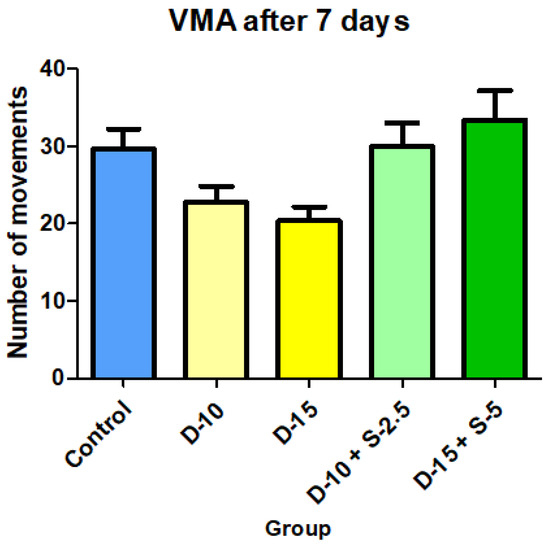
Figure 9.
Vertical motor activity + SD after 7 days of treatment.
Figure 9.
Vertical motor activity + SD after 7 days of treatment.
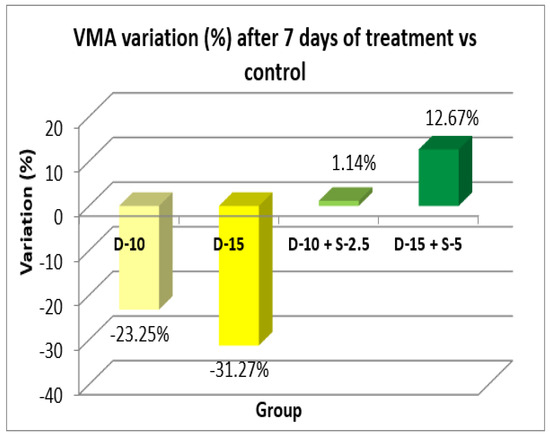
Figure 10.
Evolution of the vertical motor activity after one week compared to the control group.
Figure 10.
Evolution of the vertical motor activity after one week compared to the control group.
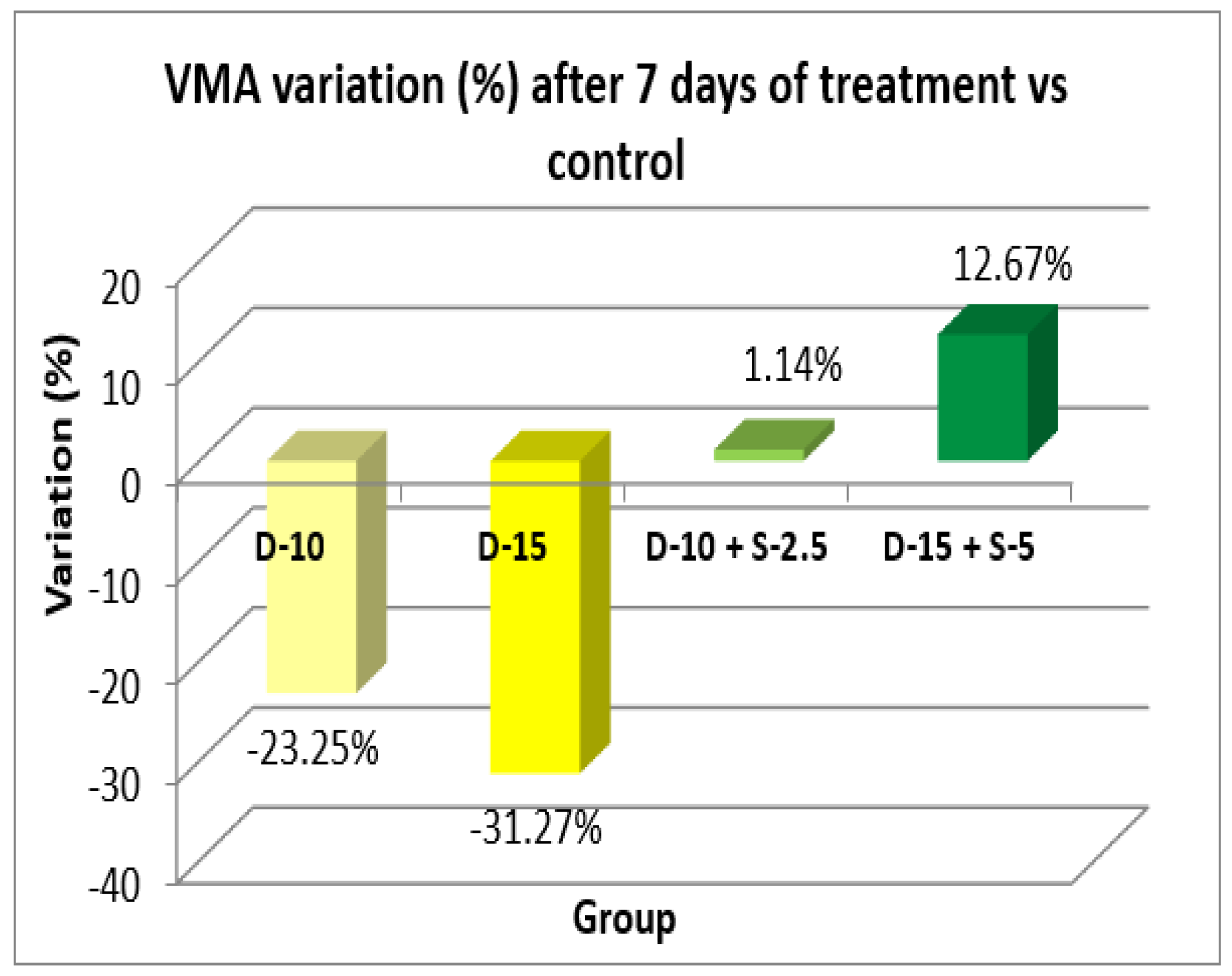
After one week of treatment, both the horizontal and the vertical motor activity decreases in the groups treated only with doxepin in a dose-dependent manner, which was expected due to its known sedative profile. However, this phenomenon is completely annulled, even reversed, in a statistically significant way (in case of HMA) for the groups treated with the doxepin + selegiline combination compared to the control group.

Table 6.
Horizontal motor activity in 5 minutes after a 14-day treatment.
Table 6.
Horizontal motor activity in 5 minutes after a 14-day treatment.
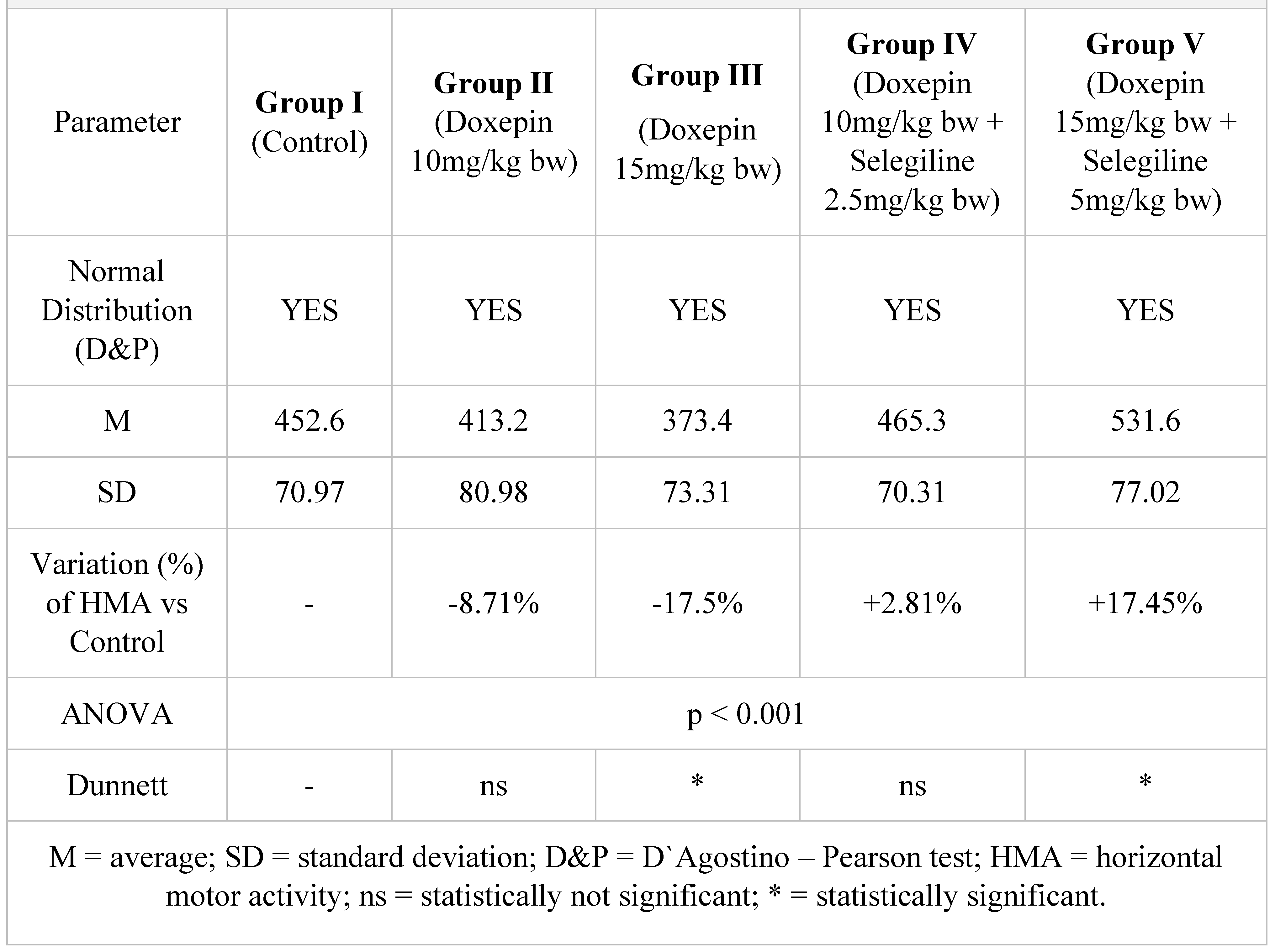 |
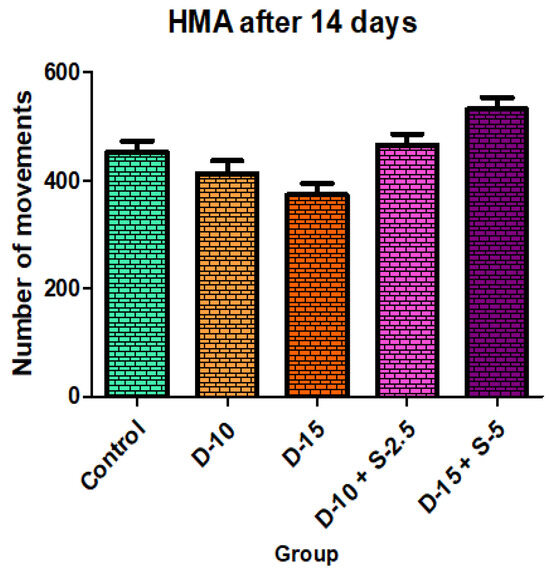
Figure 11.
Horizontal motor activity + SD after 14 days of treatment.
Figure 11.
Horizontal motor activity + SD after 14 days of treatment.
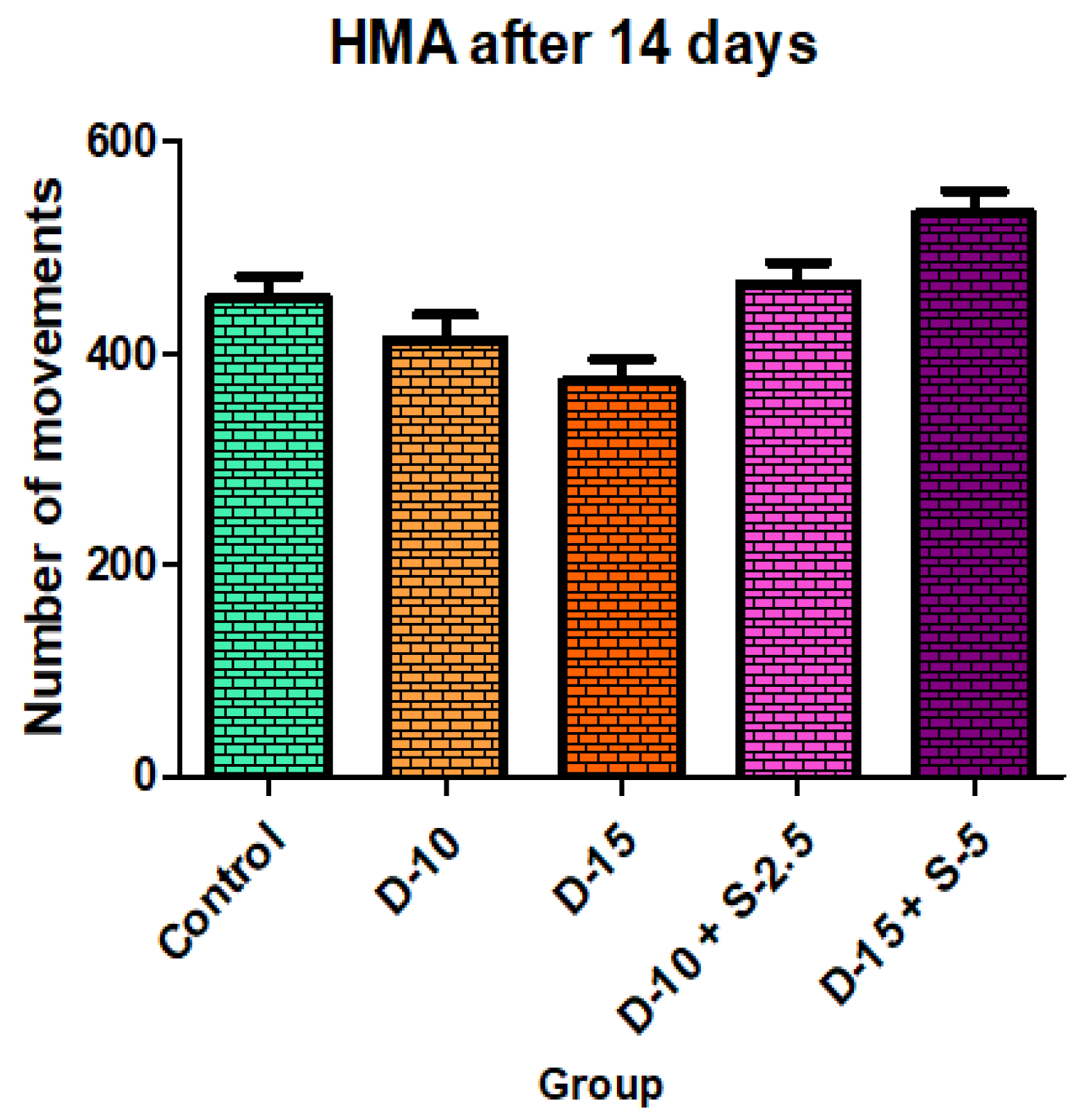
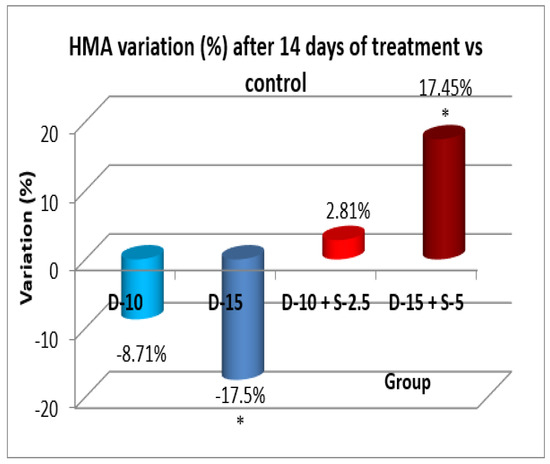
Figure 12.
Evolution of the horizontal motor activity after two weeks compared to the control group.
Figure 12.
Evolution of the horizontal motor activity after two weeks compared to the control group.
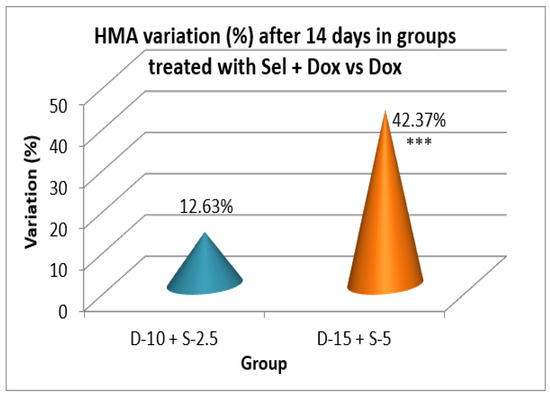
Figure 13.
Evolution of the horizontal motor activity after two weeks in the groups treated with the combination of selegiline + doxepin versus groups treated only with doxepin (*** -.p<0.001 in t-Student test)
Figure 13.
Evolution of the horizontal motor activity after two weeks in the groups treated with the combination of selegiline + doxepin versus groups treated only with doxepin (*** -.p<0.001 in t-Student test)
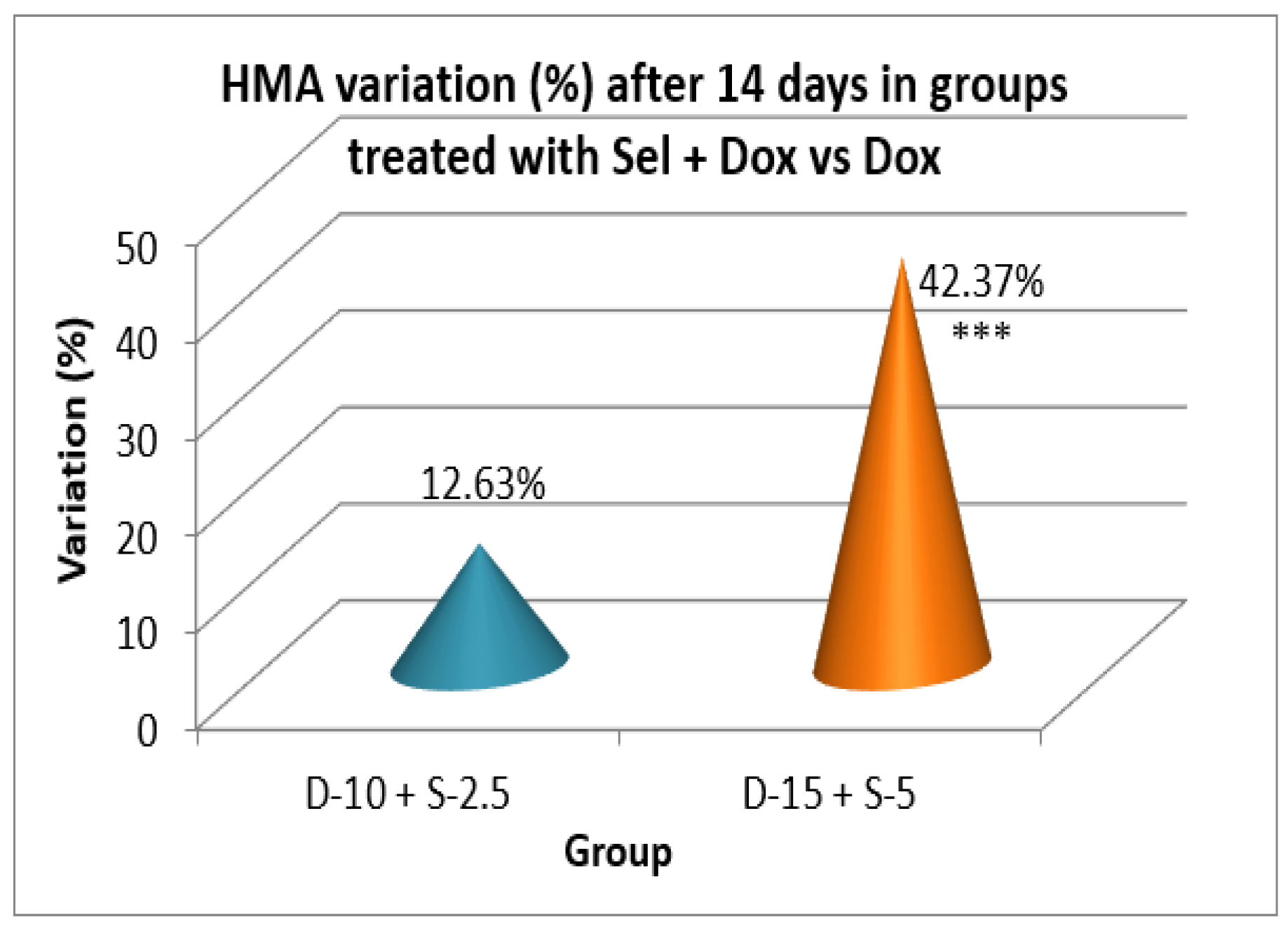
Figure 14.
Vertical motor activity + SD after 14 days of treatment.
Figure 14.
Vertical motor activity + SD after 14 days of treatment.
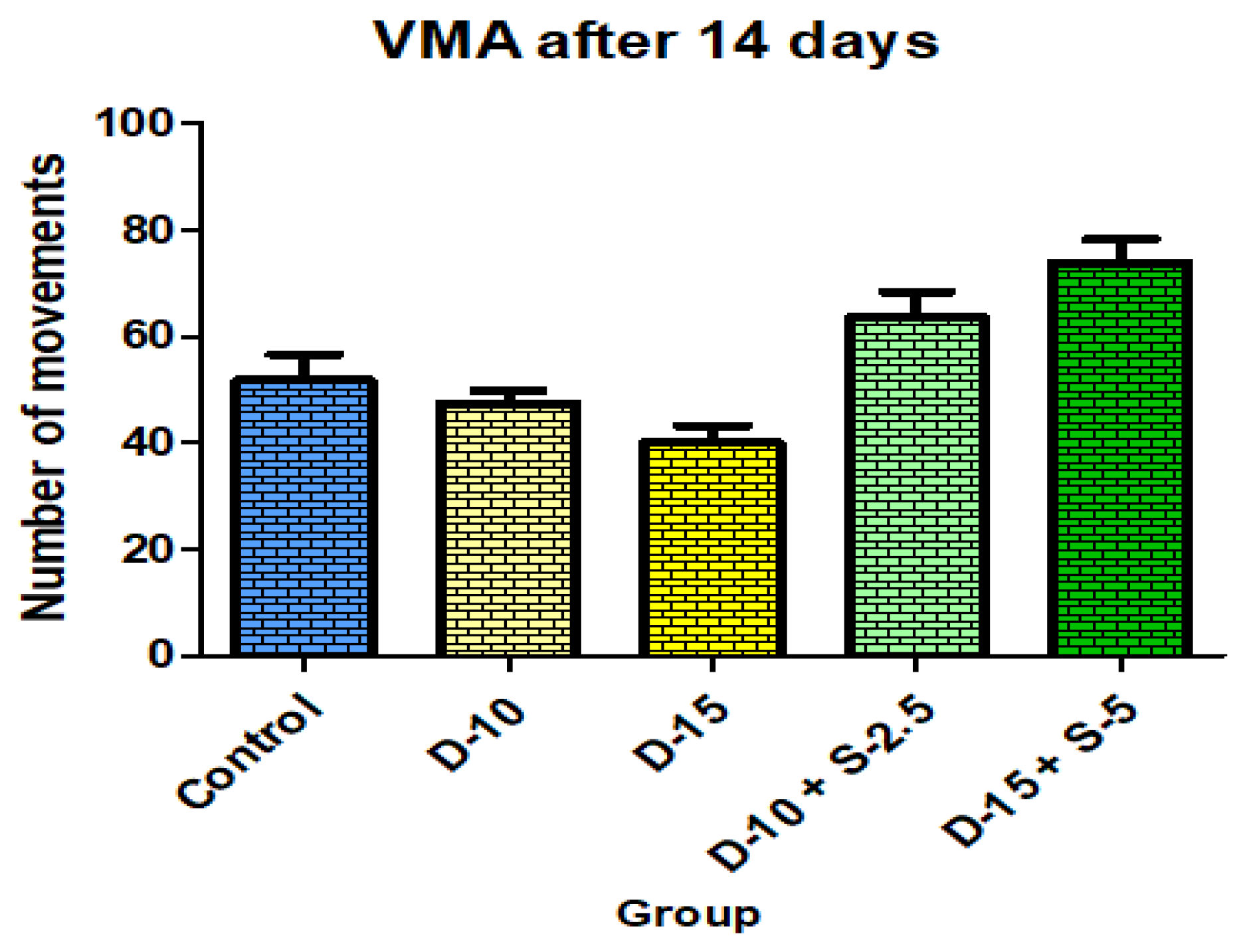

Table 7.
Vertical motor activity in 5 minutes after a 14-day treatment.
Table 7.
Vertical motor activity in 5 minutes after a 14-day treatment.
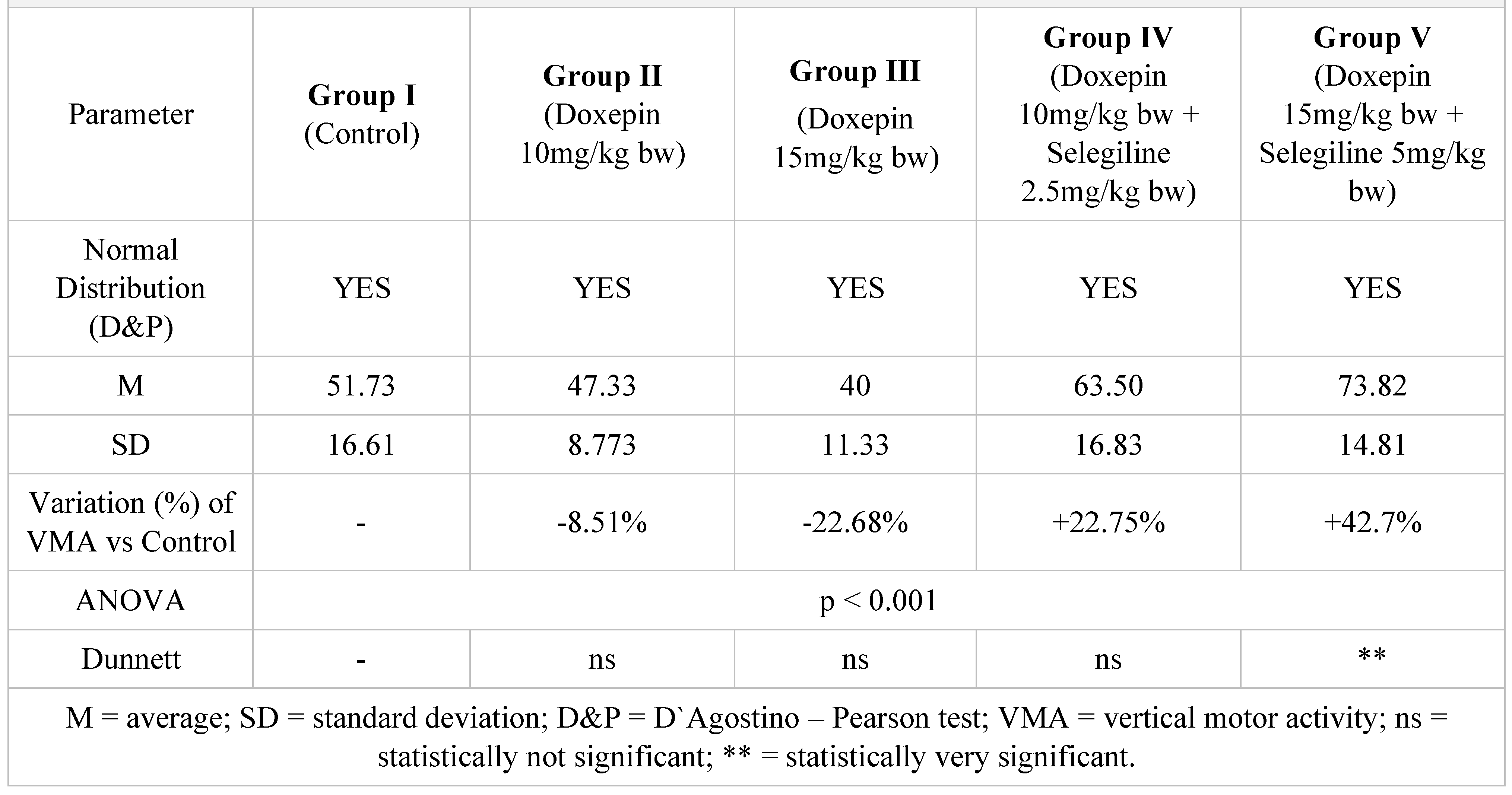 |
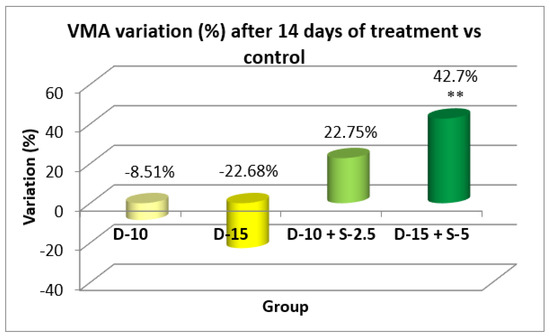
Figure 15.
Evolution of the vertical motor activity after two weeks compared to the control group.
Figure 15.
Evolution of the vertical motor activity after two weeks compared to the control group.
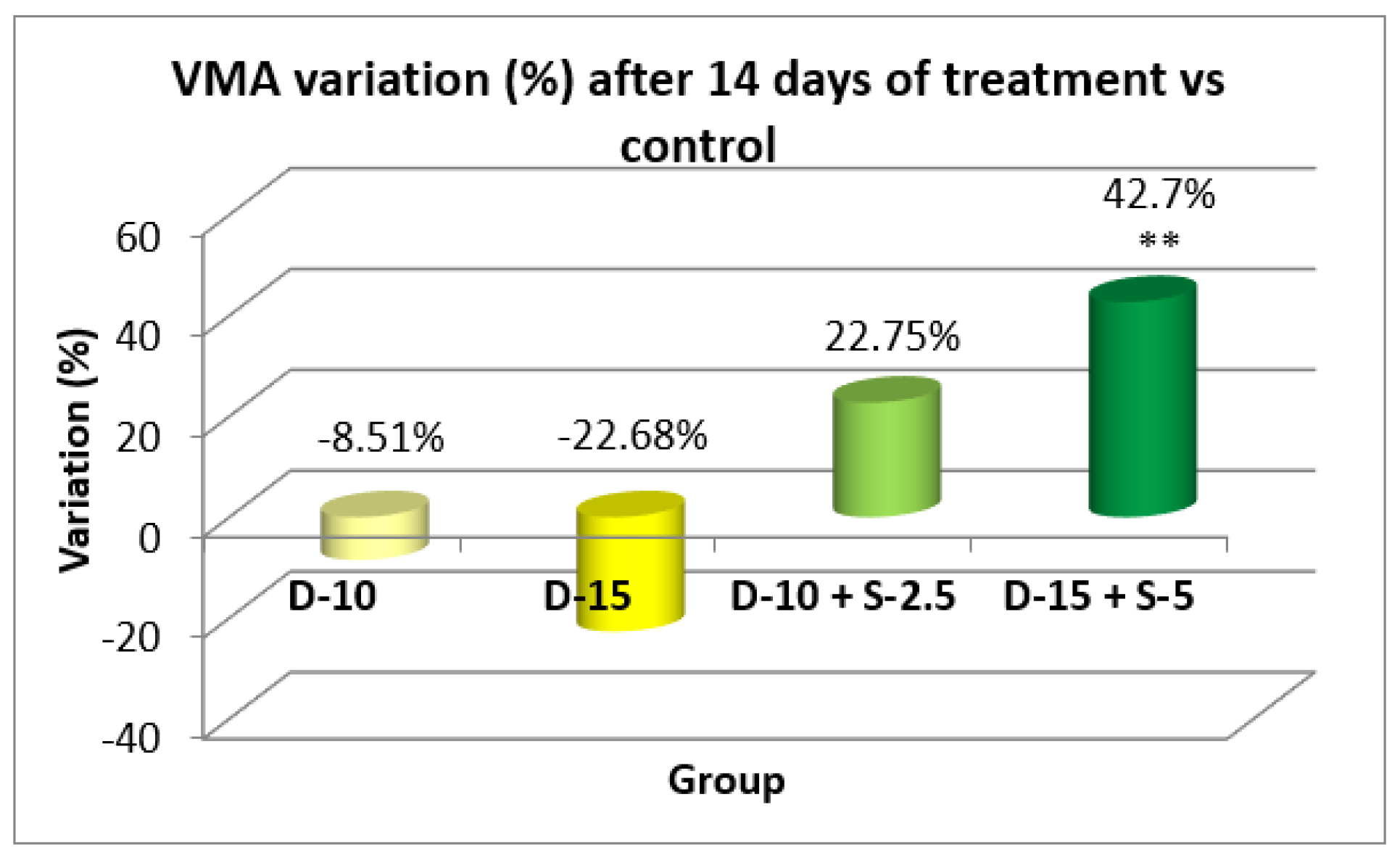
After two weeks of treatment, the increase of HMA in the groups treated with the selegiline + doxepin combination compared to the control group is maintained, although the statistical significance is lower than the one registered after the first week of treatment. VMA intensifies after 14 days of treatment in the same groups compared to control, versus the 7-day determination. The sedative effect of doxepin administered by itself decreases in the 2nd week compared to the first, which is consistent with existing data in the scientific literature regarding the use of doxepin in long time treatments
Conclusions
Selegiline in association with doxepin induced a slight reduction in the immobilization time, compared to doxepin administered alone, in FST after the acute treatment.
After a 7-day treatment, the most intense anti- depressant effect compared to the control group was registered in the groups treated with doxepin 10 mg/kg bw + selegiline 2.5 mg/kg bw (-28%; p<0.01) and doxepin 15 mg/kg bw + selegiline 5 mg/kg bw (-52.52%; p<0.001). Comparing the single drug doxepin therapy with the combined doxepin + selegiline therapy, the antidepressant effect almost doubled when using the combination in higher dosages.
After a 14-day treatment, the most intense antidepressant effect compared to the control group continued to register in the groups treated with doxepin 10 mg/kg bw + selegiline 2.5 mg/kg bw (-42.03%; p<0.001) and doxepin 15 mg/kg bw + selegiline 5 mg/kg bw (- 48.38%; p<0.001). Comparing the single drug doxepin therapy with the combined doxepin + selegiline therapy, the combination of the two antidepressants continued to be more effective, although the difference in intensity between the effect of the single drug therapy and that of the double drug therapy decreased, especially in the case of higher dosages.
Assessing the motor activity registered after 7 and 14 days of treatment, selegiline not only canceled the sedative effect of doxepin, but to some extent, reversed it. This phenomenon is more obvious and significant in HMA than in VMA.
Based on our analysis, we conclude that a selegiline + doxepin combination therapy not only significantly increases the antidepressant effect, but also prevents the sedative side effect of doxepin administered as a single drug therapy.
Conflicts of Interest
There are no known conflicts of interest in the publication of this article. The manuscript was read and approved by all authors.
Compliance with ethical standards
Any aspect of the work covered in this manuscript has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
References
- Friedrich, M.J. Depression Is the Leading Cause of Disability Around the World. JAMA. 2017, 317, 1517. [Google Scholar] [CrossRef] [PubMed]
- Schildkraut, J.J.; Kopin, I.J.; Schanberg, S.M.; Durell, J. Norepinephrine Metabolism and Psychoactive Drugs in the Endogenous Depressions. Pharmacopsychiatry. 1968, 1, 69–92. [Google Scholar] [CrossRef]
- Heninger, G.R.; Delgado, P.L.; Charney, D.S. The Revised Monoamine Theory of Depression: A Modulatory Role for Monoamines, Based on New Findings from Monoamine Depletion Experiments in Humans. Pharmacopsychiatry. 1996, 29, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Hillhouse, T.M.; Porter, J.H. A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp Clin Psychopharmacol. 2015, 23, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Shulman, K.I.; Herrmann, N.; Walker, S.E. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs. 2013, 27, 789–797. [Google Scholar] [CrossRef]
- Miklya, I. The significance of selegiline/(-)-deprenyl after 50 years in research and therapy (1965-2015). Mol Psychiatry. 2016, 21, 1499–1503. [Google Scholar] [CrossRef]
- Magyar, K. The pharmacology of selegiline. Int Rev Neurobiol. 2011, 100, 65–84. [Google Scholar] [CrossRef] [PubMed]
- Chiriță, C.; Cioroianu, D.M.; Chiriță, I.C.; Negreș, S.; Marian, B.; Zbârcea, C.E. Synthesis and pharmacological activity of new acyloximines derivatives. Farmacia. 2016, 64, 61–66. [Google Scholar]
- Chiriță, C.; Ștefănescu, E.; Marineci, C.D.; Negreș, S.; Nuță, D.C. Experimental pharmacological research regarding some newly synthesized benzamides on central nervous system functions. J Mind Med Sci. 2017, 4, 148–155. [Google Scholar] [CrossRef]
- Chiriță, C.; Ștefănescu, E.; Zbârcea, C.E.; Negreș, S.; Bratu, M.; Nuță, D.C.; Limban, C.; Chiriță, I.C.; Marineci, C.D. Experimental pharmacological research regarding some new quinazolin-4-ones derivatives. J Mind Med Sci. 2019, 6, 121–129. [Google Scholar] [CrossRef]
- Ștefănescu, E.; Scutari, C.; Păunică, I.; Junghină, A. Experimental pharmacological research regarding the potential antidepressant activity induced by some newly synthesized dibenzo [a,d] cycloheptene compounds. J Mind Med Sci. 2015, 2, 62–75. [Google Scholar] [CrossRef]
- Inoue, T.; Tsuchiya, K.; Miura, J.; Sakakibara, S.; Denda, K.; Kasahara, T.; Koyama, T. Bromocriptine treatment of tricyclic and heterocyclic antidepressant-resistant depression. Biol Psychiatry. 1996, 40, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Gangopadhyay, A.; Malakar, J.; Ghosh, A.; Deb, J.; Dey, S.; Datta, S.; Datt, P.K. The Central Nervous System Activity of Barleria prionitis Linn. on the Locomotor Activity of Swiss Albino Mice using Actophotometer. Int J Pharm Biol Sci Arch. 2012, 3, 403–405. [Google Scholar]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977, 229, 327–336. [Google Scholar] [PubMed]
- Lucki, I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997, 8, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Negreș, S.; Zbârcea, C.E.; Arsene, A.; Chiriță, C.; Buzescu, A.; Velescu, B.Ș.; Ștefănescu, E.; Șeremet, O.C.; Nicolescu, F. Experimental pharmacological model for inducing and quantifying depression in mouse. Farmacia. 2013, 61, 1102–1116. [Google Scholar]
- Perrault, G.; Morel, E.; Zivkovic, B.; Sanger, D.J. Activity of litoxetine and other serotonin uptake inhibitors in the tail suspension test in mice. Pharmacol Biochem Behav. 1992, 42, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The tail suspension test. J Vis Exp. 2012, 59, e3769. [Google Scholar] [CrossRef]
© 2019 by the author. 2019 Cornel Chiriță, Emil Ștefănescu, Cristina E. Zbârcea, Horațiu Mireșan, Simona Negreș, Diana C. Nuță, Carmen Limban, Rucsandra E. Dănciulescu Miulescu, Cristina D. Marineci
