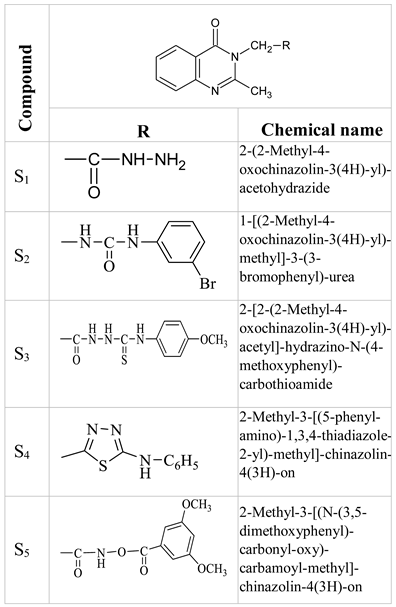
Introduction
Quinazolone derivatives have been studied in preclinical and clinical trials for many and various therapeutic purposes: antidepressant [
1], anticonvulsant [
2], analgesic [
3], antineoplastic [
4], antihypertensive [
5], or tuberculostatic [
6]. Moreover, there are several approved drugs which belong to the carbamate ester derivatives class, many of them with cholinergic actions: physostigmine, neostigmine, or rivastigmine.
The new compound series, synthesized in the Pharmaceutical Chemistry Laboratory of the Faculty of Pharmacy within UMF “Carol Davila” Bucharest, brought together the 4(3H)-quinazolinone structure and carbamic ester group [
7].
Table 1.
Chemical structure of S1- S5 compounds
Table 1.
Chemical structure of S1- S5 compounds
This synthesis resembles other similar processes that are part of the recent research directions of the Discipline of Pharmaceutical Chemistry, with the purpose of obtaining new chemical compounds with potential pharmacological actions [
8,
9,
10,
11,
12,
13].
In this paper, we present the results of the preclinical research performed on these five new compounds in order to assess the potential pharmacological actions on the central nervous system. We followed all the existing protocols of the Laboratory of Pharmacology, the Faculty of Pharmacy, UMF “Carol Davila” Bucharest. We investigated the antidepressant effect using the forced swimming test, the anxiolytic/ anxiogenic effect using the suspended plus-shaped maze (Ugo Basile), the effect on the motor activity with the Ugo Basile activity cage, and the potential analgesic effect using the hot plate test, Ugo Basile [
14].
Materials and Methods
A sample consisting of 94 white males, NMRI mice having reached maturity and weighing 25 ± 2.8 g, were supplied by the rodent farm of the University of Medicine and Pharmacy “Carol Davila”. Animals were quarantined for 3 days, and afterward, housed in ventilated cages with free access to food and water. Temperature and relative humidity were kept constant (22-24oC, 45-60%).
All experimental procedures were carried out in accordance with the Directive 2010/63/UE of September 22nd, 2010, regarding the protection of animals used for experimental and other scientific purposes. All experimental procedures were approved by the Ethical Committee of the Faculty of Pharmacy Bucharest. The experiment was conducted in June 2014.
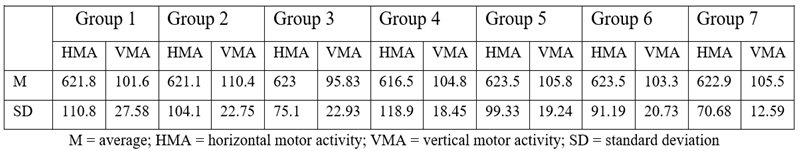
For the purpose of selecting the proper animals for the proposed pharmacological tests, 94 mice were subjected to the Ugo Basile activity cage test and divided into groups based on their horizontal motor activity, measured every 5 minutes. After exclusion of animals with extreme responses, 84 mice remained and were divided into 7 groups, each containing 12 individuals in such a manner that the average responses and the standard deviations were as similar as possible. Animals were then allowed one day for acclimation within their new groups. On the day of the experiment, each group was brought to the lab, one hour before the beginning of the experiment, to allow them to adapt to the new environment where they were kept without food.
The compounds were administered as shown below:
Group I (control) – distilled water 0.1 ml/10g orally
Group II (reference) – amitriptyline 10 mg/kg bw susp. 0.1% orally
Group III – S1 100 mg/kg bw, susp. 1% orally
Group IV – S2 100 mg/kg bw, susp. 1% orally
Group V – S3 100 mg/kg bw, susp. 1% orally
Group VI – S4 100 mg/kg bw, susp. 1% orally
Group VII – S5 100 mg/kg bw, susp. 1% orally
One hour after treatment, the mice were subjected to the activity cage test to determine their motor activity and then to the forced swimming test, in order to determine the potential antidepressant effect of the new compounds.
One week after the first set of tests (considered enough time for the compounds to have been purged from the system), all groups received the same treatment again and were subjected first to the suspended cross-maze test in order to determine the levels of anxiety, and then to the hot plate test, in order to evaluate the potential analgesic effects of the researched compounds.
All tests were conducted in accordance with the following protocol: in the testing chamber, animals were kept in artificial light, without food. Each individual was administered the treatment with a 7-minute delay from the previous one (5 minutes for the test itself and 2 minutes to clean the device before testing the next animal) so that all could be tested after the same time interval from the moment of receiving the treatment.
The determination of the motor activity to assess the effect on the central nervous system by recording the horizontal and vertical movements of mice in the Activity Cage Ugo Basile was performed by placing a mouse in a corner of the device and monitoring its movements for 5 minutes.
The
determination of the immobility time of mice in forced swimming test (FST) was accomplished by placing each mouse in a glass cylinder (25 cm height, 30 cm diameter) containing a 20 cm high column of water at a temperature of 22 ± 1
oC and recording during a 4-minute interval after a prior 2-minute interval for adaptation [
15].
The determination of curiosity used the suspended cross-shaped maze and involved placing the mouse in the center of the device and measuring the time spent in the open arms compared to the time spent in the enclosed arms during a 5-minute interval.
The evaluation of pain sensitivity was performed using the hot-plate test which consists of placing the mouse on a metal plate heated to a 53oC temperature and measuring the latency to the mouse’s reaction of licking its forepaws or of trying to escalate the Plexiglas walls of the plate.
Statistical analysis
Statistical analysis used the software GraphPad Prism 5. Comparison between groups used the Student t test (for normal distribution) whereas comparison across multiple groups used ANOVA. When significant, the Bonferroni posttest adjustment was performed for posthoc comparisons. The normality of the response distribution in collectivity was tested with the D’Agostino & Pearson test.
Results and Discussions
Motor activity determination
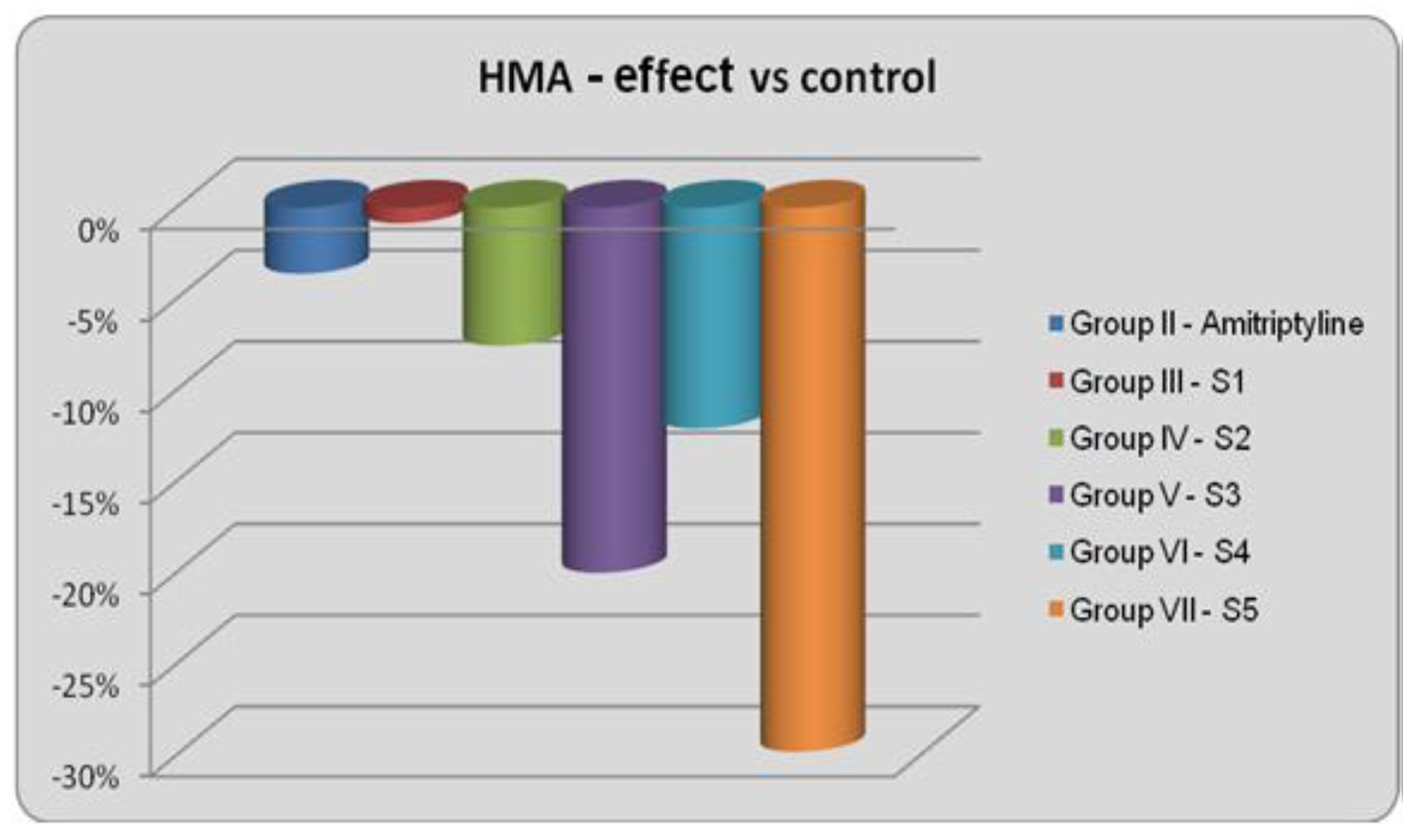
Figure 1.
HMA – effect vs Control
Figure 1.
HMA – effect vs Control
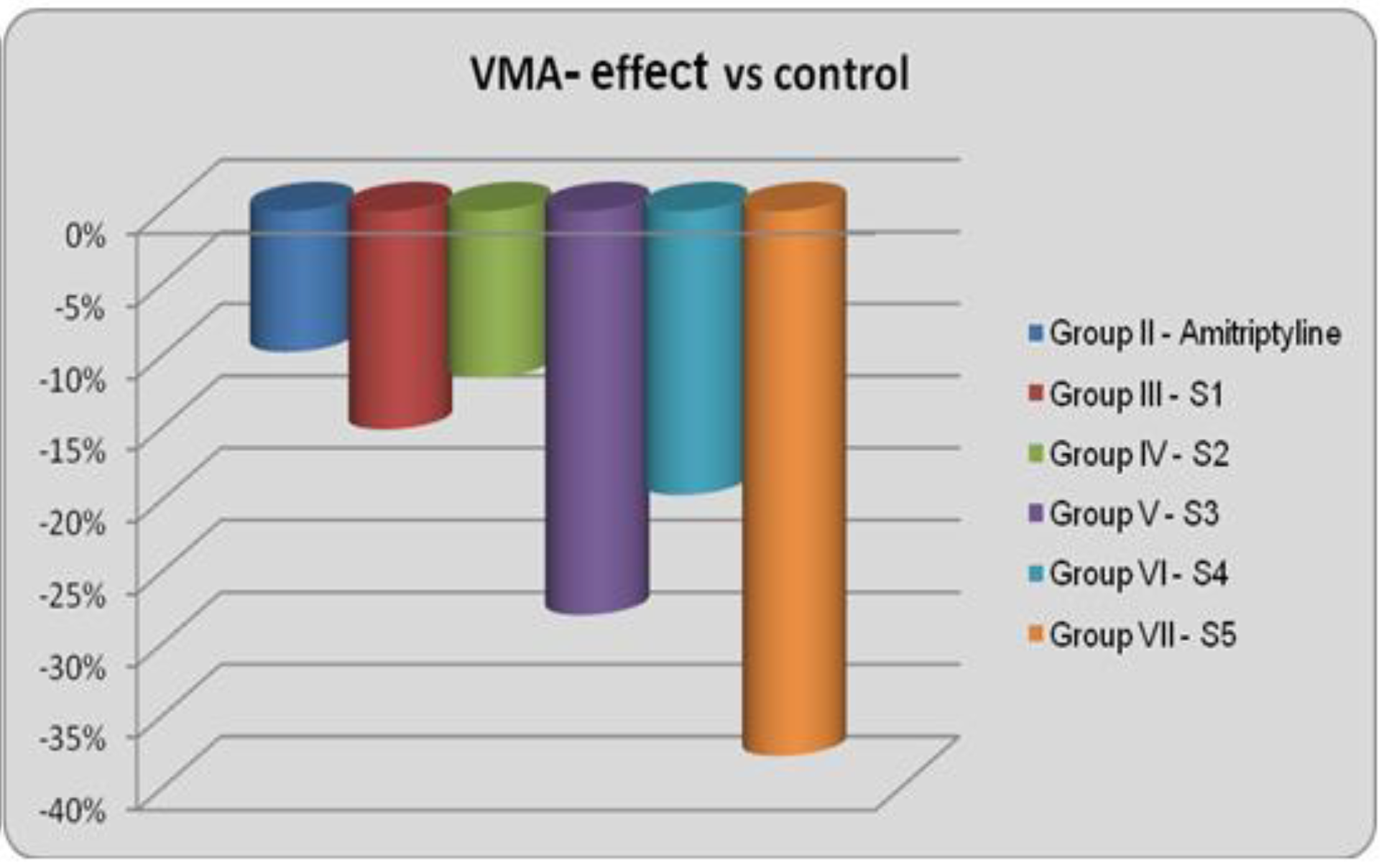
Figure 2.
VMA – effect vs Control
Figure 2.
VMA – effect vs Control
All of the newly synthesized quinasolines reduced the motor activity. S5 had the most intense effect, inducing a 29.08% reduction in HMA and a 37.78% reduction in VMA compared to the control group, which were statistically significant.
S3 also had an intense effect, but the reduction of the motor activity in this case was not statistically significant. Amitriptyline, which was used as the reference substance, did not influence significantly the motor activity after the acute administration.
Table 2.
HMA and VMA results
Table 2.
HMA and VMA results
Antidepressant effect evaluation
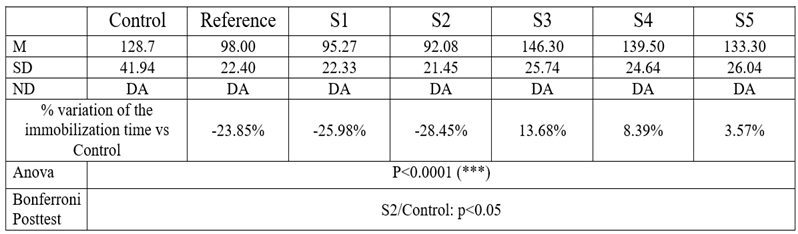
Table 3.
Immobilization time results
Table 3.
Immobilization time results
The forced swimming test was performed after acute treatment, with this test giving positive results even after a single dose, in the case of classical antidepressant drugs.
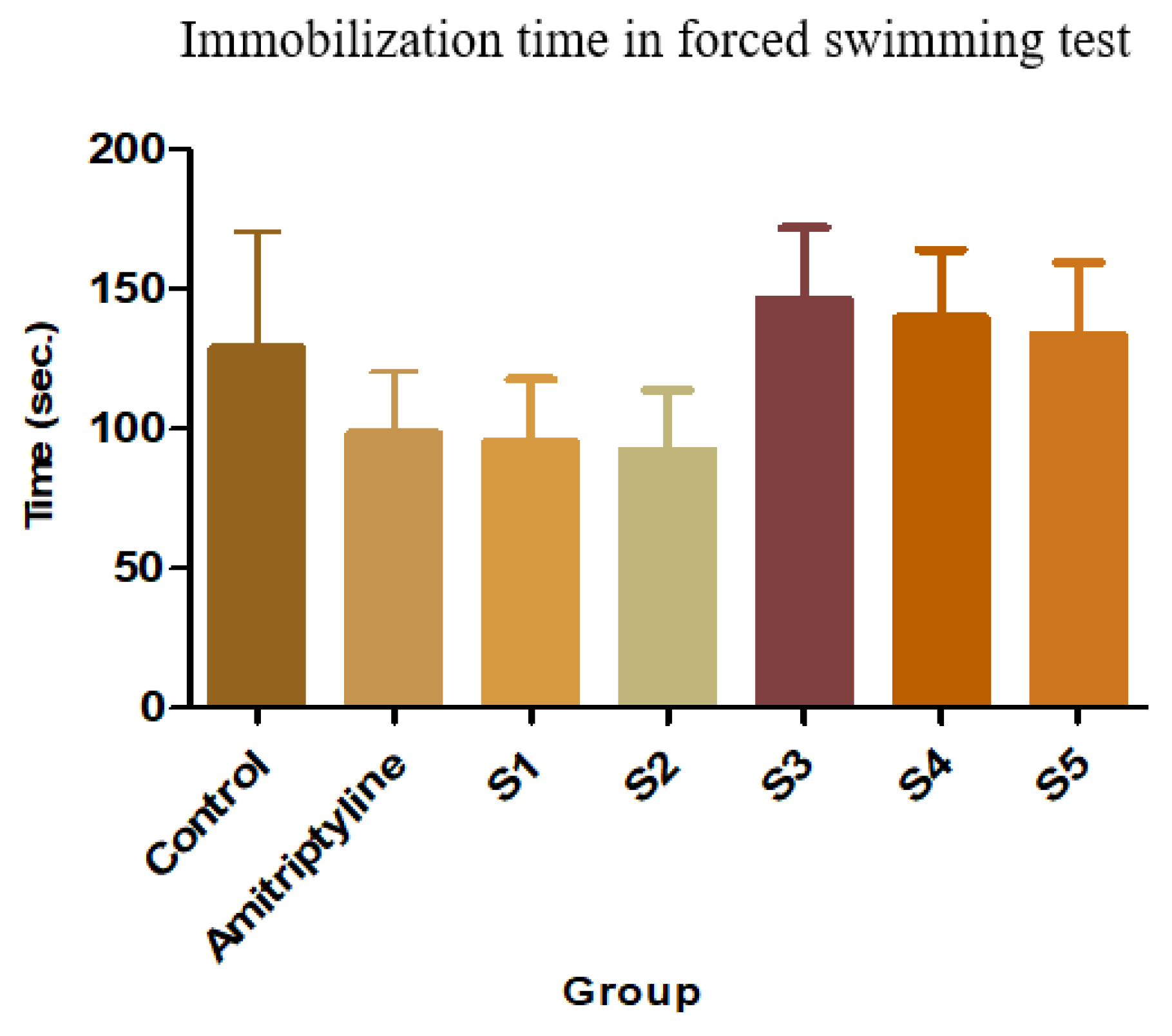
Figure 3.
FST - immobilization time + SD
Figure 3.
FST - immobilization time + SD
The average immobilization time for each group and the effect compared to the control group is shown in the following figure.
Figure 4.
The evaluation of the antidepressanteffect of the newly synthesized compounds
Figure 4.
The evaluation of the antidepressanteffect of the newly synthesized compounds
As expected initially, Amitriptyline reduced the immobilization time by 23.85% compared to controls. The result however was not statistically significant, probably due to the large within-group variance.
The antidepressant effect of Amitriptyline is well documented in the scientific literature [
7,
16]. Among the tested compounds, the highest reduction in the immobilization time was induced by S2, thus indicating that it has the most intense antidepressant effect (28.45%; p<0.05 – ANOVA + Bonferroni posttest). Similarly, S1 reduced the immobilization time by 25.98%. The other three compounds proved to have no significant antidepressant effect after only one administration.
Behavior evaluation in the suspended cross-shaped maze
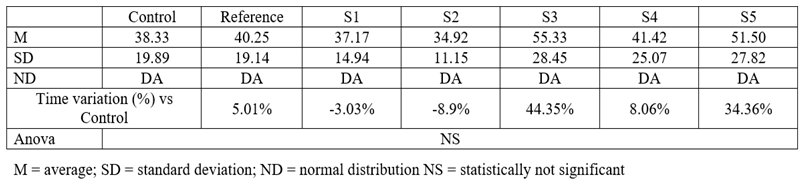
Table 4.
Time spent in the open arms of the maze
Table 4.
Time spent in the open arms of the maze
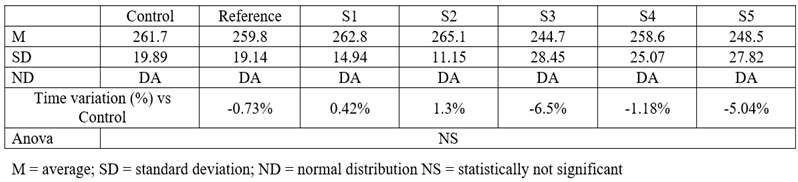
Table 5.
Time spent in the enclosed arms of the maze
Table 5.
Time spent in the enclosed arms of the maze
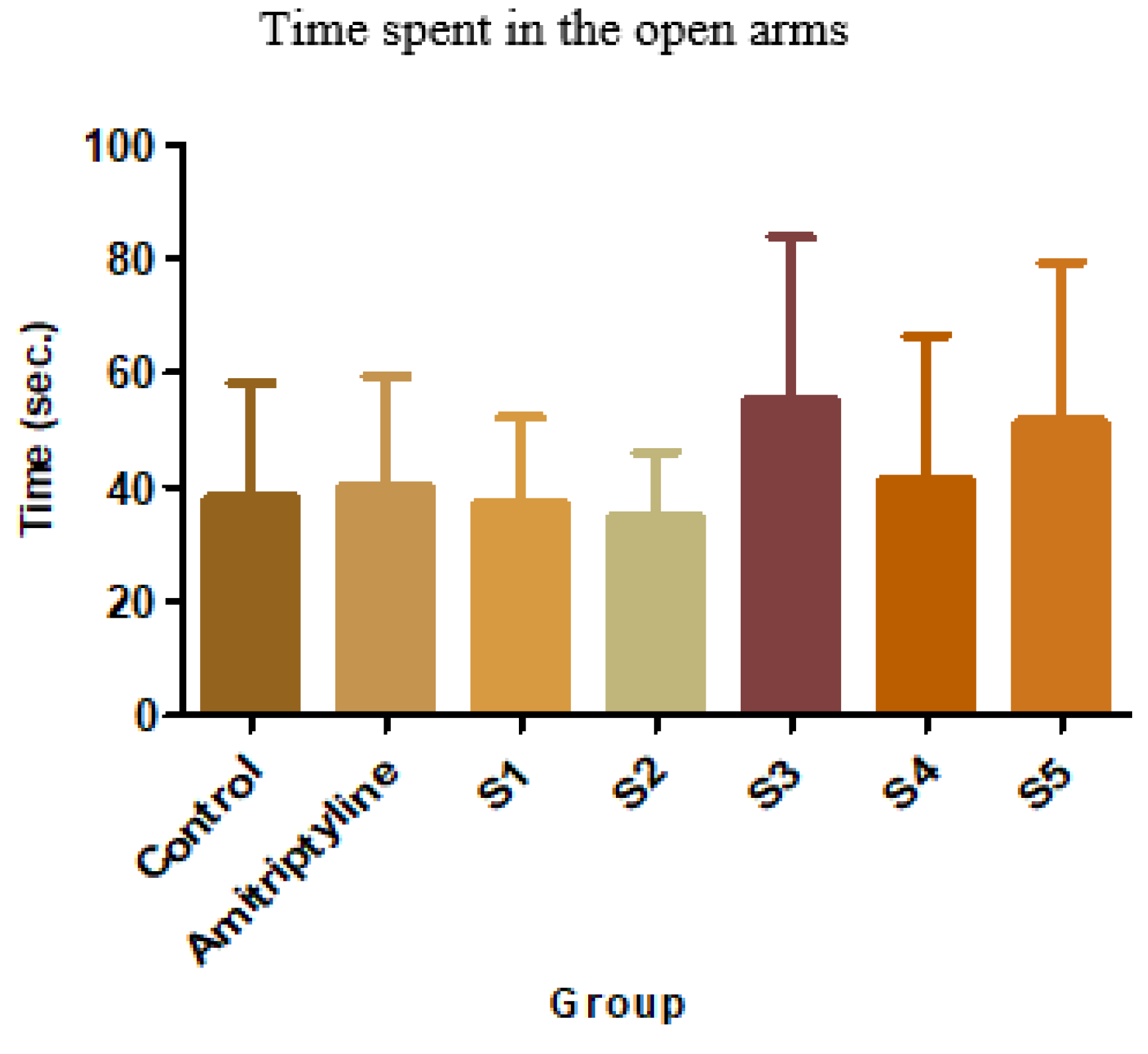
Figure 5.
Average time in the open arms +SD
Figure 5.
Average time in the open arms +SD
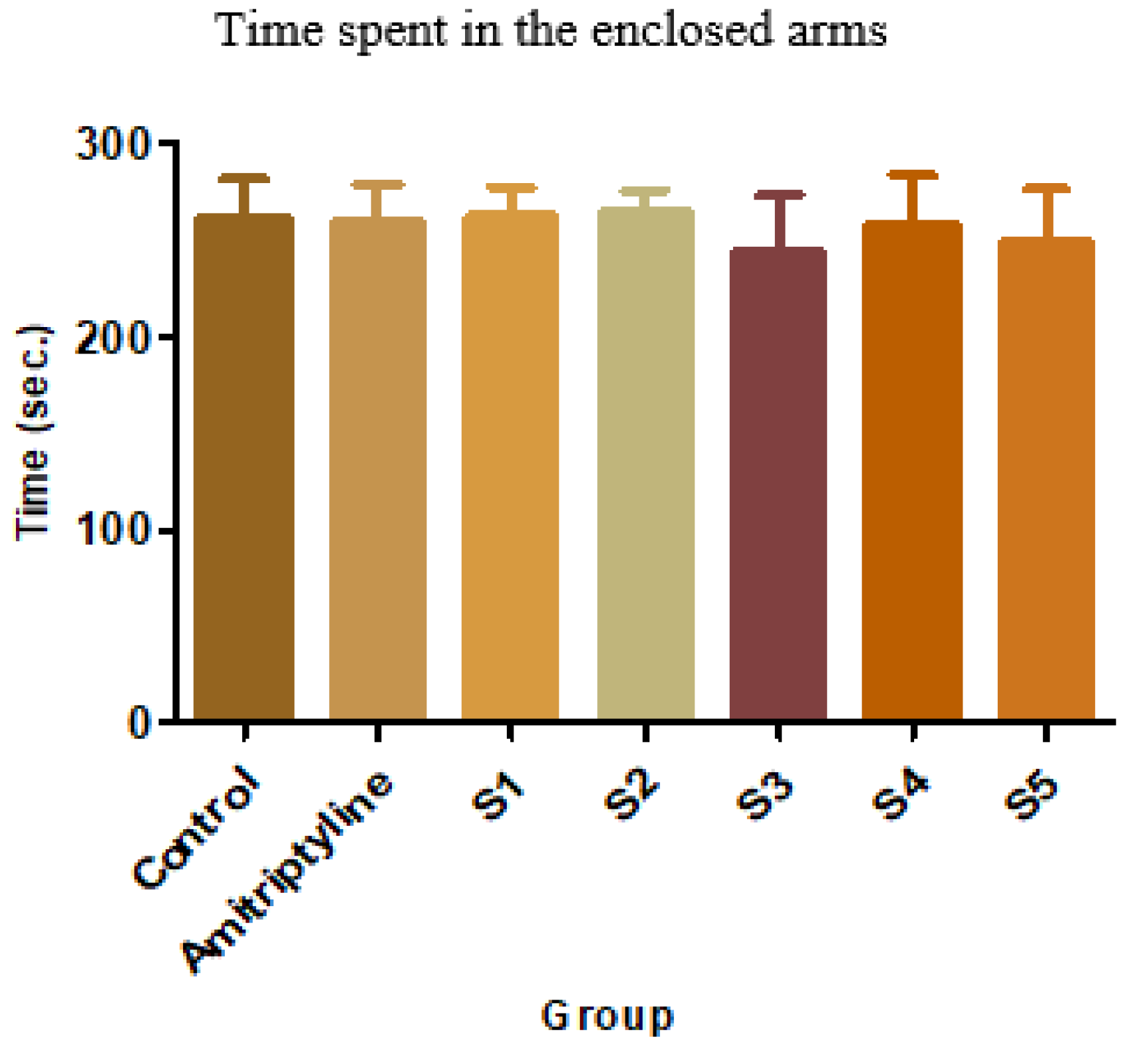
Figure 6.
Average time in the enclosed arms + SD
Figure 6.
Average time in the enclosed arms + SD
Figure 7.
Time variation (%) in open arms
Figure 7.
Time variation (%) in open arms
Figure 8.
Time variation (%)in enclosed arms
Figure 8.
Time variation (%)in enclosed arms
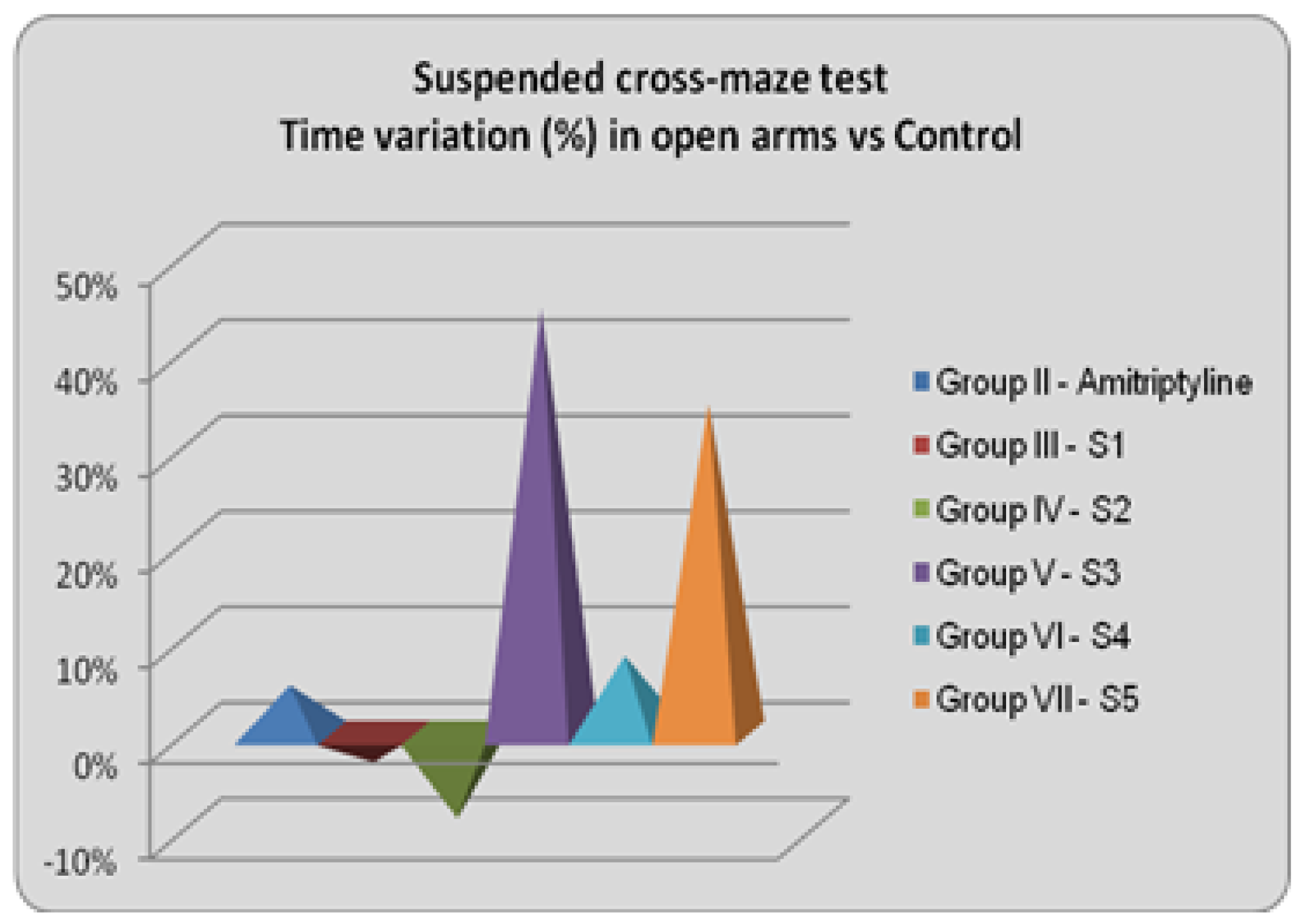
In the suspended cross-maze test, neither of the tested compounds induced statistically significant changes. However, S3 resulted in a 44.35% increase in the time spent in the open arms, and S5 had a similar effect (34.36%). Acknowledging the fact that, under the influence of anxiolytic drugs, mice tend to spend more time in the open arms than the enclosed ones further suggests that S3 and S5 might have such an effect. Another parameter that can give information regarding the anxiolytic potential of a new substance is the number of exits performed by mice outside the open arms. The mean, standard deviations, and comparison with the control group for this parameter are shown below.
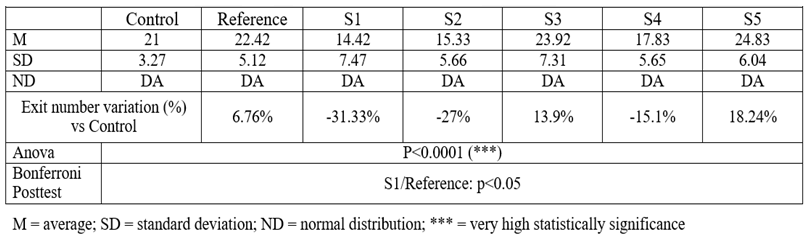
Table 6.
Number of exits outside the open arms
Table 6.
Number of exits outside the open arms
Figure 7.
Average number ofexits + SD
Figure 7.
Average number ofexits + SD
Figure 8.
Number of exits variation (%) vs Control
Figure 8.
Number of exits variation (%) vs Control
The results confirm that these 2 compounds (S3 and S5) have the highest anxiolytic potential, S3 having increased the number of exits by 13.9% compared to the control group, while S5 shows a similar and stronger pattern, 18.24%.
Analgezic effect evaluation
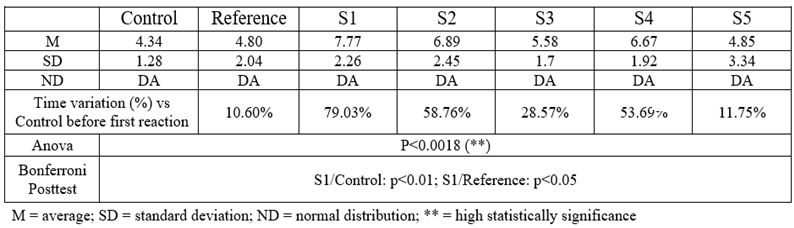
Table 7.
Time until the first reaction to the heat stimulus (53°C)
Table 7.
Time until the first reaction to the heat stimulus (53°C)
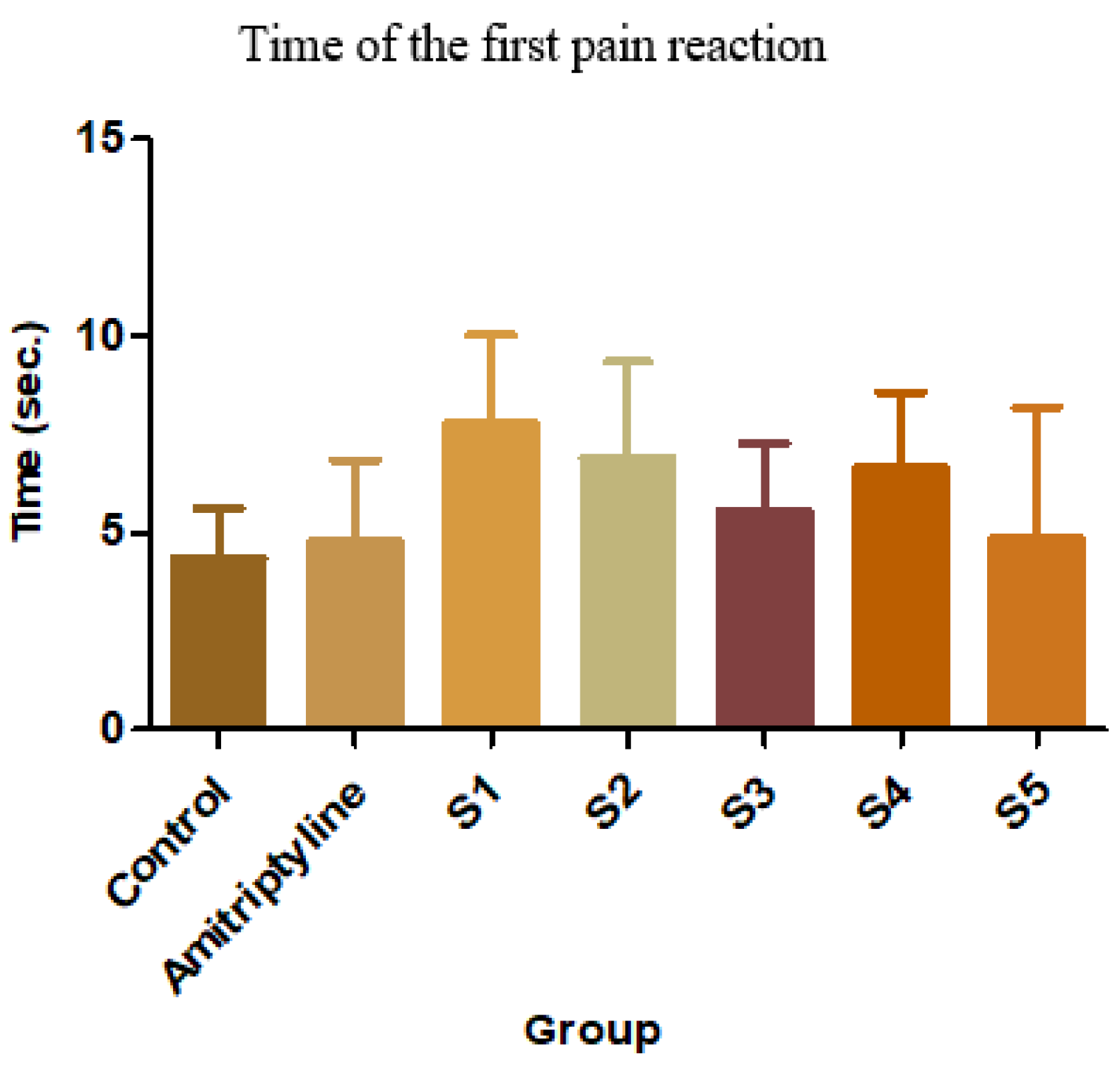
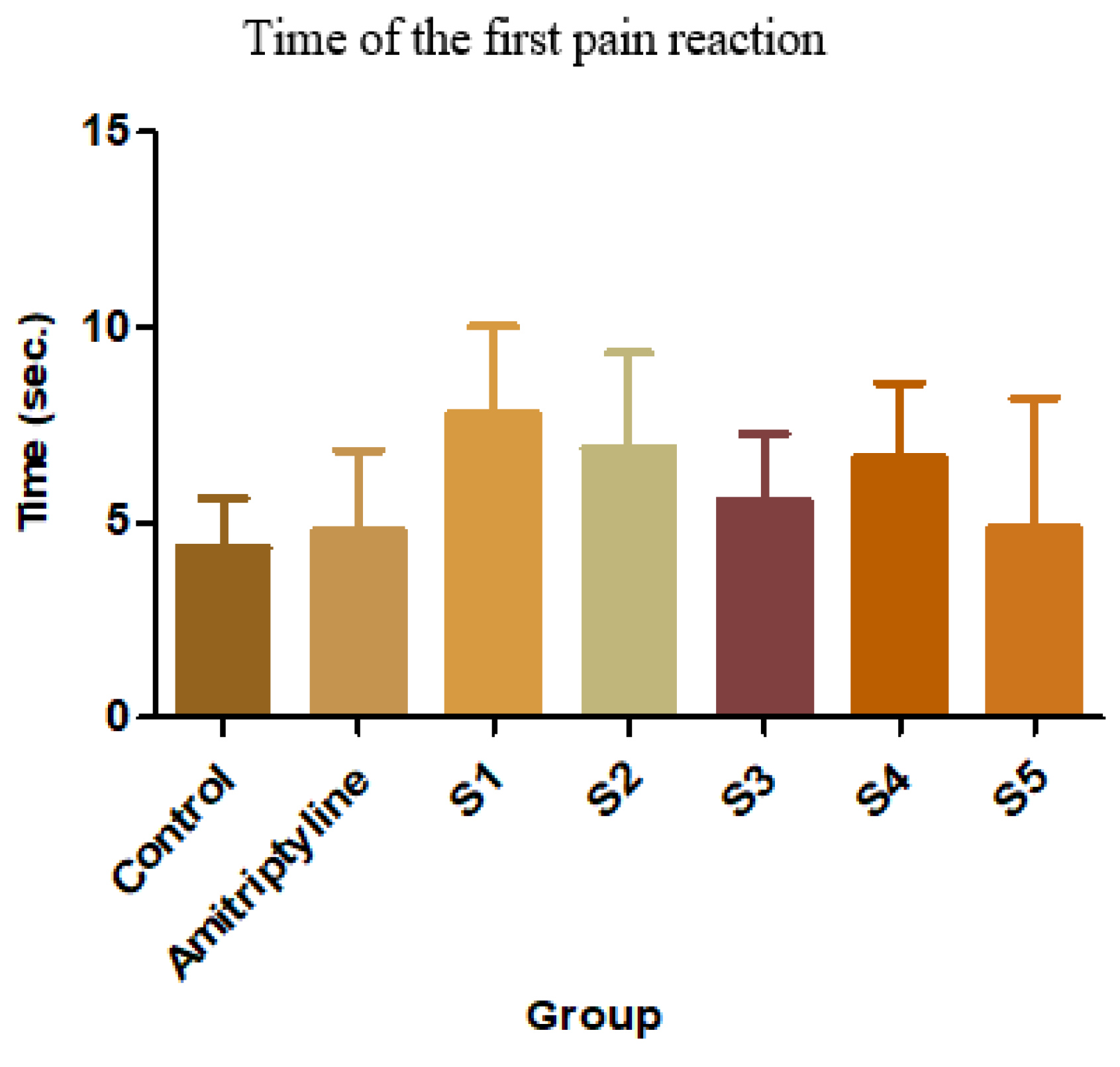
Figure 9.
Average time of the first pain reaction
Figure 9.
Average time of the first pain reaction
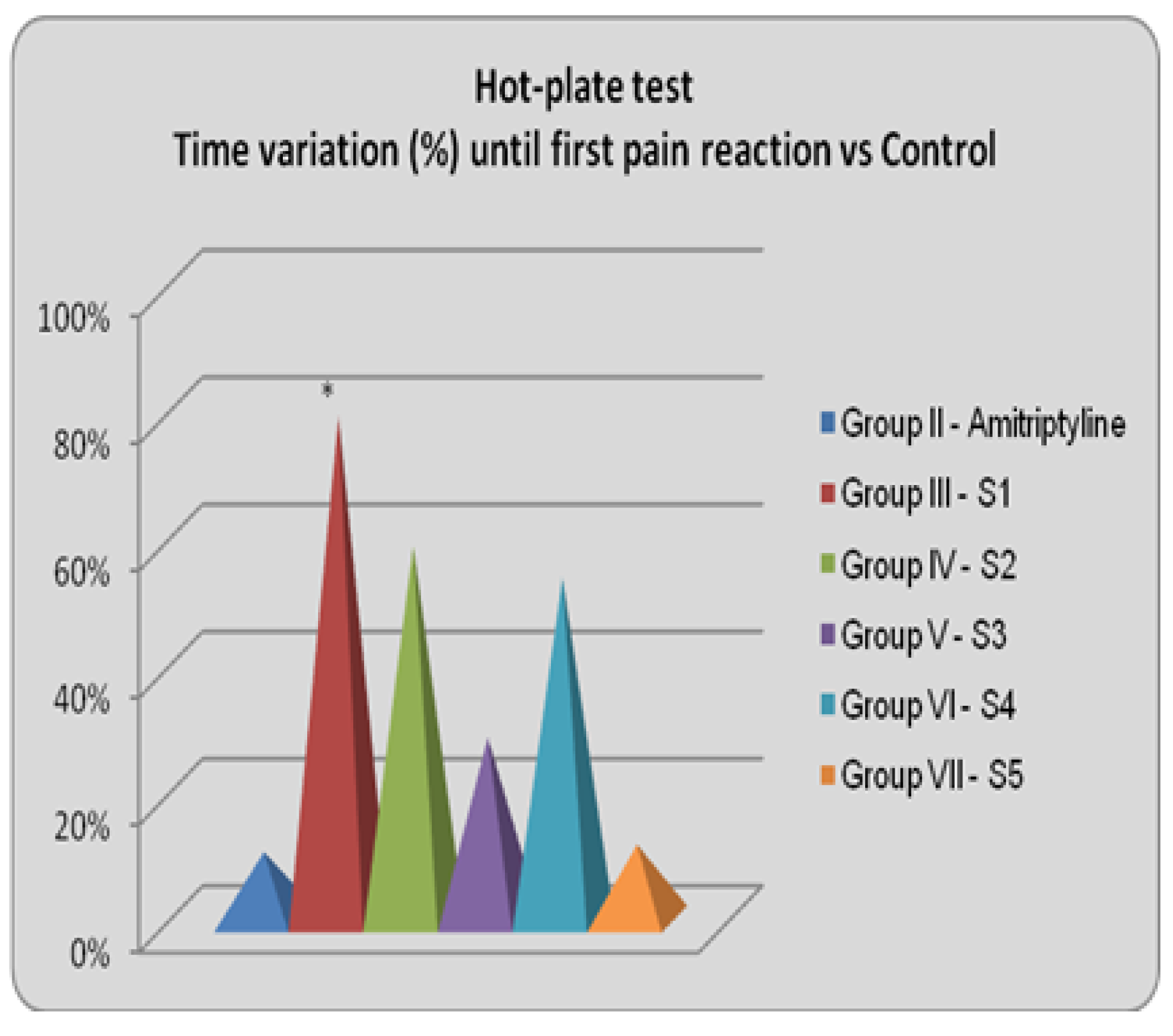
Figure 10.
Analgesic effect vs Control
Figure 10.
Analgesic effect vs Control
S1 has the most intense analgesic effect compared to the control group (79.03%, p<0.01, ANOVA + Bonferroni) and also to the reference group (61.87%, p<0.05, ANOVA + Bonferoni). S2 also delayed the first pain reaction by 58.76% compared to the control group, while S4 delayed by 53.69%. S3 and S5 had negligible analgesic effects [
17,
18].
Amitriptyline used as a reference had a low analgesic effect after a single dose of 10 mg/kg bw. This result is consistent with the other reports [
19,
20].
The scientific literature also mentions the use of tricyclic antidepressants as co-analgesics in the treatment of some forms of pain [
21].
Conclusions
The five new quinasolines tested in this study induced various changes in the parameters investigated in the preclinical tests conducted.
S3 and S5 proved to have similar pharmacological profiles in that while S3 lowered HMA by 20% and VMA by 28.01% compared to the control group, S5 had the same effect on HMA (29,8%; P<0.01 - ANOVA + Bonferroni) and to VMA, which was lowered by 37.78% compared to the control group.
The same 2 compounds showed the most intense anxiolytic potential by increasing the time spent in the open arms of the cross-maze (S3: 44.35%; S5: 34.36%) on one hand, and, on the other hand, the number of exits from the open arms (S3: 13.9%; S5: 18.24%). However, S3 and S5 had no antidepressant or analgesic effect.
S1 and S2 reduced the immobilization time in the forced swimming test by 25.98% and 28.45% respectively (p<0.05%; ANOVA + Bonferroni). The same 2 compounds also registered the most intense analgesic effects compared to the control group (S1: 79.03%, p<0.01; S2: 58.76%). S1 had a significant analgesic effect also compared to amitriptyline (61.87%, p<0.05%; ANOVA + Bonferroni).
A good correlation between the antidepressant and the analgesic effects was observed, which is consistent with the fact that analgesic drugs, by increasing the norepinephrine and serotonin levels in the pain inhibiting descendent pathways, can be used as co-analgesics in therapy.
From our analyses, we confirm that the tested compounds have high pharmacological potential worthy of further investigation.