Abstract
Introduction. According to the European Cancer Information System, melanoma is the sixth most diagnosed cancer in Europe (following breast, colorectal, prostate, lung, and bladder cancer) and ranks among the top 20 most common causes of cancer-related death. The diagnosis of melanoma can be a clinical challenge due to the variety of signs and symptoms, ranging from small, asymptomatic lesions with slow progression to large, painful tumors with rapid growth. In this paper, we will report an exceptional case of cutaneous melanoma with an atypical presentation in a patient with no personal or family history of skin cancer. Case presentation. We present the case of an 85-year-old patient who presented to our dermatology clinic with multiple nodular lesions on her right calf, extending to her thigh as isolated lesions. These violaceous lesions varied in size and displayed signs of bleeding, fibrin deposits, and purulent, foul-smelling discharge. The patient reported a five-month history of rapid tumor progression. The initial presumptive diagnosis was Kaposi's sarcoma; however, the biopsy ruled out this diagnosis, revealing the presence of malignant melanoma. Given the extensive locoregional spread and invasion of deep structures, the patient was transferred to palliative care. Conclusions. Melanoma is an aggressive form of cancer that can rapidly progress, often presenting with atypical clinical manifestations or mimicking other skin conditions. When the clinical presentation deviates from the classic patterns, a biopsy is considered the gold standard for guiding the clinician's subsequent approach. It provides valuable diagnostic information and aids in determining the prognosis as well as the appropriate therapeutic course.
Introduction
Cutaneous melanoma is one of the most aggressive cancers seen in humans, and is originating from melanocytes. These cells develop from the neural crest and are present in the basal epidermis, choroidal layer of the eye, mucosal surfaces, meninges, and hair follicles [1,2].
The incidence of cutaneous melanoma is on the rise, making it one of the most prevalent cancers among young adults. Although cutaneous melanoma comprises only 3% of all skin cancers, it accounts for 65% of skin cancer-related deaths. However, with early detection and proper treatment, the cure rate for early-stage melanoma can exceed 90% [1,2].
Epidemiologic studies have identified several risk factors considered significant in developing cutaneous melanoma. These include genetic factors such as mutations of CDKN2α, BRAF, MC1R, ultraviolet radiation from sun exposure and resulting sunburns (especially during childhood), use of tanning beds (especially before age 35), family history of dysplastic naevi or melanoma, personal history of melanoma, number (>50) and size (>5 mm) of melanocytic naevi, congenital naevi, number of dysplastic naevi (>5), dysplastic melanocytic naevus syndrome, phenotypic traits such as fair hair, eye, and skin colors (photo skin type I/II), predisposition to freckle, and higher socioeconomic status [3,4].
Ultraviolet (UV) exposure is recognized as the most significant risk factor for cutaneous melanoma, and both molecular and epidemiological evidence suggest two distinct etiological pathways. The first pathway, associated with early sun exposure and a tendency to develop naevi, is influenced by host factors and intermittent sun exposure. This pathway typically involves the B-Raf proto-oncogene serine/threonine kinase (BRAF). It is characterized by diagnosis at a young age, lack of chronic sun damage, superficial spreading melanoma, and melanoma occurring on the trunk. In contrast, accumulated sun exposure leads to the chronic sun-exposure pathway, linked to NRAS proto-oncogene GTPase (NRAS) mutations and is not associated with naevus count [5].
Almost all melanomas begin with an initial radial growth phase, followed by a vertical growth phase. The radial growth phase is characterized by a predominantly intraepidermal, preinvasive, or minimally invasive growth pattern, while the vertical growth phase involves penetration into the dermis, bringing the tumor closer to blood vessels that can facilitate metastasis. Because most melanomas produce melanin pigment, even preinvasive melanomas in the radial growth phase are usually detectable by their color patterns [2,6]. The key prognostic difference between clinical types of melanomas lies in the duration of the radial growth phase, which can last for years to decades in lentigo malignant melanoma, for months to 2 years in superficial spreading melanoma, and approximately 6 months in nodular melanoma [3,7].
Initially, four major subtypes of primary cutaneous melanoma were identified based on their predominant growth patterns: superficial spreading melanoma (SSM), nodular melanoma (NM), lentigo malign melanoma (LMM), and acral lentiginous melanoma (ALM).
Nodular melanoma (NM) represents the second most prevalent subtype of melanoma, constituting approximately 15% to 30% of all melanoma cases. The trunk is the most frequently affected site, but it can also occur on the head and neck. In women, nodular melanoma frequently presents on the legs. NM is characterized by its rapid progression, with lesions typically developing over a period of weeks to months. This subtype often bypasses the radial growth phase seen in other melanomas. NM is more commonly observed to originate de novo rather than from a preexisting nevus. Clinically, NM usually presents as a uniformly dark blue-black or bluish-red elevated lesion, although approximately 5% of cases are amelanotic [2,8]. Nodular melanoma generally has a poor prognosis because it is prone to local invasion and often leads to distant metastasis. While the clinical diagnosis of nodular melanoma is usually straightforward, extensive and infected cases can present significant challenges in terms of differential diagnosis [7].
Case Presentation
Patient V.R., an 85-year-old woman, presented to our dermatology clinic with multiple violet-hued, nodular lesions, ranging in size from 1 to 2 cm in diameter. These lesions covered the entire surface of her right lower calf and extended to her thigh as isolated lesions. The lesions exhibited signs of bleeding, thick fibrin deposits, and purulent secretions with a foul odor. The patient reported that the tumor had been rapidly developing over the past 5 months (Figure 1 and Figure 2). She denied any prior melanocytic lesions over the affected area or any family history of skin cancer.
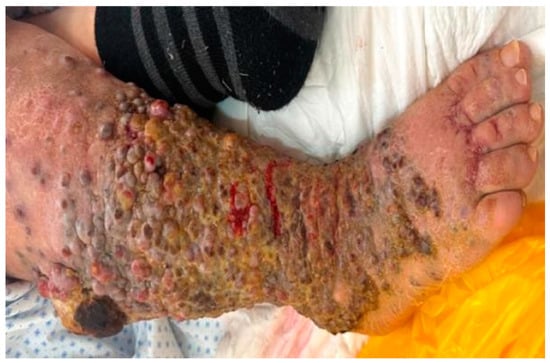
Figure 1.
Multiple violaceous nodules, covered with thick fibrin deposits. Discrete hemorrhagic areas.
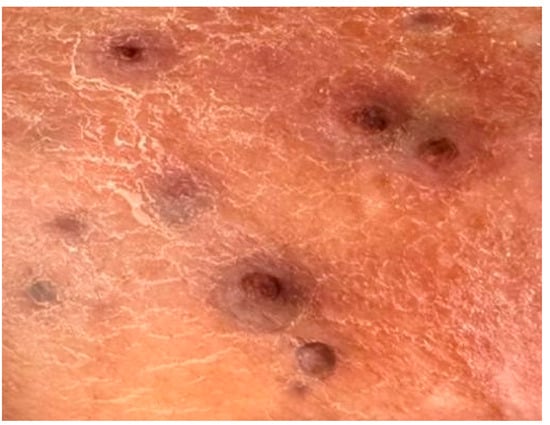
Figure 2.
Nodular, violaceous, isolated lesions located on the calf (the area from which the biopsy was taken).
The general examination did not reveal any significant abnormalities in terms of cardiovascular, respiratory, gastrointestinal, musculoskeletal, endocrinological, urogenital, or neurological systems. Additionally, no loco-regional lymphadenopathy was detected on superficial and deep palpation. The patient denied any other extracutaneous symptoms.
Laboratory investigations showed a marked increase in inflammatory markers (erythrocyte sedimentation rate, C reactive protein, fibrinogen), significant leukocytosis with neutrophilia, thrombocytosis, mild normochromic normocytic anemia, and elevated serum creatinine and uric acid levels. A fecal occult blood test was performed and the results were positive. Screening for HIV-1/2 antigen/antibody was negative. Empirical systemic treatment was initiated, including rehydration, antibiotics, and antithrombotic therapy, along with local antiseptic and antibiotic treatments.
Based on the initial skin examination, a presumptive diagnosis of infected Kaposi's sarcoma was made. However, the unusual, extensive presentation prompted additional investigations. An excisional biopsy of an isolated lesion was performed to confirm the diagnosis (Figure 3 and Figure 4). The biopsy ruled out Kaposi's sarcoma and revealed the following: moderate orthokeratosis at the epidermal level, with focal pigmentation of the basal layer, and a densely cellular intradermal tumoral proliferation composed of medium to large-sized cells with abundant, frequently retracted cytoplasm. Some cells appeared ballooned, with large, prominent eosinophilic nucleoli, arranged in nests and interconnected clusters with an alveolar pattern, separated by connective-vascular septa. There was occasional discrete deposition of brown pigment, with invasion into the hypodermis, in contact with the deep excision margin. The mitotic index was less than 1/mm². Lymphatic invasion was present. There was a focal, minimal, discontinuous peritumoral lymphomononuclear inflammatory infiltrate, with limited areas of segmental tumor regression, moderate fibrosis, rare lymphocytes, and telangiectasias. These histopathological features were consistent with nodular malignant melanoma, Clark level V, with extensive involvement of the hypodermis and lymphatic spread. The biopsy specimen was sent for immunohistochemical analysis, which confirmed the diagnosis of malignant melanoma.
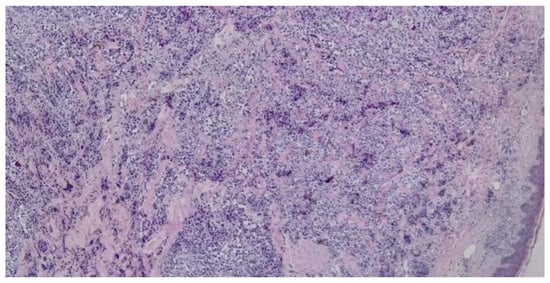
Figure 3.
Dense intradermal proliferation with the formation of nests, clusters, and cords with uneven pigmentation (haematoxilin and eosin staining, 5x).
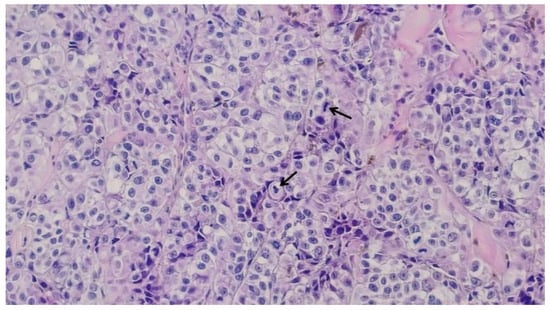
Figure 4.
Large-sized cells with irregular hypertrophic nuclei, prominent nucleoli, and focal mitotic activity, with pigment deposition; black arrows indicate active mitoses (haematoxilin and eosin staining, 20x).
Upon reviewing the advanced diagnosis with the patient and her family, a decision was made to refer her to a palliative care center. Unfortunately, the patient passed away shortly after being discharged from our clinic.
Discussions
Cutaneous melanoma is a tumor arising from the malignant transformation of melanocytes, which originate from the neural crest. Nodular melanoma is the second most common clinical subtype of melanoma (15-30%), following superficial spreading melanomas (70%). Although constituting only 15% of invasive melanomas, it significantly contributes to melanoma-related mortality, accounting for 40% of deaths. This disproportionate impact is attributed to its tendency to present with a significantly shorter radial growth phase, an early vertical growth phase, greater thickness, a higher mitotic rate, and earlier metastasis to vital organs compared to other melanoma subtypes, leading to a poor prognosis [8,9,10]. The vertical growth phase can last from a few weeks up to 6 months, and in the presented case, the patient reported the development of skin lesions over approximately 5 months, which is consistent with the diagnosis of invasive nodular melanoma. The acronym ABCDE is frequently employed to characterize typical melanoma lesions, with A representing asymmetry, B indicating irregular borders, C denoting color variation both within the lesion and relative to the patient's other nevi, D referring to a diameter exceeding 6 mm, and E signifying an evolving lesion. However, nodular melanoma may appear as rapidly growing papules or nodules and may not exhibit some of the distinguishing features found in other melanoma subtypes, thereby reducing the utility of the ABCDE mnemonic for its diagnosis [11]. Nodular melanomas typically present as nodules or papules that are symmetric, uniformly colored, with regular borders and small diameters. Due to the absence of these characteristic features, NM may remain undetected, which can result in severe outcomes since their increased thickness is associated with a poorer prognosis. Additionally, NM may present as amelanotic or hypomelanotic, further complicating the diagnostic process [12,13,14].
Skin metastases from melanoma are relatively common and can be the first indication of advanced disease or a recurrence. In 2–8% of cases, skin metastases represent the initial manifestation of melanoma. Clinically, cutaneous melanoma metastases can occur at various anatomical sites, generally within the same region as the primary melanoma. These metastases frequently present as multiple dermal or subcutaneous nodules, although they can also appear as single lesions. Typically, they are firm, pigmented, and may be reddish or skin-colored, with occasional ulceration. While skin metastases are often asymptomatic, advanced cases may present ulceration, bleeding, and secondary infection. Additionally, they may produce symptoms due to compression of surrounding tissues [15,16]. Cutaneous melanoma metastases are categorized by their distance from the primary tumor: satellite metastases (within 2 cm), in-transit metastases (between 2 cm and the first draining lymph node), and distant metastases (at any skin site). According to the eighth American Joint Committee on Cancer (AJCC) melanoma staging system, satellite and in-transit metastases are classified as stage III disease, while distant metastases indicate stage IV disease. Patients with skin-only metastases (M1a) have a relatively better prognosis with a 1-year survival rate of 59%, compared to 41% for those with visceral metastases (M1b, M1c, M1d) [17,18].
Research by Niebling et al. highlights the poor prognosis of melanomas presenting with microsatellites, showing a 5-year melanoma-specific survival rate of 53% and a higher risk of sentinel lymph node positivity and loco-regional recurrences [19]. Melanoma cells can metastasize to the skin via lymphatic invasion or by spreading directly into the adjacent epidermis, often extending beyond histologically detectable margins, as demonstrated by Bastian et al. in acral lentiginous melanoma cases. Furthermore, studies have shown that melanoma cells can spread significantly into normal skin without correlation to tumor thickness or size, complicating diagnosis and treatment [20]. In the presented case, the patient had multiple satellite and in-transit metastases, as well as distant metastases, indicating stage IV melanoma. However, the lack of additional investigations prevented precise staging, and the rapid progression of the disease resulted in her death shortly after diagnosis.
The differential diagnosis of nodular melanoma, particularly in advanced cases, can be exceedingly challenging. Nodular melanoma often presents with characteristics that overlap with other cutaneous and subcutaneous lesions, complicating accurate diagnosis. Advanced nodular melanoma may exhibit atypical features or mimic various benign and malignant conditions, making differentiation difficult. The 4 most common clinical misdiagnoses in one large study were different types of nevi, basal cell carcinoma, seborrheic keratosis, and lentigo malign. Nonetheless, there are also scenarios where malignant melanoma may present with features that are compatible with vascular lesions, such as Kaposi's sarcoma, angiokeratomas, hemangiomas, or pyogenic granulomas. These conditions may share similar features with nodular melanoma, such as nodularity, pigmentation, and rapid growth, which complicates the diagnostic process [21]. Given these similarities, a high degree of clinical suspicion is necessary when evaluating pigmented or atypically changing lesions. Accurate diagnosis relies on a thorough clinical assessment, including an extensive patient history, detailed physical examination, and, if needed, advanced diagnostic techniques like biopsy and imaging. This thorough and systematic approach is essential for distinguishing nodular melanoma from other similar conditions and ensuring appropriate and timely treatment.
In terms of differential diagnosis between advanced-stage nodular melanoma and Kaposi's sarcoma, the literature includes a single notable case described by Fernando et al. The study reported on a 48-year-old MSM (men who have sex with men) who was initially diagnosed with Kaposi’s sarcoma. The patient first presented with a subungual infection of the right middle finger, attributed to a splinter injury, which ultimately required amputation of the distal phalanx due to persistent infection. Subsequently, he developed swelling around the medial epicondyle of the right elbow, initially managed as an abscess with incision and drainage. Following a recurrence at the same site, a biopsy revealed histological features consistent with Kaposi’s sarcoma. However, the clinical presentation and negative HIV status were inconsistent with Kaposi’s sarcoma, prompting further diagnostic evaluation. A subsequent biopsy from a new lesion confirmed the diagnosis of malignant melanoma. Further imaging disclosed extensive metastases, and clinical examination revealed a pigmented area at the site of the previous amputation, suggesting that the initial ‘splinter’ injury might have been related to the primary melanoma. This case underscores the critical importance of accurate diagnosis and illustrates how an initial misdiagnosis of Kaposi’s sarcoma delayed the identification and appropriate management of malignant melanoma [22]. In the case of our patient the biopsy was conducted during her initial hospitalization, being prompted by the atypical presentation featuring extensive lesions, ulceration, and secondary infection. While the definitive diagnosis was not delayed, owing to the high level of suspicion for the pigmented lesion, the therapeutic options were severely limited by the advanced stage of the disease and the patient’s rapid deterioration. The rapid progression observed in this patient's case, with lesions covering the entire surface of her right lower calf and extending to the thigh as isolated lesions, aligns with the patterns described in the literature on nodular melanoma. Additionally, the presence of bleeding, thick fibrin deposits, and purulent secretions further underscores the aggressive nature of NM, as described in the literature. This case highlights the importance of early detection, as the extensive involvement of the calf and extension towards the thigh illustrate the devastating potential of NM when left undiagnosed for an extended period [22].
Recent advances in the treatment of metastatic melanoma have brought significant improvements in patient care, especially for those with advanced-stage disease. Historically, conventional chemotherapy for metastatic melanoma offered limited survival benefits, as evidenced by extensive research. However, recent innovations targeting specific molecular pathways and harnessing the immune system have shown promise [23].
For patients with stage IV melanoma, as seen in our patient’s case, survival rates are notably poor, with median survival times generally ranging from 8 to 10 months. Prognosis within stage IV is further stratified based on the type and location of metastases. For example, patients with only cutaneous metastases (stage IVa) have a 5-year survival rate of approximately 18.8%, while those with lung metastases (stage IVb) and other visceral metastases (stage IVc) face lower survival rates of 6.7% and 9.5%, respectively [23,24].
Treatment advancements have revolutionized the management of stage IV melanoma. Targeted therapies, such as BRAF inhibitors, have shown efficacy in patients with BRAF V600E mutations, offering improved overall and progression-free survival compared to traditional chemotherapies. Additionally, immunotherapies, including anti-CTLA-4 (cytotoxic T-lymphocyte antigen 4) and anti-PD-1 (programmed cell death protein 1) agents, have demonstrated enhanced survival outcomes by activating the immune system to better target melanoma cells. For instance, ipilimumab, an anti-CTLA-4 monoclonal antibody, and nivolumab, an anti-PD-1 agent, have demonstrated increased survival rates in patients with advanced melanoma [25,26,27].
Despite these advancements, treatment options for metastatic melanoma can be limited, particularly when the disease is in an advanced stage where curative treatment is not feasible. In such cases, palliative care becomes crucial. The focus of palliative care is to alleviate symptoms and improve the quality of life, rather than seeking to cure the disease. This approach ensures that patients receive comfort and supportive care, addressing the practical aspects of managing advanced disease.
Overall, while targeted and immune-based therapies have significantly advanced the treatment of stage IV melanoma, palliative care remains an integral part of comprehensive patient management, particularly when curative options are no longer viable.
Conclusions
The prevention of melanoma is crucial in mitigating the impact of this aggressive cancer. Key preventive measures include minimizing ultraviolet (UV) exposure and adhering to effective sun protection practices. Regular dermatological evaluations, both self-examinations by patients and professional assessments by healthcare providers, are essential for the early detection and management of melanoma.
Elderly patients face unique challenges in managing their skin health, often struggling with recognizing and reporting new or changing lesions. This highlights the importance of more frequent and proactive screening in this population to improve early detection and outcomes.
The differential diagnosis of melanoma, particularly in its atypical forms, can be quite complex. The clinical similarity between nodular melanoma and Kaposi’s sarcoma adds to the diagnostic challenge. Accurate differentiation between these conditions requires a high level of clinical suspicion and comprehensive diagnostic evaluation. In instances where melanoma is suspected or presents atypically, a biopsy remains a crucial diagnostic tool, providing definitive information that guides appropriate management.
Early detection and prompt treatment are critical in improving prognosis. The advanced stage of melanoma in the presented case led to a rapid decline and eventual death, underscoring the need for early intervention. In contrast, timely diagnosis and treatment generally offer significantly better prognostic outcomes.
In conclusion, diligent prevention, regular monitoring, and precise differential diagnosis are vital for effective melanoma management and improving patient survival rates.
Institutional Review Board Statement
Any aspect of the work covered in this manuscript has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript. Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
There are no known conflicts of interest in the publication of this article. The manuscript was read and approved by all authors.
References
- Naik, PP. Cutaneous Malignant Melanoma: A Review of Early Diagnosis and Management. World J Oncol. 2021, 12, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Arnold M, Singh D, Laversanne M; et al. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022, 158, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Garbe C, Amaral T, Peris K, Hauschild A, Arenberger P; et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics: Update 2022. Eur J Cancer. 2022, 170, 236–255. [Google Scholar] [CrossRef]
- Schadendorf D, van Akkooi ACJ, Berking C, Griewank KG; et al. Melanoma. The Lancet. 2018, 392, 971–984. [Google Scholar] [CrossRef]
- Armstrong BK, Cust AE. Sun exposure and skin cancer, and the puzzle of cutaneous melanoma: A perspective on Fears et al. Mathematical models of age and ultraviolet effects on the incidence of skin cancer among whites in the United States. American Journal of Epidemiology 1977; 105: 420-427. Cancer Epidemiol. 2017, 48, 147–156. [Google Scholar] [CrossRef]
- Hartman RI, Lin JY. Cutaneous Melanoma-A Review in Detection, Staging, and Management. Hematol Oncol Clin North Am. 2019, 33, 25–38. [Google Scholar] [CrossRef]
- Myers DJ, Hyde EA. Aggressive Nodular Malignant Melanoma. Cureus. 2021, 13, e16819. [Google Scholar] [CrossRef]
- Dessinioti C, Geller AC, Whiteman DC, Garbe C, Grob JJ; et al. Not all melanomas are created equal: A review and call for more research into nodular melanoma. Br J Dermatol. 2021, 185, 700–710. [Google Scholar] [CrossRef]
- Burton RC, Coates MS, Hersey P, Roberts G, Chetty MP; et al. An analysis of a melanoma epidemic. Int J Cancer. 1993, 55, 765–770. [Google Scholar] [CrossRef]
- Liu W, Dowling JP, Murray WK, McArthur GA, Thompson JF; et al. Rate of growth in melanomas: Characteristics and associations of rapidly growing melanomas. Arch Dermatol. 2006, 142, 1551–1558. [Google Scholar] [CrossRef]
- Abbasi NR, Shaw HM, Rigel DS; et al. Early diagnosis of cutaneous melanoma: Revisiting the ABCD criteria. JAMA. 2004, 292, 2771–2776. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain AJ, Fritschi L, Kelly JW. Nodular melanoma: patients' perceptions of presenting features and implications for earlier detection. J Am Acad Dermatol. 2003, 48, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Bergenmar M, Hansson J, Brandberg Y. Detection of nodular and superficial spreading melanoma with tumour thickness < or = 2.0 mm--an interview study. Eur J Cancer Prev. 2002, 11, 49–55. [Google Scholar] [CrossRef]
- Bergenmar M, Ringborg U, Månsson Brahme E, Brandberg Y. Nodular histogenetic type -- the most significant factor for thick melanoma: Implications for prevention. Melanoma Res. 1998, 8, 403–411. [Google Scholar] [CrossRef]
- Marcoval J, Moreno A, Peyrí J. Cutaneous infiltration by cancer. J Am Acad Dermatol. 2007, 57, 577–580. [Google Scholar] [CrossRef]
- Savoia P, Fava P, Nardò T, Osella-Abate S, Quaglino P, Bernengo MG. Skin metastases of malignant melanoma: A clinical and prognostic survey. Melanoma Res. 2009, 19, 321–326. [Google Scholar] [CrossRef]
- Wong CY, Helm MA, Helm TN, Zeitouni N. Patterns of skin metastases: A review of 25 years' experience at a single cancer center. Int J Dermatol. 2014, 53, 56–60. [Google Scholar] [CrossRef]
- Di Raimondo C, Lozzi F, Di Domenico PP, Campione E, Bianchi L. The Diagnosis and Management of Cutaneous Metastases from Melanoma. Int J Mol Sci. 2023, 24, 14535. [Google Scholar] [CrossRef]
- Niebling MG, Haydu LE, Lo SN; et al. The prognostic significance of microsatellites in cutaneous melanoma. Mod Pathol. 2020, 33, 1369–1379. [Google Scholar] [CrossRef]
- Bastian BC, Kashani-Sabet M, Hamm H; et al. Gene amplifications characterize acral melanoma and permit the detection of occult tumor cells in the surrounding skin. Cancer Res. 2000, 60, 1968–1973. [Google Scholar]
- Grant-Kels JM, Bason ET, Grin CM. The misdiagnosis of malignant melanoma. J Am Acad Dermatol. 1999, 40, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Fernando KA, Fernando JJ. Malignant melanoma masquerading as Kaposi's sarcoma. Int J STD AIDS. 2008, 19, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Maverakis E, Cornelius LA, Bowen GM; et al. Metastatic melanoma--a review of current and future treatment options. Acta Derm Venereol. 2015, 95, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Balch CM, Gershenwald JE, Soong SJ; et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009, 27, 6199–6206. [Google Scholar] [CrossRef]
- Flaherty KT, Puzanov I, Kim KB; et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010, 363, 809–819. [Google Scholar] [CrossRef]
- Wolchok JD, Kluger H, Callahan MK; et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013, 369, 122–133. [Google Scholar] [CrossRef]
- Motofei, IG. Malignant Melanoma: Autoimmunity and Supracellular Messaging as New Therapeutic Approaches. Curr Treat Options Oncol. 2019, 20, 45. [Google Scholar] [CrossRef]
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).