Associated Factors and Predictors of Medication Errors in Saudi Arabia: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Selection
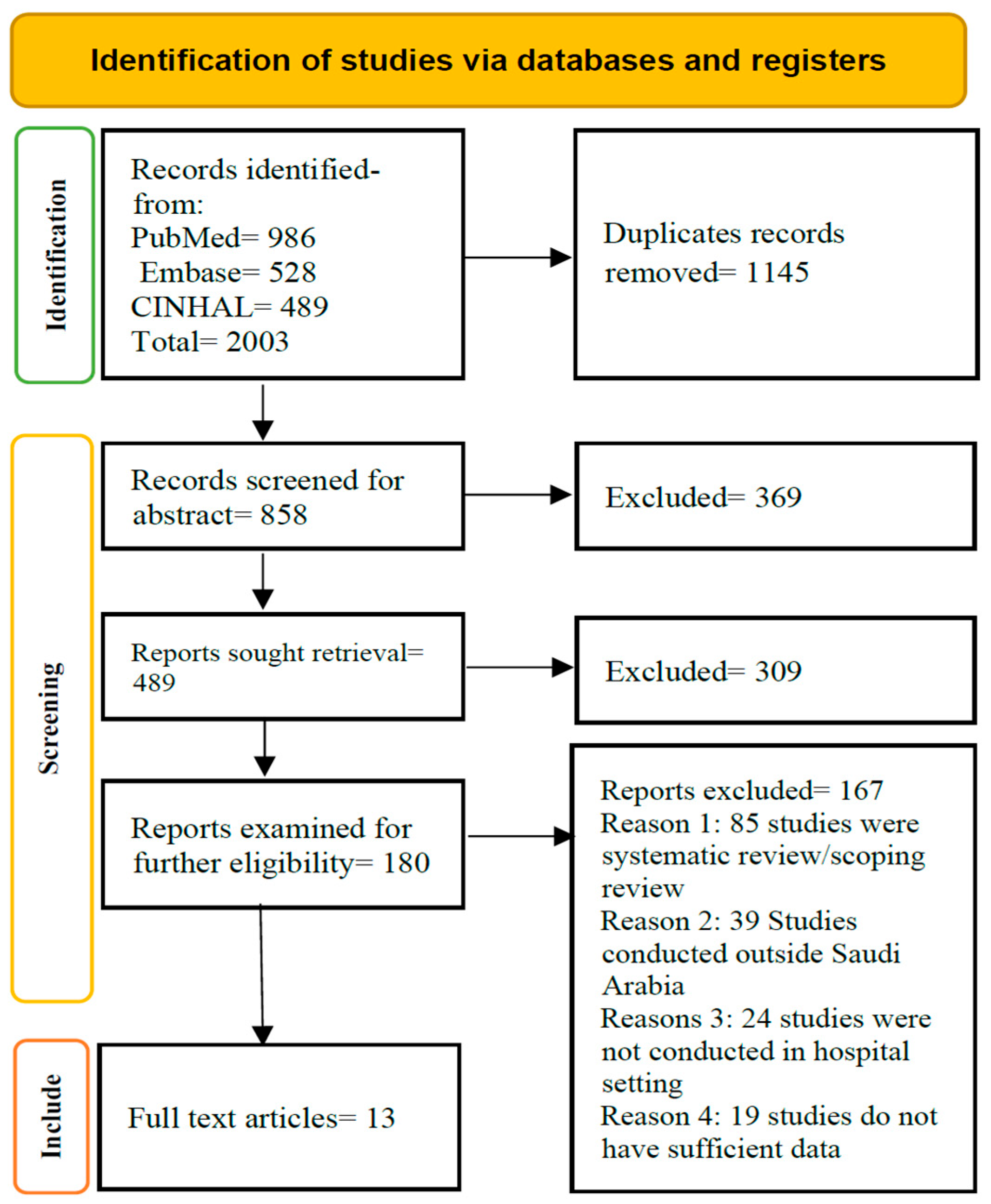
2.2. Search Outcome
2.3. Data Extraction and Methodological Quality Assessment
3. Results
3.1. Methodological Quality Appraisal
3.2. Characteristics of the Included Study
3.3. Associated Factors and Predictors of Medication Errors
3.3.1. Healthcare Workers
3.3.2. Patients
3.3.3. Institutional Settings and Culture
4. Discussion
Strengths and Weaknesses
5. Conclusions
- (i)
- Implementing targeted continuing education and simulation-based training programs on medication safety for healthcare professionals;
- (ii)
- Developing a national medication error-reporting registry to improve surveillance and benchmarking;
- (iii)
- Fostering collaborative interdisciplinary practice models;
- (iv)
- Incorporating medication safety competencies in undergraduate and postgraduate curricula.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MEs | Medical Errors |
| MAE | Medication Administration Error |
| ICU | Intensive Care Unit |
| PSC | Patient Safety Culture |
Appendix A
| No. | Question | Yes | No | Don’t Know (Comment) |
|---|---|---|---|---|
| Introduction | ||||
| 1 | Were the aims/objectives of the study clear? | |||
| 2 | Was the study design appropriate for the stated aim(s)? | |||
| 3 | Was the sample size justified? | |||
| 4 | Was the target/reference population clearly defined? (Is it clear who the research was about?) | |||
| 5 | Was the sample frame taken from an appropriate population base so that it closely represented the target/reference population under investigation? | |||
| 6 | Was the selection process likely to select subjects/participants that were representative of the target/reference population under investigation | |||
| 7 | Were measures undertaken to address and categorize non-responders? | |||
| 8 | Were the risk factor and outcome variables measured appropriate to the aims of the study | |||
| 9 | Were the risk factor and outcome variables measured correctly using instruments/measurements that had been trialed, piloted or published previously? | |||
| 10 | Is it clear what was used to determine statistical significance and/or precision estimates? (e.g., p-values, confidence intervals) | |||
| 11 | Were the methods (including statistical methods) sufficiently described to enable them to be repeated? | |||
| Results | ||||
| 12 | Were the basic data adequately described? | |||
| 13 | Does the response rate raise concerns about non-response bias? | 0 | 1 | |
| 14 | If appropriate, was information about non-responders described? | |||
| 15 | Were the results internally consistent? | |||
| 16 | Were the results presented for all the analyses described in the methods? | |||
| Discussion | ||||
| 17 | Were the authors’ discussions and conclusions justified by the results? | |||
| 18 | Were the limitations of the study discussed? | |||
| Other | ||||
| 19 | Were there any funding sources or conflicts of interest that may affect the authors’ interpretation of the results? | |||
| 20 | Was ethical approval or consent of participants attained? | |||
References
- Organisation for Economic Co-Operation and Development. Patient Safety: From Analysis to Action; Organisation for Economic Co-Operation and Development: Paris, France, 2020; Available online: http://www.oecd.org/health/health-systems/Economics-of-Patient-Safety-October-2020.pdf (accessed on 6 September 2023).
- Panagioti, M.; Khan, K.; Keers, R.N.; Abuzour, A.S.; Phipps, D.; Kontopantelis, E.; Bower, P.; Campbell, S.; Haneef, R.; Avery, A.J.; et al. Prevalence, severity, and nature of preventable patient harm across medical care settings: Systematic review and meta-analysis. BMJ 2019, 366, l4185. [Google Scholar] [CrossRef]
- Hodkinson, A.; Tyler, N.; Ashcroft, D.M.; Keers, R.N.; Khan, K.; Phipps, D.; Abuzour, A.; Bower, P.; Avery, A.; Campbell, S.; et al. Preventable medication harm across health care settings: A systematic review and meta-analysis. BMC Med. 2020, 18, 313. [Google Scholar] [CrossRef]
- Aitken, M.; Gorokhovich, L. Advancing the Responsible Use of Medicines: Applying Levers for Change. 2012. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=2222541 (accessed on 4 June 2024).
- Cronenwett, L.R.; Bootman, J.L.; Wolcott, J.; Aspden, P. (Eds.) Preventing Medication Errors; National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Alharaibi, M.A.; Alhifany, A.A.; Asiri, Y.A.; Alwhaibi, M.M.; Ali, S.; Jaganathan, P.P.; Alhawassi, T.M. Prescribing errors among adult patients in a large tertiary care system in Saudi Arabia. Ann. Saudi Med. 2021, 41, 147–156. [Google Scholar] [CrossRef]
- Alshammari, T.M.; Alenzi, K.A.; Alatawi, Y.; Almordi, A.S.; Altebainawi, A.F. Current situation of medication errors in Saudi Arabia: A nationwide observational study. J. Patient Saf. 2022, 18, e448–e453. [Google Scholar] [CrossRef]
- Aljadhey, H.; Mahmoud, M.A.; Hassali, M.A.; Alrasheedy, A.; Alahmad, A.; Saleem, F.; Sheikh, A.; Murray, M.; Bates, D.W. Challenges to and the future of medication safety in Saudi Arabia: A qualitative study. Saudi Pharm. J. 2014, 22, 326–332. [Google Scholar] [CrossRef]
- Bates, D.W.; Cullen, D.J.; Laird, N.; Petersen, L.A.; Small, S.D.; Servi, D.; Laffel, G.; Sweitzer, B.J.; Shea, B.F.; Hallisey, R.; et al. Incidence of adverse drug events and potential adverse drug events: Implications for prevention. JAMA 1995, 274, 29–34. [Google Scholar] [CrossRef]
- Al-Dhawailie, A.A. Inpatient prescribing errors and pharmacist intervention at a teaching hospital in Saudi Arabia. Saudi Pharm. J. 2011, 19, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Aljadhey, H.; Alhusan, A.; Alburikan, K.; Adam, M.; Murray, M.D.; Bates, D.W. Medication safety practices in hospitals: A national survey in Saudi Arabia. Saudi Pharm. J. 2013, 21, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Alsulami, Z.; Conroy, S.; Choonara, I. Medication errors in the Middle East countries: A systematic review of the literature. Eur. J. Clin. Pharmacol. 2013, 69, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Tobaiqy, M.; Stewart, D. Exploring health professionals’ experiences of medication errors in Saudi Arabia. Int. J. Clin. Pharm. 2013, 35, 542–545. [Google Scholar] [CrossRef]
- Isaksson, S.; Schwarz, A.; Rusner, M.; Nordström, S.; Källman, U. Monitoring preventable adverse events and near misses: Number and type identified differ depending on method used. J. Patient Saf. 2022, 18, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Hibbert, P.D.; Molloy, C.J.; Schultz, T.J.; Carson-Stevens, A.; Braithwaite, J. Comparing rates of adverse events detected in incident reporting and the Global Trigger Tool: A systematic review. Int. J. Qual. Health Care 2023, 35, mzad056. [Google Scholar] [CrossRef] [PubMed]
- Stovold, E.; Beecher, D.; Foxlee, R.; Noel-Storr, A. Study flow diagrams in Cochrane systematic review updates: An adapted PRISMA flow diagram. Syst. Rev. 2014, 3, 54. [Google Scholar] [CrossRef]
- Downes, M.J.; Brennan, M.L.; Williams, H.C.; Dean, R.S. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016, 6, e011458. [Google Scholar] [CrossRef]
- Al Khreem, S.M.; Al-khadher, M. Perceptions of Nurses about Medication Errors: A Cross-Sectional Study. J. Sci. Res. Med. Biol. Sci. 2021, 2, 30–41. [Google Scholar] [CrossRef]
- Mazhar, F.; Akram, S.; Al-Osaimi, Y.A.; Haider, N. Medication reconciliation errors in a tertiary care hospital in Saudi Arabia: Admission discrepancies and risk factors. Pharm. Pract. 2017, 15, 864. [Google Scholar] [CrossRef]
- Aljadhey, H.; Mahmoud, M.A.; Ahmed, Y.; Sultana, R.; Zouein, S.; Alshanawani, S.; Mayet, A.; Alshaikh, M.K.; Kalagi, N.; Al Tawil, E.; et al. Incidence of adverse drug events in public and private hospitals in Riyadh, Saudi Arabia: The (ADESA) prospective cohort study. BMJ Open 2016, 6, e010831. [Google Scholar] [CrossRef]
- Al-Arifi, M.N. Community pharmacists’ attitudes toward dispensing errors at community pharmacy setting in Central Saudi Arabia. Saudi Pharm. J. 2014, 22, 195–202. [Google Scholar] [CrossRef]
- Tawhari, M.M.H.; Mahlawi, A.Q.M.; Althrwi, S.N.A.; Noshily, M.A.; Tawhari, M.A.; Bakri, H.A. Evaluation of Medication Error Incident Reports at a Tertiary Care Hospital. Int. J. Med. Dev. Ctries. 2024, 8, 7–13. [Google Scholar] [CrossRef]
- Al-Maghrabi, M.; Mamede, S.; Schmidt, H.G.; Omair, A.; Al-Nasser, S.; Alharbi, N.S.; Magzoub, M.E.M.A. Overconfidence, Time-on-Task, and Medical Errors: Is There a Relationship. Adv. Med. Educ. Pract. 2024, 15, 133–140. [Google Scholar] [CrossRef]
- Alsafi, E.; Baharoon, S.; Ahmed, A.; Al-Jahdali, H.H.; Al Zahrani, S.; Al Sayyari, A. Physicians’ knowledge and practice towards medical error reporting: A cross-sectional hospital-based study in Saudi Arabia. East. Mediterr. Health J. 2015, 21, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Alduais, A.M.S.; Mogali, S.; Al Shabrain, B.; Al Enazi, A.; Al-awad, F. Barriers and strategies of reporting medical errors in public hospitals in Riyadh city: A survey-study. IOSR J. Nurs. Health Sci. 2014, 3, 72–85. [Google Scholar] [CrossRef]
- Alyaemni, A. Patient safety and medical error disclosure: Evidence from across-sectional study at a tertiary hospital in saudi arabia. Res. Sq. 2023; preprint. [Google Scholar] [CrossRef]
- Alrasheeday, A.M.; Alkubati, S.A.; Alqalah, T.A.H.; Alrubaiee, G.G.; Pasay-An, E.; Alshammari, B.; Abdullah, S.O.; Loutfy, A. Nurses’ perceptions of patient safety culture and adverse events in Hail City, Saudi Arabia: A cross-sectional approach to improving healthcare safety. BMJ Open 2024, 14, e084741. [Google Scholar] [CrossRef]
- Alandajani, A.; Khalid, B.; Ng, Y.G.; Banakhar, M. Knowledge and Attitudes Regarding Medication Errors among Nurses: A Cross-Sectional Study in Major Jeddah Hospitals. Nurs. Rep. 2022, 12, 1023–1039. [Google Scholar] [CrossRef]
- Berk, W.A.; Welch, R.D.; Levy, P.D.; Jones, J.T.; Arthur, C.; Kuhn, G.J.; King, J.J.; Bock, B.F.; Sweeny, P.J. The effect of clinical experience on the error rate of emergency physicians. Ann. Emerg. Med. 2008, 52, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Reason, J. Human error: Models and management. BMJ 2000, 320, 768–770. [Google Scholar] [CrossRef]
- Marx, D. Patient Safety and the Just Culture. Obstet. Gynecol. Clin. N. Am. 2019, 46, 239–245. [Google Scholar] [CrossRef]
- Keohane, C.A.; Bates, D.W. Medication safety. Obstet. Gynecol. Clin. N. Am. 2008, 35, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Olds, D.M.; Clarke, S.P. The effect of work hours on adverse events and errors in health care. J. Saf. Res. 2010, 41, 153–162. [Google Scholar] [CrossRef]
- Lockley, S.W.; Barger, L.K.; Ayas, N.T.; Rothschild, J.M.; Czeisler, C.A.; Landrigan, C.P. Effects of health care provider work hours and sleep deprivation on safety and commitance. Jt. Comm. J. Qual. Patient Saf. 2007, 33, 7–18. [Google Scholar]
- Barger, L.K.; Ayas, N.T.; Cade, B.E.; Cronin, J.W.; Rosner, B.; Speizer, F.E.; Czeisler, C.A. Impact of extended-duration shifts on medical errors, adverse events, and attentional failures. PLoS Med. 2006, 3, e487. [Google Scholar] [CrossRef]
- Alshammari, F.M.; Alanazi, E.J.; Alanazi, A.M.; Alturifi, A.K.; Alshammari, T.M. Medication error concept and reporting practices in Saudi Arabia: A multiregional study among healthcare professionals. Risk Manag. Healthc. Policy 2021, 14, 2395–2406. [Google Scholar] [CrossRef]
- Teoh, B.; Alrasheedy, A.; Hassali, M.; Tew, M.; Samsudin, M. Perceptions of doctors and pharmacists towards medication error reporting and prevention in Kedah, Malaysia: A Rasch model analysis. Adv. Pharmacoepidemiol. Drug Saf. 2015, 4, 1052–2167. [Google Scholar]
- Hu, K.T.; Matayoshi, A.; Stevenson, F.T. Calculation of the estimated creatinine clearance in avoiding drug dosing errors in the older patient. Am. J. Med. Sci. 2001, 322, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Won, H.-J.; Chung, G.; Lee, K.J.; Lee, E.; Son, S.; Choi, S.; Park, S.C.; Lee, Y.J. Evaluation of medication dosing errors in elderly patients with renal impairment. Int. J. Clin. Pharmacol. Ther. 2018, 56, 358–365. [Google Scholar] [CrossRef]
- AlQashqri, H. Renally Inappropriate Medications in the Old Population: Prevalence, Risk Factors, Adverse Outcomes, and Potential Interventions. Cureus 2023, 15, e49111. [Google Scholar] [CrossRef]
- Fialová, D.; Onder, G. Medication errors in elderly people: Contributing factors and future perspectives. Br. J. Clin. Pharmacol. 2009, 67, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Teo, S.P. Reducing medication error and polypharmacy in older people. Asian J. Gerontol. Geriatr. 2020, 15, 86–90. [Google Scholar]
- Picone, D.M.; Titler, M.G.; Dochterman, J.; Shever, L.; Kim, T.; Abramowitz, P.; Kanak, M.; Qin, R. Predictors of medication errors among elderly hospitalized patients. Am. J. Med. Qual. 2008, 23, 115–127. [Google Scholar] [CrossRef]
- Liu, P.; Yang, Y.; Cheng, J. Gender differences in medical errors among older patients and inequalities in medical compensation compared with younger adults. Front. Public Health 2022, 10, 883822. [Google Scholar] [CrossRef] [PubMed]
- Oertelt-Prigione, S.; Regitz-Zagrosek, V. (Eds.) Sex and Gender Aspects in Clinical Medicine; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Keers, R.N.; Williams, S.D.; Cooke, J.; Ashcroft, D.M. Prevalence and nature of medication administration errors in health care settings: A systematic review of direct observational evidence. Ann. Pharmacother. 2013, 47, 237–256. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, J.M.; Landrigan, C.P.; Cronin, J.W.; Kaushal, R.; Lockley, S.W.; Burdick, E.; Stone, P.H.; Lilly, C.M.; Katz, J.T.; Czeisler, C.A.; et al. The Critical Care Safety Study: The incidence and nature of adverse events and serious medical errors in intensive care. Crit. Care Med. 2005, 33, 1694–1700. [Google Scholar] [CrossRef] [PubMed]
| Authors | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 * | Q14 | Q15 | Q16 | Q17 | Q18 | Q19 | Q20 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alshammari et al. [7] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 18 |
| Al Khreem & Al-khadher [18] | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 14 |
| Mazhar et al. [19] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 18 |
| Aljadhey et al. [20] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 18 |
| Al-Arifi [21] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 18 |
| Tawhari et al. [22] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 18 |
| Alharaibi et al. [6] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 18 |
| Al-Maghrabi et al. [23] | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 15 |
| Alsafi et al. [24] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 16 |
| Alduais et al. [25] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 18 |
| Alyaemni [26] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 17 |
| Alrasheeday et al. [27] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 18 |
| Alandajani et al. [28] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 18 |
| Author | Study Objectives | Study Design | Setting | Materials and Methods | Findings | Study Recommendations |
|---|---|---|---|---|---|---|
| Alshammari et al. [7] | To investigate predictors for healthcare professionals to report medication errors in Saudi Arabia | Observational cross-sectional study | Conducted in 6 regions: Hail, Al-Qassim, Al-Jouf, Al-Madinah, the eastern region, and the western region | Validated questionnaire with 37 close-ended questions on a five-point Likert scale | Significant predictors of medication error reporting:
| Need for interventions to address medication errors in Saudi Arabia, including regular education and training for healthcare professionals, mandatory training for new employees, and participation in symposiums on patient safety. |
| Al Khreem & Al-khadher [18] | To assess nurses’ perceptions of causes and reporting of medication errors | Cross-sectional study | Maternity and Children Hospital in Najran City, Saudi Arabia | Questionnaires on demographic data and reporting of medication errors | Significant factors associated with medication error reporting:
| The implementation of “No Blame, No Punishment” campaigns. Adopting a medication safety officer and incorporating pharmacovigilance in health college curricula is also crucial. |
| Mazhar et al. [19] | To describe the frequency and type of medication reconciliation errors
| Prospective cross-sectional study | Tertiary care teaching hospital in the eastern province of Saudi Arabia | Comprehensive-structured interviews using a standard form | Significant factors associated with medication reconciliation error:
| A higher authority at each institution should also encourage participation in symposiums on patient safety and drug safety. |
| Aljadhey et al. [20] | To determine the incidence of adverse drug events (ADEs) and their severity and preventability | Prospective cohort study | Setting | Trained clinical pharmacists using a study manual for incident assessment, including severity and preventability classifications | Significant factors associated with ADEs (adjusted):
| It is recommended that the factors that lead to medication errors must be considered by policy makers, and they should set policies and strategies to overcome the factors. |
| Al-Arifi [21] | To assess pharmacists’ attitudes toward dispensing errors | Cross-sectional study | Conducted in 6 regions: Hail, Al-Qassim, Al-Jouf, Al-Madinah, the eastern region, and the western region | Structured self-administered questionnaire | Significant factors associated with dispensing error:
| Decision makers consider the factors that lead to medication errors. |
| Tawhari et al. [22] | To assess incidents of medical errors at a hospital | Retrospective study |
|
| Significant factors associated with medical error:
| Policies and strategies must be developed to overcome the factors that are the causes of medication errors. |
| Alharaibi et al. [6] | To assess the prevalence, type, severity, and factors associated with prescribing errors | Retrospective database review | Tertiary care teaching hospital in the eastern province of Saudi Arabia | Medication Error Electronic Report Forms database from January 2017 to December 2018. | Significant factors associated with prescribing errors (adjusted):
| Staffing of clinical pharmacists in committing structured medication and reducing the risk of medication errors; |
| Al-Maghrabi et al. [23] | To investigate the effects of high confidence on diagnostic accuracy | Randomized controlled experiment | Setting | Eight written clinical vignettes were used for this study with the following diagnoses: stomach cancer, vitamin B12 deficiency, pulmonary thromboembolism, celiac disease, acute viral pericarditis, acute myeloid leukemia, and acute bacterial endocarditis | The study failed to display relationships between overconfidence and premature closure with diagnostic accuracy | Collaboration between healthcare providers, including nurses and physicians, on medication reconciliation to prevent unintentional discrepancies at admission in these high-risk patients. |
| Alsafi et al. [24] | To identify reasons for underreporting among physicians | Cross-sectional study | Conducted in 6 regions: Hail, Al-Qassim, Al-Jouf, Al-Madinah, the eastern region, and the western region | Self-administered questionnaire | Significant factors associated with medication error reporting:
| Study Recommendations |
| Alduais et al. [25] |
| Cross-sectional study | Maternity and Children Hospital in Najran City, Saudi Arabia | Self-administered questionnaire | Significant factors associated with medical error reporting:
| Need for interventions to address medication errors in Saudi Arabia, including regular education and training for healthcare professionals, mandatory training for new employees, and participation in symposiums on patient safety. |
| Alyaemni [26] | To assess the knowledge and attitudes about medical error disclosure and explore the factors that facilitate or hinder the disclosure | Cross-sectional survey | Tertiary care teaching hospital in the eastern province of Saudi Arabia | Self-administered questionnaire | Significant factors associated with medical error reporting:
| The implementation of “No Blame, No Punishment” campaigns. Adopting a medication safety officer and incorporating pharmacovigilance in health college curricula is also crucial. |
| Alrasheeday et al. [27] | To assess nurses’ perceptions of patient safety culture and its relationship with adverse events | Cross-sectional study | Setting | Three self-administered questionnaires on demographic factors, patient safety culture, and adverse events | Significant factors associated with adverse medical events | A higher authority at each institution should also encourage participation in symposiums on patient safety and drug safety. |
| Alandajani et al. [28] | To investigate nurses’ knowledge and attitudes toward medication errors in Saudi Arabia | Cross-sectional study | Conducted in 6 regions: Hail, Al-Qassim, Al-Jouf, Al-Madinah, the eastern region, and the western region | Online self-administered questionnaire from January to March 2022 | Significant predictors of medication errors (adjusted):
| It is recommended that the factors that lead to medication errors must be considered by policy makers, and they should set policies and strategies to overcome the factors. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the JMMS. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mushi, M.H.; Nazan, A.I.N.M.; Ibrahim, M.I.; Rasdi, I.; Alsharqi, O.Z.; Albalawi, M.A. Associated Factors and Predictors of Medication Errors in Saudi Arabia: A Systematic Review. J. Mind Med. Sci. 2025, 12, 43. https://doi.org/10.3390/jmms12020043
Mushi MH, Nazan AINM, Ibrahim MI, Rasdi I, Alsharqi OZ, Albalawi MA. Associated Factors and Predictors of Medication Errors in Saudi Arabia: A Systematic Review. Journal of Mind and Medical Sciences. 2025; 12(2):43. https://doi.org/10.3390/jmms12020043
Chicago/Turabian StyleMushi, Mugapish Hussain, Ahmad Iqmer Nashriq Mohd Nazan, Mohd Ismail Ibrahim, Irniza Rasdi, Omar Zayyan Alsharqi, and Majed Awad Albalawi. 2025. "Associated Factors and Predictors of Medication Errors in Saudi Arabia: A Systematic Review" Journal of Mind and Medical Sciences 12, no. 2: 43. https://doi.org/10.3390/jmms12020043
APA StyleMushi, M. H., Nazan, A. I. N. M., Ibrahim, M. I., Rasdi, I., Alsharqi, O. Z., & Albalawi, M. A. (2025). Associated Factors and Predictors of Medication Errors in Saudi Arabia: A Systematic Review. Journal of Mind and Medical Sciences, 12(2), 43. https://doi.org/10.3390/jmms12020043







