Early Childhood Nutrition and Development in Atopic Families from Northeastern Bulgaria
Abstract
1. Introduction
Research Gaps and Future Directions
2. Objective of the Study
3. Methodology
3.1. Study Design and Setting
3.2. Participants and Recruitment
3.3. Eligibility Criteria
3.3.1. Inclusion Criteria
3.3.2. Exclusion Criteria
4. Study Measurements and Outcomes
4.1. Demographic and Social Data:
4.1.1. Medical Data
4.1.2. Smoking Status
4.1.3. Breastfeeding and Early Feeding Practices
4.1.4. Assessment of Neuropsychological Development
4.2. Clinical Data
4.3. Nutritional Status Assessment
5. Data Management and Analysis
6. Results
6.1. Socio-Demographic Profile of the Study Participants
6.2. Medical Data and Smoking Status
6.3. Developmental Outcomes (DP-3)
7. Discussion
7.1. Smoking in the Family
7.2. Early Feeding, Parental Age, and Education
7.3. Early Feeding, Allergic Diseases, Growth, and Development
7.4. Neuropsychological Development (DP-3) in Atopic Families
7.4.1. Factors Related to Children’s Neuropsychological Development (NPD)
7.4.2. Physical and Adaptive Development
7.4.3. Social–Emotional and Cognitive Development
7.4.4. Communication and Overall Development
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schoos, A.-M.M. Atopic diseases—Diagnostics, mechanisms, and exposures. Pediatr. Allergy Immunol. 2024, 35, e14198. [Google Scholar] [CrossRef] [PubMed]
- Adeyeye, T.E.; Yeung, E.H.; McLain, A.C.; Lin, S.; Lawrence, D.A.; Bell, E.M. Wheeze and Food Allergies in Children Born via Cesarean Delivery: The Upstate KIDS Study. Am. J. Epidemiol. 2019, 188, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Bantz, S.K.; Zhu, Z.; Zheng, T. The Atopic March: Progression from Atopic Dermatitis to Allergic Rhinitis and Asthma. J. Clin. Cell. Immunol. 2014, 5, 202. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Mach, N. Role of Vitamin D in the Hygiene Hypothesis: The Interplay between Vitamin D, Vitamin D Receptors, Gut Microbiota, and Immune Response. Front. Immunol. 2016, 7, 627. [Google Scholar] [CrossRef]
- Sandini, U.; Kukkonen, A.K.; Poussa, T.; Sandini, L.; Savilahti, E.; Kuitunen, M. Protective and Risk Factors for Allergic Diseases in High-Risk Children at the Ages of Two and Five Years. Int. Arch. Allergy Immunol. 2011, 156, 339–348. [Google Scholar] [CrossRef]
- Bigman, G. Exclusive breastfeeding for the first 3 months of life may reduce the risk of respiratory allergies and some asthma in children at the age of 6 years. Acta Paediatr. 2020, 109, 1627–1633. [Google Scholar] [CrossRef]
- Kull, I.; Almqvist, C.; Lilja, G.; Pershagen, G.; Wickman, M. Breast-feeding reduces the risk of asthma during the first 4 years of life. J. Allergy Clin. Immunol. 2004, 114, 755–760. [Google Scholar] [CrossRef]
- Lodge, C.; Tan, D.; Lau, M.; Dai, X.; Tham, R.; Lowe, A.; Bowatte, G.; Allen, K.; Dharmage, S. Breastfeeding and asthma and allergies: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 38–53. [Google Scholar] [CrossRef]
- Bernard, J.Y.; Armand, M.; Peyre, H.; Garcia, C.; Forhan, A.; De Agostini, M.; Charles, M.-A.; Heude, B.; EDEN Mother-Child Cohort Study Group. Breastfeeding, Polyunsaturated Fatty Acid Levels in Colostrum and Child Intelligence Quotient at Age 5–6 Years. J. Pediatr. 2017, 183, 43–50. [Google Scholar] [CrossRef]
- Georgieff, M.K.; Ramel, S.E.; Cusick, S.E. Nutritional influences on brain development. Acta Paediatr. 2018, 107, 1310–1321. [Google Scholar] [CrossRef]
- Horta, B.L.; de Sousa, B.A.; de Mola, C.L. Breastfeeding and neurodevelopmental outcomes. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Wallenborn, J.T.; Levine, G.A.; Carreira Dos Santos, A.; Grisi, S.; Brentani, A.; Fink, G. Breastfeeding, Physical Growth, and Cognitive Development. Pediatrics 2021, 147, e2020008029. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, A.E.; Bisiacchi, P.; Tarantino, V.; Chiarotti, F.; D’elia, L.; De Mei, B.; Romano, M.; Gesualdo, F.; Salmaso, S. Effect of duration of breastfeeding on neuropsychological development at 10 to 12 years of age in a cohort of healthy children. Dev. Med. Child Neurol. 2012, 54, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.; Fujiwara, T. Breastfeeding and risk of atopic dermatitis up to the age 42 months: A birth cohort study in Japan. Ann. Epidemiol. 2014, 24, 267–272. [Google Scholar] [CrossRef]
- Lin, B.; Dai, R.; Lu, L.; Fan, X.; Yu, Y. Breastfeeding and Atopic Dermatitis Risk: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Dermatology 2020, 236, 345–360. [Google Scholar] [CrossRef]
- Kramer, M.S.; Aboud, F.; Mironova, E.; Vanilovich, I.; Platt, R.W.; Matush, L.; Igumnov, S.; Fombonne, E.; Bogdanovich, N.; Ducruet, T.; et al. Breastfeeding and Child Cognitive Development: New Evidence from a Large Randomized Trial. Arch. Gen. Psychiatry 2008, 65, 578–584. [Google Scholar] [CrossRef]
- Meldrum, S.J.; D’Vaz, N.; Dunstan, J.A.; Mori, T.A.; Hird, K.; Simmer, K.; Prescott, S.L. Allergic disease in the first year of life is associated with differences in subsequent neurodevelopment and behavior. Early Hum. Dev. 2012, 88, 567–573. [Google Scholar] [CrossRef]
- Kim, J.H.; Yi, Y.Y.; Ha, E.K.; Cha, H.R.; Han, M.Y.; Baek, H.-S. Neurodevelopment at 6 years of age in children with atopic dermatitis. Allergol. Int. 2023, 72, 116–127. [Google Scholar] [CrossRef]
- Easey, K.E.; Sharp, G.C. The impact of paternal alcohol, tobacco, caffeine use and physical activity on offspring mental health: A systematic review and meta-analysis. Reprod. Health 2021, 18, 214. [Google Scholar] [CrossRef]
- Polańska, K.; Muszyński, P.; Sobala, W.; Dziewirska, E.; Merecz-Kot, D.; Hanke, W. Maternal lifestyle during pregnancy and child psychomotor development—Polish Mother and Child Cohort study. Early Hum. Dev. 2015, 91, 317–325. [Google Scholar] [CrossRef]
- Koletzko, B.; Godfrey, K.M.; Poston, L.; Szajewska, H.; Van Goudoever, J.B.; De Waard, M.; Brands, B.; Grivell, R.M.; Deussen, A.R.; Dodd, J.M.; et al. Nutrition During Pregnancy, Lactation and Early Childhood and its Implications for Maternal and Long-Term Child Health: The Early Nutrition Project Recommendations [Review of Nutrition During Pregnancy, Lactation and Early Childhood and its Implications for Maternal and Long-Term Child Health: The Early Nutrition Project Recommendations]. Ann. Nutr. Metab. 2019, 74, 93. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management [Review of Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management]. J. Allergy Clin. Immunol. 2017, 141, 41. [Google Scholar] [CrossRef] [PubMed]
- Turcanu, V.; Brough, H.A.; Toit, G.D.; Foong, R.; Marrs, T.; Santos, A.F.; Lack, G. Immune mechanisms of food allergy and its prevention by early intervention [Review of Immune mechanisms of food allergy and its prevention by early intervention]. Curr. Opin. Immunol. 2017, 48, 92. [Google Scholar] [CrossRef]
- Filaci, G.; Rizzi, M.; Setti, M.; Fenoglio, D.; Fravega, M.; Basso, M.; Ansaldo, G.; Ceppa, P.; Borgonovo, G.; Murdaca, G.; et al. Non-antigen-specific CD8(+) T suppressor lymphocytes in diseases characterized by chronic immune responses and inflammation. Ann. New York Acad. Sci. 2005, 1050, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, K.N.; Eskenazi, B.; Schantz, S.; Yolton, K.; Rauh, V.A.; Johnson, C.B.; Alkon, A.; Canfield, R.L.; Pessah, I.N.; Berman, R.F. Principles and practices of neurodevelopmental assessment in children: Lessons learned from the Centers for Children’s Environmental Health and Disease Prevention Research. Environ. Health Perspect. 2005, 113, 1437–1446. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Julvez, J.; Ribas-Fitó, N.; Torrent, M.; Forns, M.; Garcia-Esteban, R.; Sunyer, J. Maternal smoking habits and cognitive development of children at age 4 years in a population-based birth cohort. Int. J. Epidemiol. 2007, 36, 825–832. [Google Scholar] [CrossRef]
- Tanaka, K.; Arakawa, M.; Miyake, Y. Perinatal smoking exposure and risk of asthma in the first three years of life: A prospective prebirth cohort study. Allergol. Et Immunopathol. 2020, 48, 530–536. [Google Scholar] [CrossRef]
- National Statistical Institute of Bulgaria. Population and Demographic Processes; NSI: Sofia, Bulgaria, 2024; Available online: https://nsi.bg/bg (accessed on 1 March 2025).
- Leung, T.; Tam, W.; Hung, E.; Fok, T.; Wong, G. Sociodemographic and atopic factors affecting breastfeeding intention in Chinese mothers. J. Paediatr. Child Health 2003, 39, 460–464. [Google Scholar] [CrossRef]
- Dragneva, P. Factors Influencing Pregnant Women’s Choice of an Elective Delivery. The midwife’s role. Ph.D. Thesis, Medical University of Varna, Varna, Bulgaria, 2023. [Google Scholar]
- Leventakou, V.; Roumeliotaki, T.; Koutra, K.; Vassilaki, M.; Mantzouranis, E.; Bitsios, P.; Kogevinas, M.; Chatzi, L. Breastfeeding duration and cognitive, language and motor development at 18 months of age: Rhea mother–child cohort in Crete, Greece. J. Epidemiol. Community Health 2015, 69, 232–239. [Google Scholar] [CrossRef]
- Paller, A.S.; Spergel, J.M.; Mina-Osorio, P.; Irvine, A.D. The atopic march and atopic multimorbidity: Many trajectories, many pathways. J. Allergy Clin. Immunol. 2019, 143, 46–55. [Google Scholar] [CrossRef]
- Kull, I.; Wickman, M.; Lilja, G.; Nordvall, S.L.; Pershagen, G. Breast feeding and allergic diseases in infants—A prospective birth cohort study. Arch. Dis. Child. 2002, 87, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Paduraru, D. The evidence for the benefits from breast milk in the neurodevelopment of premature babies—A review of the recent literature. JMMS 2018, 5, 151–157. [Google Scholar] [CrossRef]
- Polidano, C.; Zhu, A.; Bornstein, J.C. The relation between cesarean birth and child cognitive development. Sci. Rep. 2017, 7, 11483. [Google Scholar] [CrossRef] [PubMed]
- Blazkova, B.; Pastorkova, A.; Solansky, I.; Veleminsky, M., Jr.; Veleminsky, M.; Rossnerova, A.; Honkova, K.; Rossner, P., Jr.; Sram, R.J. The Impact of Cesarean and Vaginal Delivery on Results of Psychological Cognitive Test in 5 Year Old Children. Medicina 2020, 56, 554. [Google Scholar] [CrossRef]
- Zaigham, M.; Hellström-Westas, L.; Domellöf, M.; Andersson, O. Prelabour caesarean section and neurodevelopmental outcome at 4 and 12 months of age: An observational study. BMC Pregnancy Childbirth 2020, 20, 564. [Google Scholar] [CrossRef]
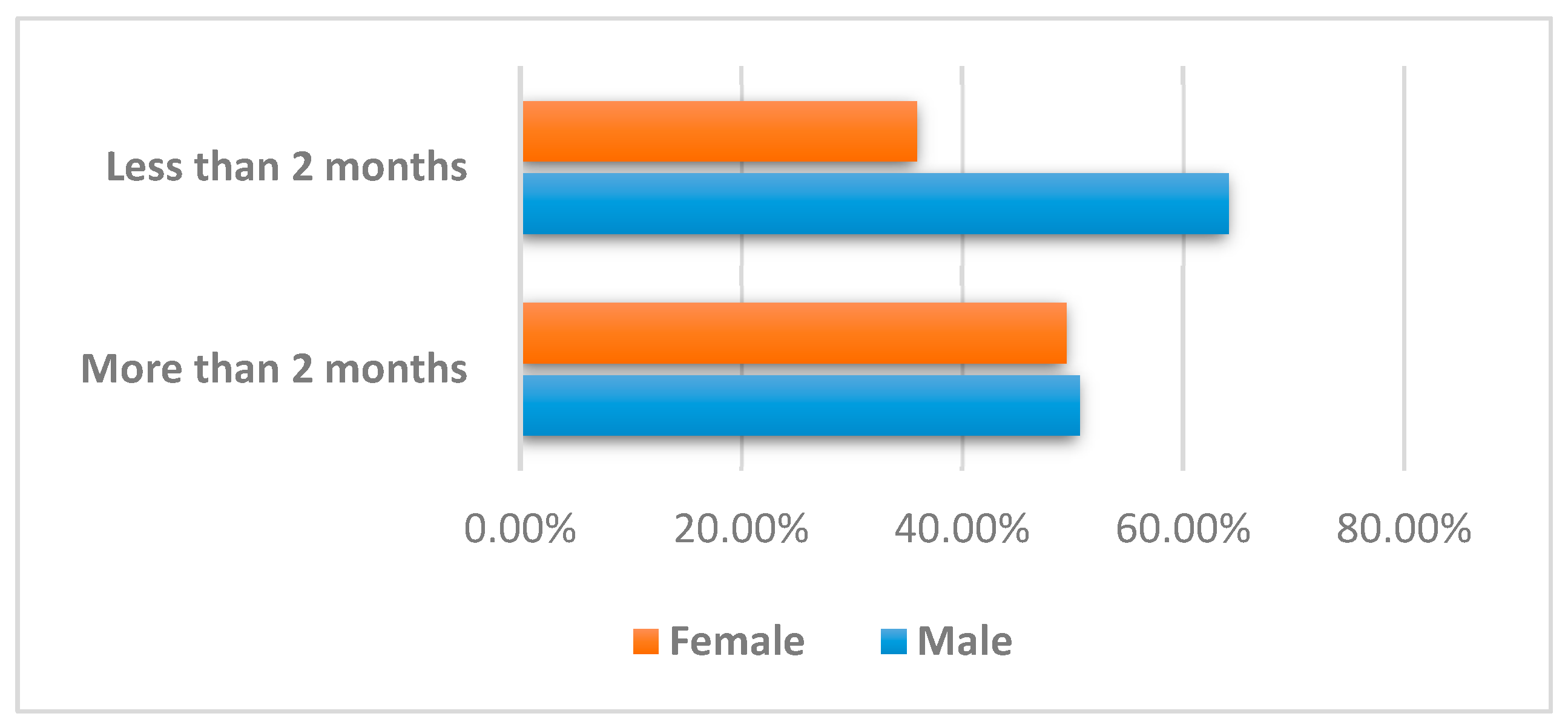
| Category | Variable | Group A More than 2 Months (n = 81) | Group B Less than 2 Months (n = 39) |
|---|---|---|---|
| Demographics | Gender | ||
| Male | 41 (50.6%) | 25 (64.1%) | |
| Female | 40 (49.4%) | 14 (35.9%) | |
| Mother’s Age (years) | 30.7 (4.6) | 30.4 (4.5) | |
| Father’s Age (years) | 33.3 (4.6) | 33.8 (4.8) | |
| Parental Factors | Mother’s Education | ||
| Till Secondary | 25 (31.65%) | 15 (38.5%) | |
| Bachelor/Master | 54 (68.35%) | 24 (61.5%) | |
| Father’s Education | |||
| Till Secondary | 36 (44.4%) | 23 (59.0%) | |
| Bachelor/Master | 44 (54.3%) | 15 (38.5%) |
| Category | Variable | Group A More than 2 Months (n = 81) | Group B Less than 2 Months (n = 39) | T-Value/Chi-square | df | p-Value |
|---|---|---|---|---|---|---|
| Health Indicators | Cesarean Section | 0.104 | ||||
| Yes | 37 (45.7%) | 24 (61.5%) | ||||
| No | 44 (54.3%) | 15 (38.5%) | ||||
| Birth Weight (grams) | 3385.3 (±337.1) | 3356.2 (±483.7) | 0.383 | 118 | 0.702 | |
| Child’s Age (months) | 24.8 (3.6) | 22.4 (3.9) | 3.259 | 118 | 0.001 | |
| Allergy at 2 Years | 0.244 | 1 | 0.621 | |||
| Yes | 35 (43.2%) | 15 (38.5%) | ||||
| No | 46 (56.8%) | 24 (61.5%) | ||||
| Nutritional Status | HAZ | 0.43 (1.33) | 0.51 (1.05) | −0.327 | 118 | 0.744 |
| WAZ | 0.62 (0.99) | 0.57 (0.85) | 0.253 | 118 | 0.800 | |
| BMIAZ | 0.49 (1.34) | 0.35 (1.35 |
| Category | Variable | Group A More than 2 Months (n = 81) | Group B Less than 2 Months (n = 39) | T-Value/Chi-square | df | p-Value |
|---|---|---|---|---|---|---|
| Developmental Outcomes | Physical Development (DP-3) | 91.2 (15.6) | 94.6 (14.3) | −1.134 | 118 | 0.259 |
| Social-Emotional Development (DP-3) | 85.5 (16.1) | 87.9 (16.9) | −0.742 | 118 | 0.459 | |
| Cognitive Development (DP-3) | 98.4 (13.2) | 97.5 (12.8) | 0.346 | 118 | 0.730 | |
| Adaptive Behavior (DP-3) | 96.7 (14.8) | 96.2 (18.6) | 0.173 | 118 | 0.863 | |
| Communication (DP-3) | 96.0 (17.8) | 100.2 (20.0) | −1.160 | 118 | 0.248 | |
| Overall Development (DP-3) | 91.0 (16.9) | 92.8 (17.5) | −0.540 | 118 | 0.590 |
| Correlation | Spearman’s rho | Physical Development | Adaptive Behavior | Social-Emotional | Cognitive Development | Communication | Overall Development |
|---|---|---|---|---|---|---|---|
| p-Value | |||||||
| Food allergy 2 years Y/N | Spearman’s rho | −0.069 | −0.129 | −0.172 | −0.105 | 0.010 | −0.082 |
| p-value | 0.540 | 0.251 | 0.126 | 0.353 | 0.927 | 0.468 | |
| Allergy at 2 years Y/N | Spearman’s rho | −0.038 | −0.140 | −0.002 | 0.074 | 0.044 | 0.013 |
| p-value | 0.735 | 0.212 | 0.989 | 0.510 | 0.698 | 0.906 | |
| Atopic dermatitis | Spearman’s rho | −0.205 | −0.250 * | −0.246 * | −0.179 | −0.142 | −0.206 |
| p-value | 0.067 | 0.024 | 0.027 | 0.110 | 0.205 | 0.066 | |
| Mother’s age | Spearman’s rho | 0.263 * | 0.141 | 0.087 | 0.224 * | 0.117 | 0.139 |
| p-value | 0.018 | 0.208 | 0.438 | 0.044 | 0.300 | 0.216 | |
| Father’s age | Spearman’s rho | 0.261 * | 0.155 | 0.065 | 0.091 | 0.042 | 0.082 |
| p-value | 0.019 | 0.167 | 0.562 | 0.417 | 0.712 | 0.468 | |
| Father’s education | Spearman’s rho | −0.079 | −0.143 | −0.134 | −0.238 * | 0.001 | −0.056 |
| p-value | 0.485 | 0.201 | 0.234 | 0.032 | 0.992 | 0.619 | |
| Smoking (mother) per day | Spearman’s rho | −0.352 ** | −0.235 * | −0.234 * | −0.282 * | −0.250 * | −0.143 |
| p-value | 0.001 | 0.034 | 0.035 | 0.011 | 0.025 | 0.204 | |
| Smoking during pregnancy | Spearman’s rho | −0.179 | −0.129 | −0.135 | −0.266 * | −0.187 | −0.329 ** |
| p-value | 0.109 | 0.252 | 0.230 | 0.016 | 0.095 | 0.003 | |
| Cesarean section Y/N | Spearman’s rho | 0.292 ** | 0.294 ** | 0.203 | 0.195 | 0.126 | −0.244 * |
| p-value | 0.008 | 0.008 | 0.069 | 0.081 | 0.261 | 0.028 |
| Correlation | Spearman’s rho | Physical Development | Adaptive Behavior | Social-Emotional | Cognitive Development | Communication | Overall Development |
|---|---|---|---|---|---|---|---|
| p-Value | |||||||
| Food allergy 2 years Y/N | Spearman’s rho | −0.181 | −0.103 | −0.120 | −0.077 | −0.086 | −0.098 |
| p-value | 0.269 | 0.532 | 0.467 | 0.641 | 0.604 | 0.551 | |
| Allergy at 2 years Y/N | Spearman’s rho | −0.419 ** | −0.228 | −0.347 * | −0.279 | −0.363 * | −0.342 * |
| p-value | 0.008 | 0.162 | 0.030 | 0.085 | 0.023 | 0.033 | |
| Atopic dermatitis (AD) | Spearman’s rho | −0.358 * | −0.185 | −0.334 * | −0.351 * | −0.372 * | −0.368 * |
| p-value | 0.025 | 0.260 | 0.038 | 0.028 | 0.020 | 0.021 | |
| Mother’s age | Spearman’s rho | −0.007 | 0.137 | 0.006 | 0.040 | −0.042 | −0.011 |
| p-value | 0.965 | 0.404 | 0.969 | 0.811 | 0.798 | 0.947 | |
| Father’s age | Spearman’s rho | 0.116 | 0.060 | 0.156 | 0.074 | −0.019 | 0.119 |
| p-value | 0.482 | 0.716 | 0.344 | 0.653 | 0.908 | 0.469 | |
| Mother’s education | Spearman’s rho | 0.076 | 0.141 | −0.002 | −0.094 | 0.024 | 0.007 |
| p-value | 0.645 | 0.390 | 0.989 | 0.569 | 0.886 | 0.968 | |
| Father’s education | Spearman’s rho | 0.043 | 0.073 | −0.011 | −0.052 | 0.017 | 0.038 |
| p-value | 0.796 | 0.657 | 0.947 | 0.754 | 0.916 | 0.817 | |
| Smoking_ mother_ cigars/day | Spearman’s rho | −0.098 | −0.386 * | −0.303 | −0.310 | −0.331 * | −0.318 * |
| p-value | 0.553 | 0.015 | 0.061 | 0.055 | 0.040 | 0.049 | |
| Smoking during pregnancy | Spearman’s rho | −0.510 *** | −0.268 | −0.390 * | −0.247 | −0.345 * | −0.398 * |
| p-value | <0.001 | 0.099 | 0.014 | 0.130 | 0.032 | 0.012 | |
| Cesarean section Y/N | Spearman’s rho | 0.208 | 0.221 | 0.192 | 0.169 | 0.077 | 0.190 |
| p-value | 0.203 | 0.176 | 0.240 | 0.304 | 0.640 | 0.247 |
| NPD Areas (DP-3) | Group A (More than 2 Months) | Group B (Less than 2 Months) |
|---|---|---|
| Physical Development | Parents’ age | Allergy at 2 years |
| Cesarean section | Atopic dermatitis | |
| Mother’s daily cigarette consumption | Maternal smoking during pregnancy | |
| Adaptive Behavior | Cesarean section | Mother’s daily cigarette consumption |
| Atopic dermatitis | ||
| Mother’s daily cigarette consumption | ||
| Social-Emotional Development | Atopic dermatitis | Allergy at 2 years |
| Mother’s daily cigarette consumption | Atopic dermatitis | |
| Cognitive Development | Parents’ age | Atopic dermatitis |
| Father’s education level | ||
| Mother’s daily cigarette consumption | ||
| Communication | Mother’s daily cigarette consumption | Allergy at 2 years |
| Atopic dermatitis | ||
| Maternal smoking during pregnancy | ||
| Overall Development | Cesarean section | Allergy at 2 years |
| Maternal smoking during pregnancy | Atopic dermatitis | |
| Mother’s daily cigarette consumption | Mother’s daily cigarette consumption |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toneva, A.; Hachmeriyan, A.; Pancheva, R.; Marinova-Achkar, M. Early Childhood Nutrition and Development in Atopic Families from Northeastern Bulgaria. J. Mind Med. Sci. 2025, 12, 4. https://doi.org/10.3390/jmms12010004
Toneva A, Hachmeriyan A, Pancheva R, Marinova-Achkar M. Early Childhood Nutrition and Development in Atopic Families from Northeastern Bulgaria. Journal of Mind and Medical Sciences. 2025; 12(1):4. https://doi.org/10.3390/jmms12010004
Chicago/Turabian StyleToneva, Albena, Antoniya Hachmeriyan, Rouzha Pancheva, and Miglena Marinova-Achkar. 2025. "Early Childhood Nutrition and Development in Atopic Families from Northeastern Bulgaria" Journal of Mind and Medical Sciences 12, no. 1: 4. https://doi.org/10.3390/jmms12010004
APA StyleToneva, A., Hachmeriyan, A., Pancheva, R., & Marinova-Achkar, M. (2025). Early Childhood Nutrition and Development in Atopic Families from Northeastern Bulgaria. Journal of Mind and Medical Sciences, 12(1), 4. https://doi.org/10.3390/jmms12010004






