The Effect of Glucosamine, Chondroitin and Harpagophytum Procumbens on Femoral Hyaline Cartilage Thickness in Patients with Knee Osteoarthritis—An MRI Versus Ultrasonography Study
Highlights
- The combination with HPc could be able to delay progression of the knee osteoarthritis.
- US and MRI represent important techniques with comparable results on patients with osteoarthritis, but with the remark that US is a much cheaper and more accessible tool.
Highlights
- The combination with HPc could be able to delay progression of the knee osteoarthritis.
- US and MRI represent important techniques with comparable results on patients with osteoarthritis, but with the remark that US is a much cheaper and more accessible tool.
Abstract
Introduction
Materials and Methods
Objective
Patients and Methods
The ultrasound (US) evaluation
The MRI examination
Statistical analysis
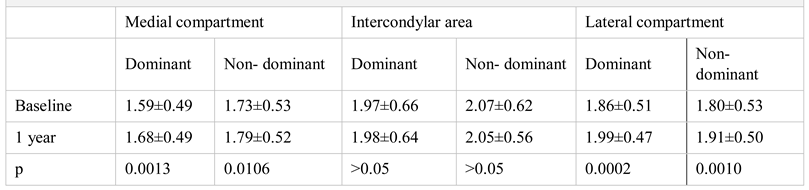
Results
Imaging evaluation of treatment response after 1 year
Discussions
Conclusions
Acknowledgement
Conflict of interest disclosure
Compliance with ethical standards
References
- Biro, A.; Georgescu, L.; Nedelcut, C.; Marinescu, C.; Bolosiu, H. Effect of the combination glucosamine hydrochloride and chondroitin sulphate on knee osteoarthritis symptoms: a randomized, double blind, placebo-controlled study. Ro J Rheumatol. 2009, 18, 105–113. [Google Scholar]
- Jordan, K.; Arden, N.; Doherty, M.; et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the standing committee for international clinical studies including therapeutic trials (ESCISIT). Ann Rheum Dis. 2003, 62, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Moskowitz, R.W.; Nuki, G.; et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008, 16, 137–162. [Google Scholar] [CrossRef] [PubMed]
- Kloppenburg, M.; Kroon, F.P.; Blanco, F.J.; et al. 2018 update of the EULAR recommendations for the management of hand osteoarthritis. Ann Rheum Dis. 2019, 78, 16–24. [Google Scholar] [CrossRef]
- Henrotin, Y.; Marty, M.; Mobasheri, A. What is the current status of chondroitin sulfate and glucosamine for the treatment of knee osteoarthritis? Maturitas. 2014, 78, 184–187. [Google Scholar] [CrossRef]
- Roman-Blas, J.A.; Mediero, A.; Tardío, L.; et al. The combined therapy with chondroitin sulfate plus glucosamine sulfate or chondroitin sulfate plus glucosamine hydrochloride does not improve joint damage in an experimental model of knee osteoarthritis in rabbits. Eur J Pharmacol. 2017, 794, 8–14. [Google Scholar] [CrossRef]
- Silva, F.S., Jr.; Yoshinari, N.H.; Castro, R.R.; et al. Combined glucosamine and chondroitin sulfate provides functional and structural benefit in the anterior cruciate ligament transection model. Clin Rheumatol. 2009, 28, 109–117. [Google Scholar] [CrossRef]
- Hochberg, M.C.; Altman, R.D.; April, K.T.; et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012, 64, 465–474. [Google Scholar] [CrossRef]
- Sawitzke, A.D.; Shi, H.; Finco, M.F.; et al. The effect of glucosamine and/or chondroitin sulfate on the progression of knee osteoarthritis: a report from the glucosamine/chondroitin arthritis intervention trial. Arthritis Rheum. 2008, 58, 3183–3191. [Google Scholar] [CrossRef]
- Malattia, C.; Damasio, M.B.; Magnaguagno, F.; Pistorio, A.; Valle, M.; Martinoli, C.; et al. Magnetic resonance imaging, ultrasonography and conventional radiography in the assessment of bone erosions in juvenile idiopathic arthritis. Arthritis Rheum. 2008, 59, 1764–1772. [Google Scholar] [CrossRef]
- Zhang, W.; Doherty, M.; Peat, G.; et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis. 2010, 69, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Torp-Pedersen, S.; Bartels, E.M.; Wilhjelm, J.; Bliddal, H. Articular cartilage thickness measured with US is not as easy as it appears: a systematic review of measurement techniques and image interpretation. Ultraschall Med. 2011, 32, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.G.; Zheng, Y.P.; Wu, J.Y.; Shi, J. Measurement of depth-dependence and anisotropy of ultrasound speed of bovine articular cartilage in vitro. Ultrasound Med Biol. 2004, 30, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.Q.; Seedhom, B.B. Ultrasonic measurement of the thickness of human articular cartilage in situ. Rheumatology 1999, 38, 1269–1271. [Google Scholar] [CrossRef]
- Colebatch, A.N.; Edwards, C.J.; Ostergaard, M.; et al. EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann Rheum Dis. 2013, 72, 804–814. [Google Scholar] [CrossRef]
- Altman, R.; Asch, E.; Bloch, D.; et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986, 29, 1039–1049. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef]
- Bellamy, N.; Buchanan, W.W.; Goldsmith, C.H.; Campbell, J.; Stitt, L.W. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988, 15, 1833–1840. [Google Scholar]
- Brown, A.K.; O'Connor, P.J.; Roberts, T.E.; Wakefield, R.J.; Karim, Z.; Emery, P. Recommendations for musculoskeletal ultrasonography by rheumatologists: setting global standards for best practice by expert consensus. Arthritis Rheum. 2005, 53, 83–92. [Google Scholar] [CrossRef]
- Wakefield, R.J.; Balint, P.V.; Szkudlarek, M.; et al. Musculoskeletal ultrasound including definitions for u ltrasonographic pathology. J Rheumatol 2005, 32, 2485–2487. [Google Scholar]
- Gavrilă, M.T.; Ștefan, C. Arthroscopic treatment for elbow intraarticular loose bodies. J Clin Invest Surg. 2018, 3, 100–104. [Google Scholar] [CrossRef]
- Reginster, J.Y.; Deroisy, R.; Rovati, L.C.; et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet 2001, 357, 251–6. [Google Scholar] [CrossRef] [PubMed]
- Pavelká, K.; Gatterová, J.; Olejarová, M.; Machacek, S.; Giacovelli, G.; Rovati, L.C. Glucosamine sulfate use and delay of progression of knee osteoarthritis: a 3-year, randomized, placebo-controlled, double-blind study. Arch Intern Med. 2002, 162, 2113–2123. [Google Scholar] [CrossRef] [PubMed]
- Bruyere, O.; Honore, A.; Ethgen, O.; et al. Correlation between radiographic severity of knee osteoarthritis and future disease progression. Results from a 3-year prospective, placebo-controlled study evaluating the effect of glucosamine sulfate. Osteoarthritis Cartilage 2003, 11, 1–5. [Google Scholar] [CrossRef]
- Brandt, K.D. Effects of nonsteroidal anti-inflammatory drugs on chondrocyte metabolism in vitro and in vivo. Am J Med. 1987, 83, 29–34. [Google Scholar] [CrossRef]
- Chrubasik, S.; Model, A.; Black, A.; Pollak, S. A randomised double-blind pilot study comparing Doloteffin and Vioxx in the treatment of low back pain. Rheumatology 2003, 42, 141–148. [Google Scholar] [CrossRef]
- Grant, L.; McBean, D.; Fyfe, L.; Warnock, M. ffects of Harpagophytum procumbens (Devils claw) on the cyclooxygenase and lipoxygense pathways of the arachidonic acid cascade. In: Govil JN, Singh VK, Bhardwaj R (eds). Recent progress in medicinal plants volume 24, Studium Press, 2009: 203-19.
- Schulsse, S.; Wiggers, L.; Daix, M.; Kirschvink, N. Effects of an oral supplementation with glucosamine and chondroitin sulphate on MMP-2 activity in supernatants of IL-1 stimulated-equine chondrocytes. Acta physiologica. 2008, 194, P–15. [Google Scholar]
- Pradsgaard, D.Ø.; Fiirgaard, B.; Spannow, A.H.; Heuck, C.; Herlin, T. Cartilage thickness of the knee joint in juvenile idiopathic arthritis: comparative assessment by ultrasonography and magnetic resonance imaging. J Rheumatol. 2015, 42, 534–540. [Google Scholar] [CrossRef]
- Filippou, G.; Scirè, C.A.; Adinolfi, A.; et al. Identification of calcium pyrophosphate deposition disease (CPPD) by ultrasound: reliability of the OMERACT definitions in an extended set of joints-an international multiobserver study by the OMERACT Calcium Pyrophosphate Deposition Disease Ultrasound Subtask Force. Ann Rheum Dis. 2018, 77, 1194–1199. [Google Scholar] [CrossRef]
- Vreju, A.F.; Ciurea, M.E.; Popa, D.; et al. Ultrasonography in diagnosis and management of non-inflammatory conditions of the hand and wrist. Med Ultrason. 2016, 18, 90–95. [Google Scholar] [CrossRef]
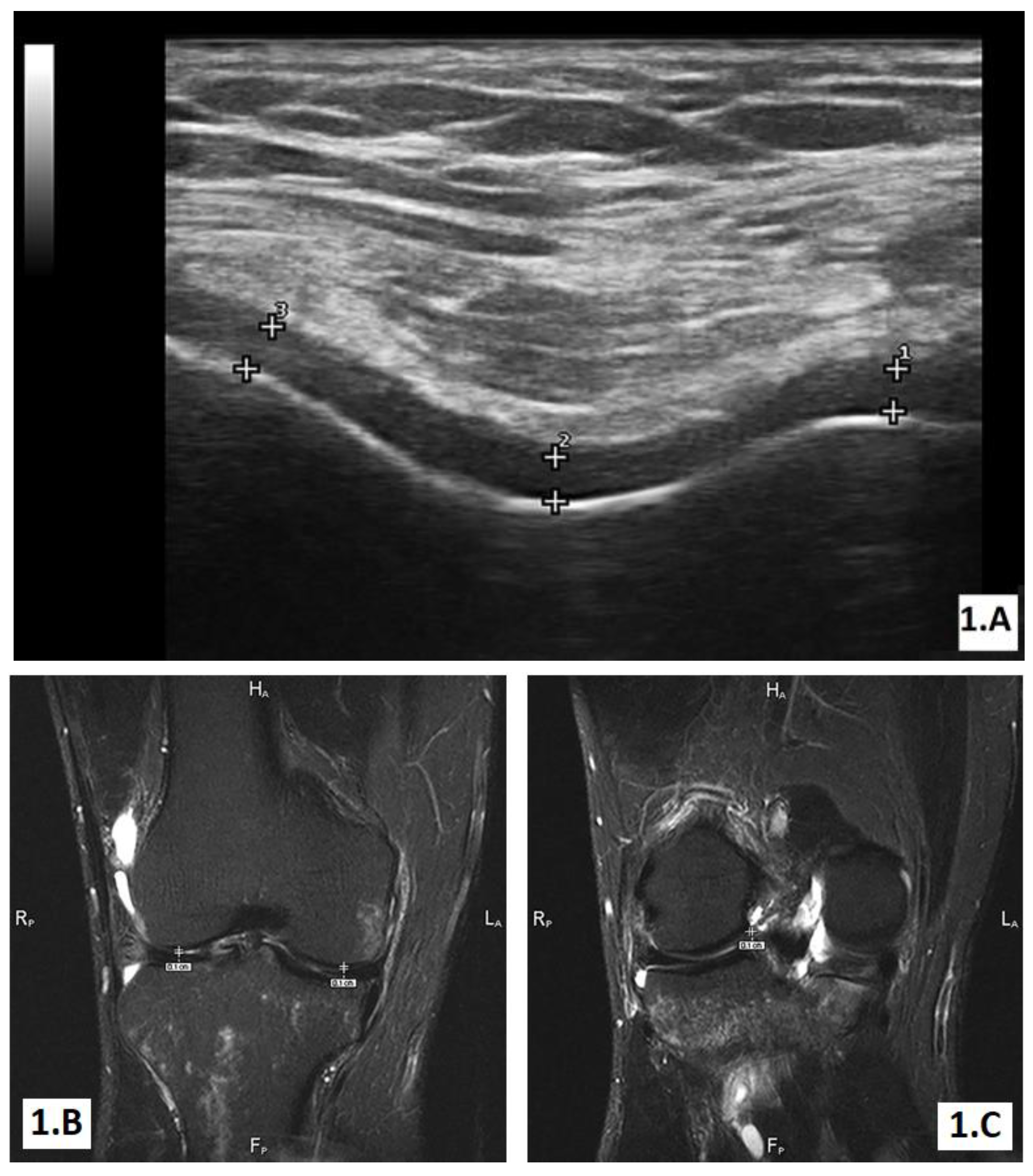
 |
© 2019 by the author. 2019 Florentin A. Vreju, Paulina L. Ciurea, Anca Rosu, Beatrice A. Chisalau, Cristina D. Parvanescu, Sineta C. Firulescu, Adina Turcu-Stiolica, Andreea L. Barbulescu, Stefan C. Dinescu, Cristiana I. Dumitrescu, Roxana Mihaela Dumitrascu, Criveanu Cristina, Lucretiu Radu, Mihai Tusaliu, Daniela Dumitrescu
Share and Cite
Vreju, F.A.; Ciurea, P.L.; Rosu, A.; Chisalau, B.A.; Parvanescu, C.D.; Firulescu, S.C.; Turcu-Stiolica, A.; Barbulescu, A.L.; Dinescu, S.C.; Dumitrescu, C.I.; et al. The Effect of Glucosamine, Chondroitin and Harpagophytum Procumbens on Femoral Hyaline Cartilage Thickness in Patients with Knee Osteoarthritis—An MRI Versus Ultrasonography Study. J. Mind Med. Sci. 2019, 6, 162-168. https://doi.org/10.22543/7674.61.P162168
Vreju FA, Ciurea PL, Rosu A, Chisalau BA, Parvanescu CD, Firulescu SC, Turcu-Stiolica A, Barbulescu AL, Dinescu SC, Dumitrescu CI, et al. The Effect of Glucosamine, Chondroitin and Harpagophytum Procumbens on Femoral Hyaline Cartilage Thickness in Patients with Knee Osteoarthritis—An MRI Versus Ultrasonography Study. Journal of Mind and Medical Sciences. 2019; 6(1):162-168. https://doi.org/10.22543/7674.61.P162168
Chicago/Turabian StyleVreju, Florentin A., Paulina L. Ciurea, Anca Rosu, Beatrice A. Chisalau, Cristina D. Parvanescu, Sineta C. Firulescu, Adina Turcu-Stiolica, Andreea L. Barbulescu, Stefan C. Dinescu, Cristiana I. Dumitrescu, and et al. 2019. "The Effect of Glucosamine, Chondroitin and Harpagophytum Procumbens on Femoral Hyaline Cartilage Thickness in Patients with Knee Osteoarthritis—An MRI Versus Ultrasonography Study" Journal of Mind and Medical Sciences 6, no. 1: 162-168. https://doi.org/10.22543/7674.61.P162168
APA StyleVreju, F. A., Ciurea, P. L., Rosu, A., Chisalau, B. A., Parvanescu, C. D., Firulescu, S. C., Turcu-Stiolica, A., Barbulescu, A. L., Dinescu, S. C., Dumitrescu, C. I., Dumitrascu, R. M., Cristina, C., Radu, L., Tusaliu, M., & Dumitrescu, D. (2019). The Effect of Glucosamine, Chondroitin and Harpagophytum Procumbens on Femoral Hyaline Cartilage Thickness in Patients with Knee Osteoarthritis—An MRI Versus Ultrasonography Study. Journal of Mind and Medical Sciences, 6(1), 162-168. https://doi.org/10.22543/7674.61.P162168



