Highlights
- Patients with ADHD have significantly higher scores from the EDE-Q- eating concern, EDE-Q- shape concern and all CPRS-RSF subscales than individuals without ADHD.
- EDE-Q shape concern and CPRS-RSF subscale scores seem to be correlated with inattention and cognitive problems.
Highlights
- Patients with ADHD have significantly higher scores from the EDE-Q- eating concern, EDE-Q- shape concern and all CPRS-RSF subscales than individuals without ADHD.
- EDE-Q shape concern and CPRS-RSF subscale scores seem to be correlated with inattention and cognitive problems.
Abstract
Objectives. This study aims to evaluate the effects of methylphenidate (MPH) on eating patterns and body mass index (BMI) in children with attention deficit/hyperactivity disorder (ADHD). The secondary aim of this study is the comparison between weight and eating behavior of children with ADHD undergoing an MPH treatment, and of children without ADHD. Methods. One hundred fourty three children and adolescents who diagnosed with ADHD were enrolled, and the effects of MPH on the eating patterns and BMI were evaluated. All participants completed a number of tests to analyze eating patterns and clinical psychopathological profiles. Results. Children and adolescents with ADHD had significantly higher scores on the EDE-Q- eating concern, EDE-Q- shape concern, and all CPRS-RSF subscales than individuals without ADHD (p < 0.05). MPH treatment was associated with a notional reduction in height-sds and weight-sds. The results of the correlation analysis which assessed the possible contribution of the different treatment-related factors revealed no significant correlations between MPH mean dose [mg/(kg/d)], the duration of use (months), and the core characteristics of eating disorders except the restraint subscale of EDE Q. Conclusions. Our findings add to the growing research suggesting that MPH may be associated with disordered eating behaviors. Although the literature is limited, our findings conclude that MPH may not be associated with the reduction of growth velocity and disordered eating behaviors.
Introduction
Attention Deficit Hyperactivity Disorder (ADHD) is defined as a neurodevelopmental disorder that reflects the persistence of ADHD symptoms such as inattention, overactivity, and impulsivity across the lifespan [1]. Children with ADHD are at elevated risk of comorbid psychopathology such as mood disorders, anxiety disorders, substance use disorders, learning disorders, and conduct disorders [2]. Some studies also suggest a possible link between ADHD and an increased risk of eating disorders, specifically that eating disorders and ADHD may share common clinical features and that ADHD rates may increase eating disorders and/or contribute to the seriousness of pathological eating behaviors [3].
Biederman et al. (2010) reported that girls treated for ADHD in childhood and adolescence were 3.5 times more likely to be diagnosed with eating disorders than girls without ADHD in young adulthood (95% CI: 1.6-7.3) [4]. Similarly, Yoshimasu et al. found that children with ADHD were 5.7 times more likely to have eating disorders than those without ADHD (95% CI: 1.1-28.2) in late adolescence, based on data from a population-based birth cohort [5]. Childhood ADHD symptoms have been associated with the development of irregular eating behaviors including bulimic symptoms, binge eating, and restrictive eating in the present and subsequent period [6,7]. However, in ADHD studies with both subjective and objective eating disorder examination instruments, consistent results are lacking, although it should be noted that the effects of long-term methylphenidate (MPH) on both appetite and weight have been minimally studied. A recently published, double-blind, drug-placebo, cross-over design trial found improvements, using MPH compared to placebo, in rates of binge eating cessation [8].
Decreased appetite is the most frequent adverse effect of stimulants, but it is not necessarily related to a decrease in height and BMI. The association between stimulants and a delay in growth is still unclear and controversial. Although a recent review and meta-analysis reported the prevalence of obesity in children/adolescents with ADHD is 40% higher than in healthy children/adolescents, a notable side effect of MPH is thought to be growth delay [9].
This study evaluated the effects of MPH on eating patterns in Turkish children and adolescents with ADHD and also in healthy controls. The secondary aim of this study was the comparison of weight and eating behavior patterns between children with ADHD and MPH treatment and children without ADHD. In this context, we hypothesized that (I) MPH and ADHD would show a negative effect on eating patterns, and (II) would be associated with reduction of height, weight, and body mass index (BMI) in Turkish children and adolescents.
Materials and Methods
Study setting and subjects
We reviewed the medical records of 152 children and adolescents (aged 6-18 years) with ADHD who received treatment with MPH for at least 1 year at the Department of Child and Adolescent Psychiatry at Dokuz Eylül University Medical School. The exclusion criteria via medical records included (I) positive history of diseases that can suppress growth, (II) past and/or current history of autistic spectrum disorders or mental retardation, (III) past and/or current history of epilepsy, brain injury, and cerebral palsy, and (IV) use of chronic medications which could affect growth (e.g. cortisol, stimulants, mood stabilizers). Nine participants with missing or erroneous entries in the data collection instruments were excluded from the study. 144 children and adolescents (aged 6-18 years) who were brought to our pediatric outpatient clinic by parents for causes such as headaches or acute infections, but did not meet any diagnostic criteria, formed the healthy sample group. Data from both groups—287 cases—were analyzed after approval was obtained from the The Dokuz Eylul University Ethics Committee. After participiants were informed about the aim and method of the research, written consent was obtained.
Height, weight, and BMI measurements of ADHD cases were obtained from hospital records. Participants diagnosed with K-SADS-PL by blinded professionals completed a data form containing questions regarding sociodemographic and clinical features (data about MPH treatment by age, duration of MPH treatment etc.), Wechsler Intelligence Scale for Children-Revised (WISC- R) and Conners Parent Rating Scale-Revised Short Form (only for ADHD cases to support the diagnosis), and The Eating Disorder Examination Questionnaire (EDE-Q) (all participants). Weight, height, and BMI z-scores (age- and gender- adjusted) were collected at baseline and final follow-up. We also recorded weight, height, and BMI z- scores of the healthy sample group [10] for statistical comparisons.
Assessment instruments
Sociodemographic Data Form: This form obtained information about age, gender, education, family type, socioeconomic level, home conditions, status of parents, background, and family history.
Conners Parent Rating Scale-Revised Short Form (CPRS-RSF): This form is widely used for the assessment of the prevalence of ADHD and its effect on diagnosis and treatment. Studies on the new version are described in the U.S. and Canada. The validity and reliability study of the scale were assessed by Kaner (2013) [11].
Eating Disorder Examination Questionnaire (EDE-Q): The EDE-Q is the self-report version of the Eating Disorder Assessment Interview [12]. In Turkish psychometric evaluation, the internal consistency coefficient was .93 and the test-retest reliability was .91 [13].
Stastistical Analysis
Differences in all study variables were analysed using SPSS (IBM, NY) version 22. The Shapiro–Wilk test was used initially to ascertain that variables met the conditions for parametric tests. Variables that did not show normal distribution were evaluated by the Mann-Whitney U test. In the interpretation of the variables, descriptive statistical techniques and quantitative data analyses were used. Chi- square analysis was used to compare categorical variables between groups. The Pearson correlation was used to determine the direction, level, and significance of correlations between the variables. P<0.05 was considered statistically significant.
Results
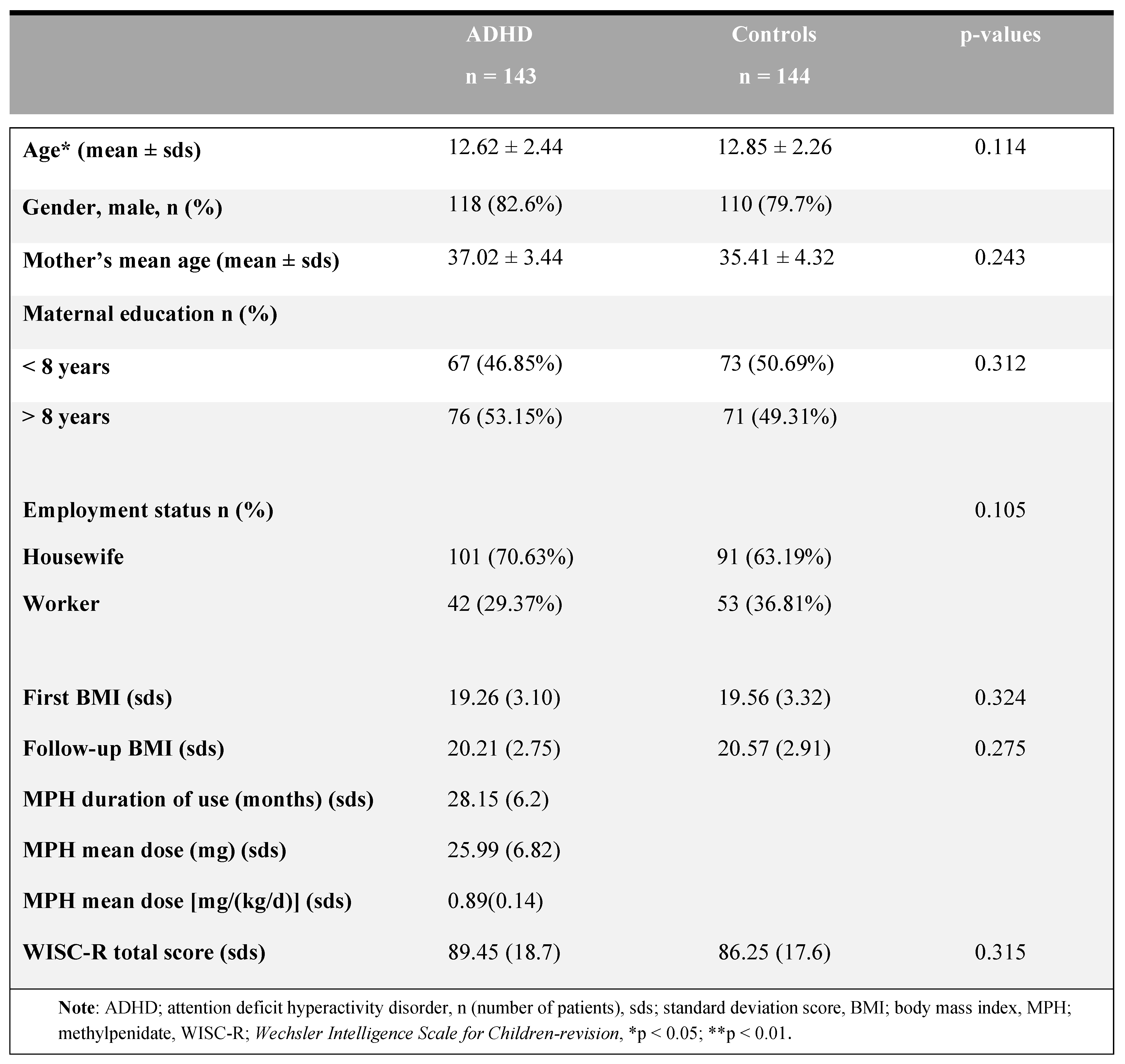
Table 1 summarizes the main features of the participants and the identification of the clinical characteristics between groups. The mean age of the patient group was 9.40 ± 2.60 and the mean age of the control group was 9.85 ± 2.26, with no difference between groups (t=1.544, p = 0.114). Table 1 summarizes the first and last follow-up visit BMIs, WISC-R scores, MPH duration of use (months), and MPH doses for ADHD cases. No differences between groups were reported in terms of sex, parental education level, and employment status (all groups).

Table 1.
The sociodemographic data of the ADHD patients and the control groups.
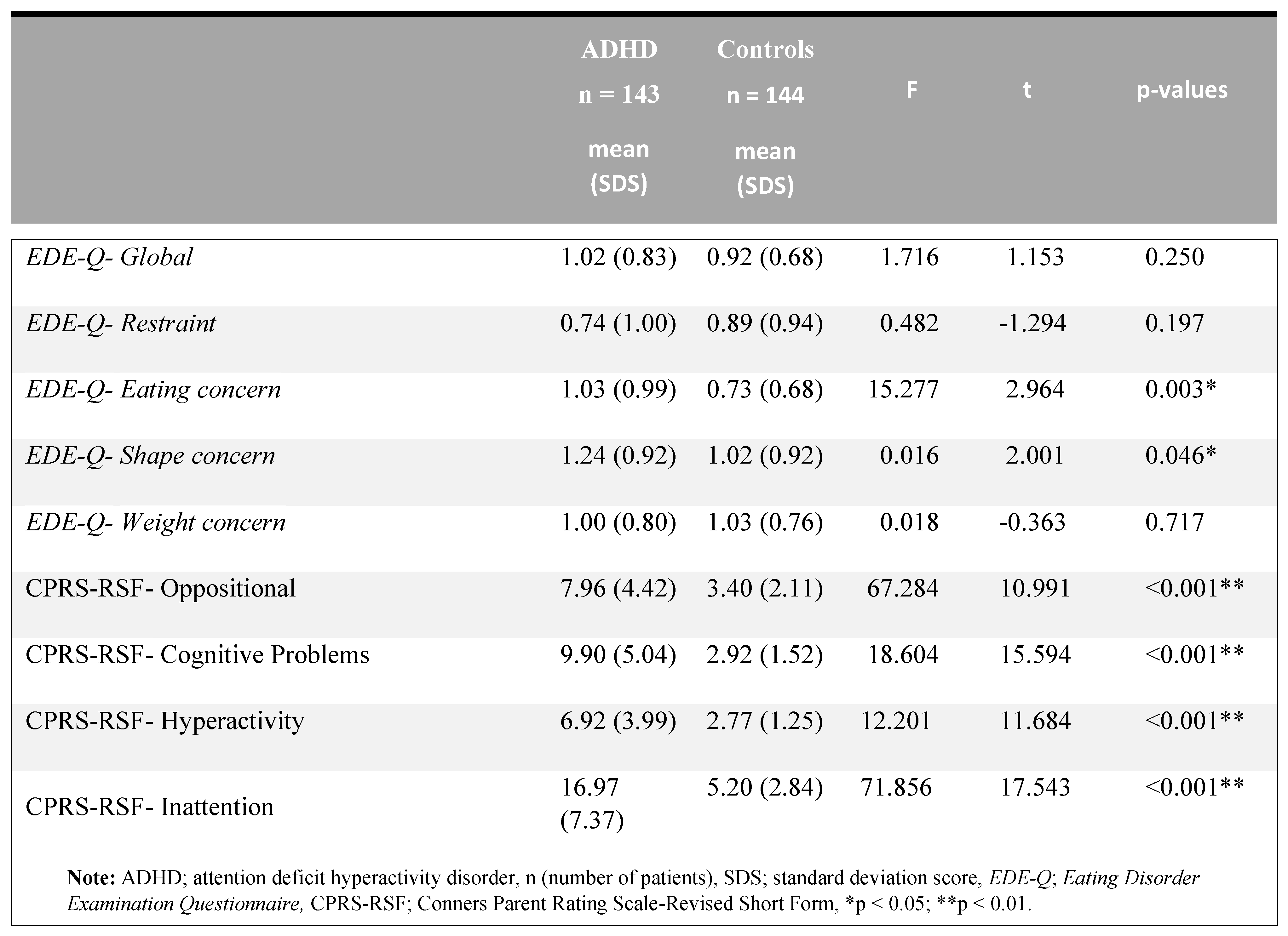
Table 2 summarizes the total and subscale scores of ADHD and control subjects on the Eating Disorder Examination Questionnaire (EDE-Q) and Conners Parent Rating Scale-Revised Short Form (CPRS-RSF). A significant difference was found between the two groups in terms of EDE-Q- eating concerns, EDE-Q- shape concern, and all CPRS-RSF subscales (p <.05). There were no differences in other subtests between EDE-Q- weight concern, EDE-Q- restraint, and EDE-Q- global score (p> 0.05).

Table 2.
Conners Parent Rating Scale-Revised Short Form (CPRS-RSF) scores of ADHD and the control group.
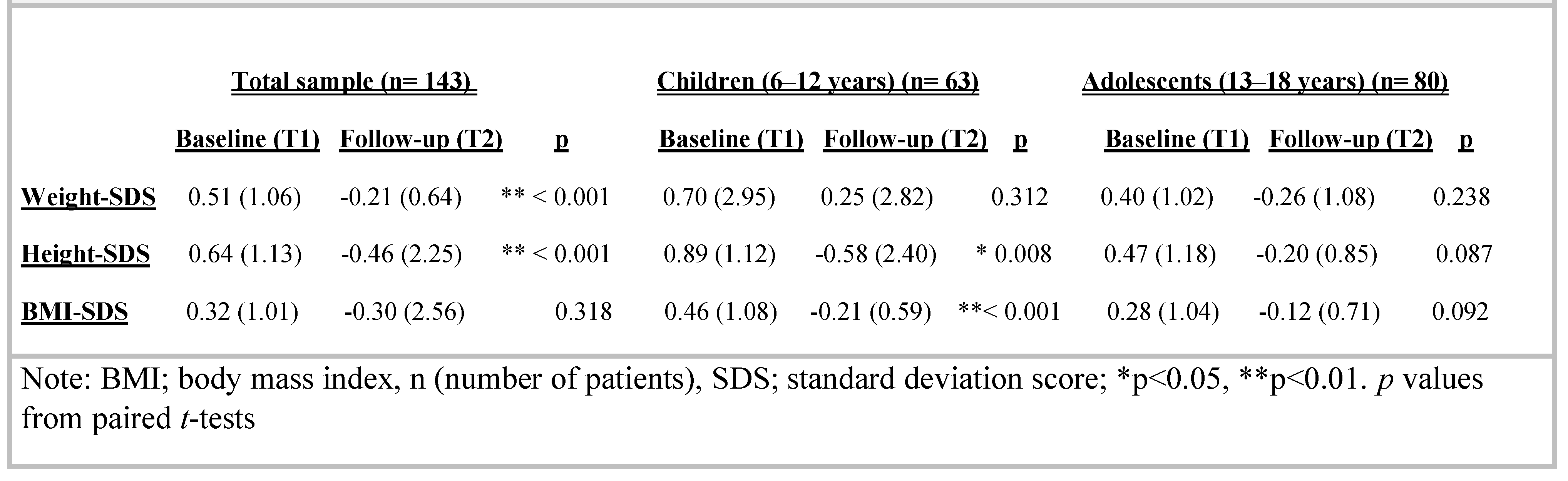
Considering the entire sample, weight-SDS significantly decreased at follow-up (baseline weight-SDS [SDS] 0.51 [1.06], follow-up: -0.21 [0.64]; p** < 0.001). Height-SDS [SDS] was also affected: 0.64 [1.13] at baseline and -0.46 (2.25) at follow-up (p** < 0.001). There were no significant differences in pre- and post-treatment BMI-SDS [SDS] z scores: baseline 0.32 [1.01], follow-up: -0.30 (2.56), p = 0.318. The effect of MPH on growth is described in detail in Table 3, including pre- and postdata on weight, height and BMI z-scores.

Table 3.
Comparison of height, weight and BMI at baseline and follow up data of Methylphenidate treatment by age.
However, considering whether the patients were children (6–12 years) or adolescents (13–18 years) when they began medication, in the group of children, height was slightly affected by the treatment (baseline height- SDS [SDS]: 0.89 [1.12]; follow-up: -0.58 [2.40]; p* = 0.008), but this effect was not observed when MPH began during adolescence. In those cases, height-SDS was slightly above the average at follow-up (baseline height- SDS [SDS]: 0.47 [1.18], follow-up: -0.20 [0.85]; p = 0.087).
In the group of MPH children, weight was not affected by the treatment (baseline weight-SDS [SDS]: 0.70 [2.95]; follow-up: 0.25 [2.82]; p = 0.312), and no effect was also observed when MPH was started during adolescence. In such cases, weight-SDS was slightly above the average at follow-up (baseline weight-SDS [SDS]: 0.40 [1.02], follow-up: -0.26 [1.08]; p = 0.238).
In the group of children, BMI was significantly affected by the treatment (baseline BMI-SDS [SDS]: 0.46 [1.08]; follow-up: -0.21 [0.59]; p** < 0.001). This effect was not observed when MPH began during adolescence. In such cases, BMI-SDS was slightly above the average at follow- up (baseline BMI-SDS [SDS]: 0.28 [1.04], follow-up: - 0.12 [0.71]; p = 0.092) (Table 3).
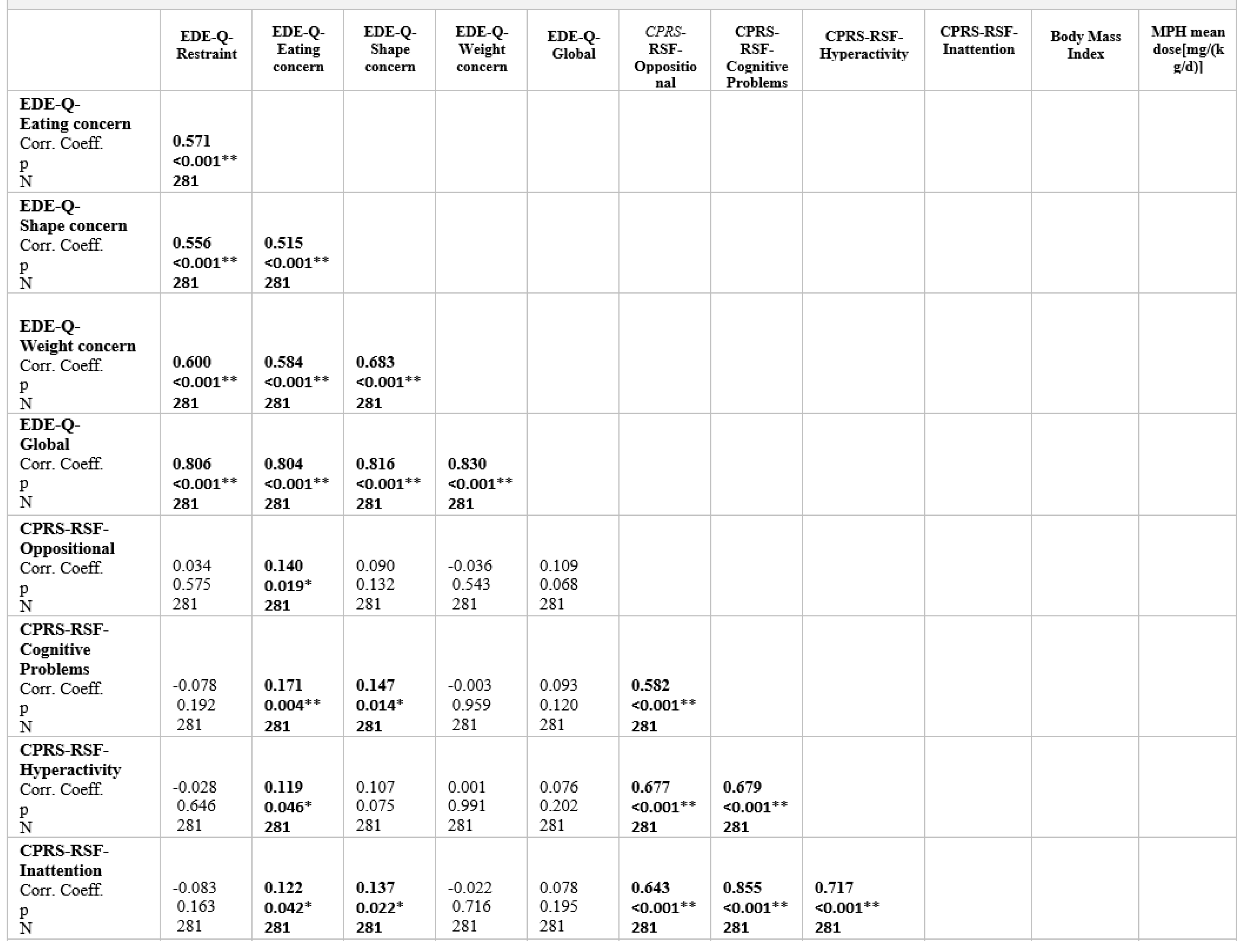
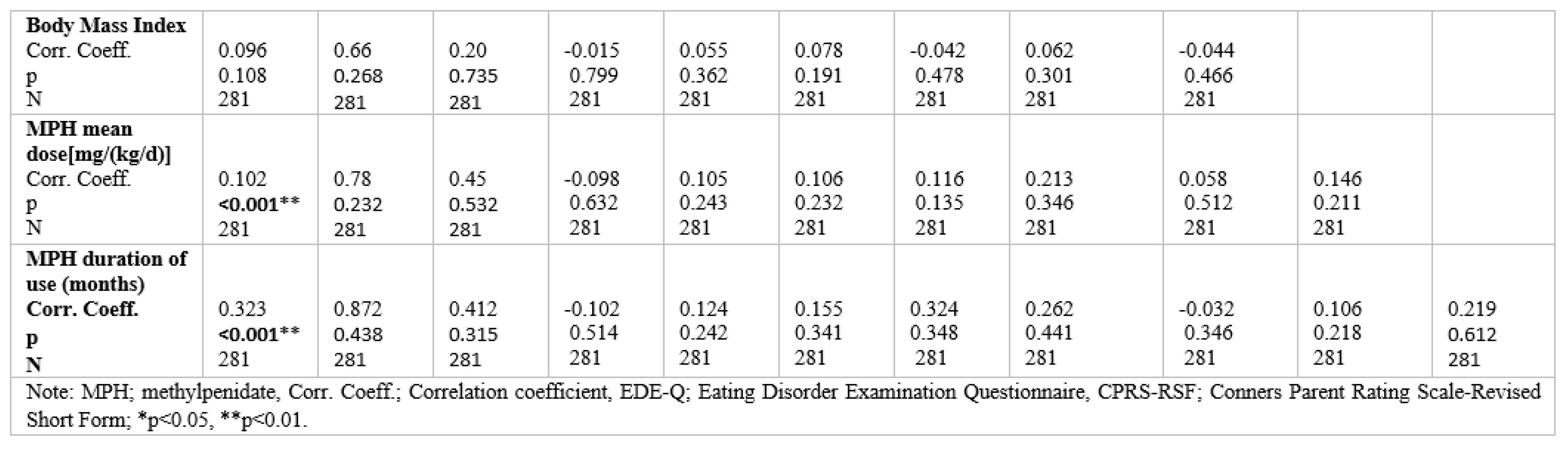
Table 4 shows correlations between Eating Disorder Examination Questionnaire (EDE-Q) subscale scores, Conners Parent Rating Scale-Revised Short Form (CPRS- RSF), Body mass index, MPH mean dose [mg/(kg/d)], and duration of use (months) for the ADHD sample. Correlations were significant between all CPRS-RSF subscale scores and the scores for EDE-Q eating concern (positive).mThe EDE-Q subscale scores correlated with one other. A statistically significant positive correlation was found between EDE-Q shape concern and CPRS-RSF subscale scores assessing inattention and cognitive problems. No correlations were found with BMI, MPH mean dose [mg/(kg/d)], and duration of use (months) except restraint subscale.

Table 4.
Two-tailed Spearman's rank-order correlations between Eating Disorder Examination Questiomnaire (EDE-Q) subscale seores, Conners Parent Rating Scale-Revised Short Form (CPRS-RSF) and Body mass index for the ADHD sample.
Discussions
This study evaluated the effects of MPH on eating patterns in attention deficit/hyperactivity disorder (ADHD) children and adolescents. A secondary aim was to compare the weight and eating behavior in children with ADHD and MPH treatment with that of children without ADHD. We had expected to find that individuals who take MPH for ADHD would be more likely to show negative effects on eating patterns. Moreover, we explored the association between MPH and growth parameters of weight, height, and BMI standard z-scores, but only minimal effects were noted in our sample.
Previous research has associated ADHD with global eating disorder pathology, restraint, eating, shape, and weight concerns. Several mechanisms have been advanced to explain ADHD effects on eating patterns. According to one recent study (2018), children with ADHD may have less control while eating and thus consume more calories than healthy subjects. In addition, children diagnosed with ADHD may eat more food even when they are satiated compared to healthy subjects [14]. Faster eating in children with ADHD, the inability to focus on hunger-satiety cycles, and the inability to perceive body stimuli may all lead to impaired eating-feeding patterns.
Alternatively, the eating problems may reflect a complex interaction among a number of functions, including deterioration in executive function, eating- appetite problems, eating problems, obesity, eating disorders (especially bulimia nervosa and binge eating disorder), attachment and family relation problems. As predicted, this interaction is more frequent and more complicated in women with ADHD than in men. Therefore, it is important to focus on the problems related to nutrition-appetite-eating and not to overlook a possible ADHD diagnosis, especially in female children and adolescents, given that obesity, under-threshold eating disorders (ADHD, bulimia nervosa and binge eating disorder risk especially during adolescence), and manifest eating disorders are more common in female adolescents. For this reason, early diagnosis and treatment are vitally importance for those with ADHD.
Several interpretations of our results are possible. It is well-known that ADHD may not directly cause eating disorders but, in many cases, may cause subthreshold eating disorders and impaired eating patterns [15]. Therefore, the associations between EDEQ and all CPRS- RSF subscale scores that hint at increased disordered eating patterns as another comorbidity in individuals with ADHD might initially seem legitimate. However, it remains uncertain whether the altered eating patterns that result from MPH reflect a deliberate decision to compensate for it, or whether the compensation response is automatic (rather than deliberate). For example, shape and weight concerns among these individuals are consistent with prior research in those individuals who engage in disordered eating behaviors and tend to have higher body image concerns [16]. In our study, there was no correlation between the MPH mean dose [mg/(kg/d)], the duration of use (months), and the core characteristics of eating disorders except restraint subscale of EDE Q.
Whereas MPH raises brain synaptic dopamine, which has been shown to induce anorexia and weight loss, it is generally considered a safe medication. Research on energy intake during the administration of MPH has been limited, but studies have shown effects on body weight and/or eating behavior that are consistent with the hypothesis that dopamine may play a role in the development or perpetuation of human obesity [17]. Attention deficit and impulsivity symptom clusters in ADHD may increase the development of obesity and binge eating. The early treatment of ADHD with psychostimulants or atomoxetine may reduce nutritional difficulties associated with obesity and binge eating, loss of control while eating, and impairments in reward- motivation systems [18].
The significant decrease in z-scores for height and weight was observed in the overall sample, but the decline was not observed in all subgroups suggested in the clinical literature [19,20]. Several possible mechanisms that might explain the effects of MPH on height and weight include inhibition of growth hormone, nutritional state differences, and parental height values.
Our study focused on the effect of psychostimulants in the different age subgroups, i.e., children (6–12 years) and adolescents (13–18 years). In the children subgroup, height and BMI were significantly reduced by the treatment. Given this finding, young patients using MPH should be closely monitored. Few studies have examined the relationships between the age of onset, the duration, and the growth parameters for the ADHD sample. The present study suggests that younger age at first stimulant use was associated with a decline in anthropometric scores. Our findings that there was no significant delay in the rate of physical maturation do not align with the findings of Gustafsson et al. (2010), which suggests that children with ADHD are less mature at baseline as they show a rapid maturation catch-up [21]. The possible reasons for these differences may include nutritional status or hormonal mechanisms which can modify the relationship between growth and physical maturation.
Limitations
Limitations of our study need to be considered. Participants with no clinically diagnosed possible conditions associated with growth retardation were selected for the study sample, but differences between the health conditions of the participants, which may affect the anthropometric values, may have occurred. Also, we did not evaluate parental anthropometric values, socio- economic status, ethnicity, and genetic factors of the ADHD group, which may have influenced our results. Finally, we did not consider comorbid features such as mood disorders or mental retardation that can be associated with eating patterns. Because other medications can also influence growth parameters; the impact of such factors remains unknown.
Future prospective and longitudinal studies would allow a deeper understanding of whether and how complexities of growth parameters are affected by ADHD.
Conclusions
The current study evaluated the effects of MPH and ADHD on eating patterns and growth patterns in Turkish Children and Adolescents. The results may have clinical implications for monitoring the core characteristics of eating disorders and growth parameters in ADHD patients. Our findings add to the growing literature regarding MPH, eating disorders, and anthropometric values, with our findings concluding that MPH may not be associated with the reduction of growth velocity and disordered eating behaviors.
Acknowledgments
All authors have contributed equally to this paper.
Conflicts of Interest
There are no known conflicts of interest in the publication of this article. The manuscript was read and approved by all authors.
Compliance with ethical standards
Any aspect of the work covered in this manuscript has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Rommelse, N.N.; Altink, M.E.; Fliers, E.A.; Martinm, N.C.; Buschgens, C.J.; Hartman, C.A.; et al. Comorbid problem in ADHD: degree of association, shared endophenotypes, and formation of distinct subtypes. Implications for a future DSM. J Abnorm Child Psychol. 2009, 37, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Nazar, B.P.; Bernardes, C.; Peachey, G.; Sergeant, J.; Mattos, P.; Treasure, J. The risk of eating disorders comorbid with attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Int J Eat Disord. 2016, 49, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Biederman, J.; Spencer, T.J.; Monuteaux, M.C.; Faraone, S.V. A naturalistic 10-year prospective study of height and weight in children with attention-deficit hyperactivity disorder grown up: sex and treatment effects. J Pediatr. 2010, 157, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Yoshimasu, K.; Barbaresi, W.J.; Colligan, R.C.; Voigt, R.G.; Killian, J.M.; Weaver, A.L.; et al. Childhood ADHD is strongly associated with a broad range of psychiatric disorders during adolescence: a population-based birth cohort study. J Child Psychol Psychiatry. 2012, 53, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Bleck, J.; DeBate, R.D.; Olivardia, R. The Comorbidity of ADHD and Eating Disorders in a Nationally Representative Sample. J Behav Health Serv Res. 2015, 42, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Sonneville, K.R.; Calzo, J.P.; Horton, N.J.; Field, A.E.; Crosby, R.D.; Solmi, F.; Micali, N. Childhood hyperactivity/inattention and eating disturbances predict binge eating in adolescence. Psychol Med. 2015, 45, 2511–20. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Levitan, R.D.; Kaplan, A.S.; Carter-Major, J.C.; Kennedy, J.L. Sex differences in subjective and objective responses to a stimulant medication (methylphenidate): Comparisons between overweight/obese adults with and without binge-eating disorder. Int J Eat Disord. 2016, 49, 473–81. [Google Scholar] [CrossRef] [PubMed]
- Cortese, S.; Moreira-Maia, C.R.; St Fleur, D.; Morcillo- Pen˜alver, C.; Rohde, L.A.; Faraone, S.V. Association between ADHD and obesity: A systematic review and meta-analysis. Am J Psychiatry. 2016, 173, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Neyzi, O.; Bundak, R.; Gökçay, G.; Günöz, H.; Furman, A.; Darendeliler, F.; Baş, F. Reference values for weight, height, head circumference, and body mass index in Turkish children. J Clin Res Pediatr Endocrinol. 2015, 7, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Kaner, S.; Büyüköztürk Ş ve İşeri, E. Conners Anababa Dereceleme Ölçeği-Yenilenmiş Kısa: Türkiye stardardizasyon çalışması. Noropsikiyatri Arşivi. 2013, 50, 100–109. [Google Scholar] [CrossRef]
- Fairburn, C.G.; Cooper, Z.; O’Connor, M. Eating disorder examination (16.0D). In Cognitive behavior therapy and eating disorders; Fairburn, C.G., Ed.; Guilford Press: New York, NY, USA, 2008. [Google Scholar]
- Yucel, B.; Polat, A.; Ikiz, T.; Dusgor, B.P.; Elif Yavuz, A.; Sertel Berk, O. The Turkish Version of the Eating Disorder Examination Questionnaire: Reliability and Validity in Adolescents. Eur Eat Disorders Rev. 2011, 19, 509–11. [Google Scholar] [CrossRef] [PubMed]
- Hilbert, A.; Kurz, S.; Dremmel, D.; Blüher, S.W.; Munsch, S.; Schmidt, R. Cue reactivity, habituation, and eating in the absence of hunger in children with loss of control eating and attention-deficit/hyperactivity disorder. Int J Eat Disord. 2018, 51, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Bleck, J.; DeBate, R.D. Exploring the co-morbidity of attention-deficit/hyperactivity disorder with eating disorders and disordered eating behaviors in a nationally representative community-based sample. Eat Behav. 2013, 14, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.K.; Heinberg, L.J.; Altabe, M.; Tantleff-Dunn, S. Exacting beauty: Theory, assessment, and treatment of body image disturbance; American Psychological Association: Washington, DC, USA, 1999. [Google Scholar]
- Leddy, J.J.; Epstein, L.H.; Jaroni, J.L.; Roemmich, J.N.; Paluch, R.A.; Goldfield, G.S.; Lerman, C. Influence of Methylphenidate on Eating in Obese Men. Obes Res. 2004, 12, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Cortese, S.; Bernardina, B.D.; Mouren, M.C. Attention- deficit/hyperactivity disorder (ADHD) and binge eating. Nutr Rev. 2007, 65, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Lentferink, Y.E.; van de Garde, E.M.W.; Knibbe, C.A.J.; van der Vorst, M.M.J. Psychostimulants: Influence on Body Mass Index and Height in a Pediatric Population with Attention-Deficit/Hyperactivity Disorder? J Child Adolesc Psychopharmacol. 2018, 28, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Dubnov-Raz, G.; Perry, A.; Berger, I. Body mass index of children with attention-deficit/hyperactivity disorder. J Child Neurol. 2011, 26, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, P.; Holmström, E.; Besjakov, J.; Karlsson, M.K. ADHD symptoms and maturity - a follow-up study in school children. Acta Paediatr. 2010, 99, 1536–9. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the author. 2019 Serkan Turan, Aynur Pekcanlar Akay