Abstract
Paraneoplastic acral vascular syndrome (PAVS) is a captivating enigma in the landscape of oncological pathology, characterized by vascular disturbances of the extremities. PAVS is linked to rapidly progressing trajectory and understanding the intricate interplay between cancer and this vascular dysfunction is imperative for timely diagnosis. We describe the case of a 45-year-old cachectic male who presented at the ICU with bilateral tumefied, cyanotic digits, accompanied by acute pain and numbness in both hands. A Digital Angiography described complete obstruction of the digital arteries. Imaging showed a laterocervical tumor mass with pulmonary metastases. This form of digital ischemia likely stems from endothelial dysfunction and prothrombotic effects induced by the tumor through a paraneoplastic mechanism. This case underscores the importance of recognizing acral vascular syndrome, prompting thorough investigation for an underlying malignancy. Ultimately, collective efforts aimed at enhancing our understanding of PAVS and optimizing its management hold promise for improving outcomes and alleviating the burden of this condition on affected patients.
Introduction
Paraneoplastic syndromes represent uncommon clinical signs associated with cancer, affecting a spectrum of anatomical sites ranging from internal organs to the skin. Among these syndromes, Paraneoplastic Acral Vascular Syndrome (PAVS) stands out, characterized by vascular irregularities in the extremities concurrent with an underlying malignancy. Clinical features are often nonspecific and may include digital ischemia, acrocyanosis, and digital gangrene [1]. What is the most concerning about this phenomenon is that it typically appears only after metastases already occurred [2].
Le Besnerais et al. suggest a diligent investigation of male patients over 50 presenting with digital ischemia to rule out malignancy [3]. Their cohort study, comprising 100 patients with digital ischemia, revealed 15 cases of associated malignancy and estimated an incidence of 2 PAVS phenomenons in 100,000 per year. The calculated sex ratio (male: female) was 1.14, with a higher susceptibility observed among smokers. Although the prevalence of PAVS remains relatively low, it warrants attention. A search on PubMed yielded only 73 articles in total, spanning all available records.
Herein, we present a case of PAVS in a patient experiencing a recurrence of oropharyngeal squamous cell carcinoma. This article aims to increase awareness of this rare manifestation of malignancy and summarize essential considerations regarding PAVS and paraneoplastic syndromes related to the oropharynx.
Case Presentation
We present the case of a 45-year-old male who presented to the Emergency Room in March 2022 with bilateral cyanosis of the fingers, which had progressively worsened over the past 4 days. His medical history includes keratinized invasive squamous cell carcinoma of the oropharynx (cT3N2M0), diagnosed in September 2021, along with a pulmonary abscess, essential hypertension, and moderate anemia. His treatment for the oropharyngeal malignancy included radiotherapy and six cycles of Paclitaxel-Carboplatin chemotherapy. The patient also underwent percutaneous gastrostomy for nutritional support which was later removed. He had a 25 pack-year smoking history and consumed alcohol occasionally. His home medication included Tramadol for pain management and there was no notable family medical history.
Upon presentation, the patient reported bilateral hand pain, functional impairment, and cyanosis of the fingertips bilaterally, with acral distribution and intact peripheral pulses. Clinical examination also revealed a palpable left-sided submandibular mass, cutaneous pallor, and stable vital signs. Cachexia was prominently, with a calculated body mass index (BMI) of 16 kg/m2.
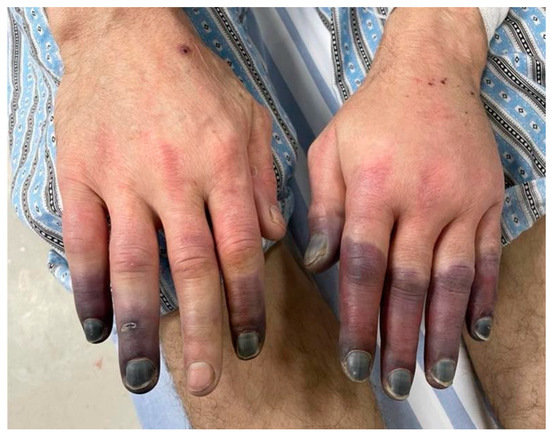
Figure 1.
Patient’s fingers at presentation (hospital archive).
Laboratory investigations revealed severe normochromic normocytic anemia (Hb: 6.9 g/dL), and inflammatory syndrome characterized by an elevated white blood cell count, heightened C-reactive protein levels, and hypoalbuminemia. Consequently, the patient received 2 units of red blood cells, and low molecular weight heparin therapy was initiated.
The electrocardiogram (ECG) exhibited sinus rhythm and echocardiography did not identify any valvular vegetations, myxoma, or patent foramen ovale. A routine evaluation including complete blood cell count, peripheral smear, renal function tests, and urinalysis with eosinophil smear, yielded no significant findings.
Subsequent evaluations included a comprehensive autoimmune panel comprising serum complement levels, cryoglobulins, rheumatoid factor (RF), anti-cyclic citrullinated peptide (CCP) antibodies, antinuclear antibodies (ANA), anti-neutrophil cytoplasmic antibodies (ANCA), anti-phospholipid antibodies, anti-Sjögren syndrome A and B antibodies. The results of these tests, along with serological screenings for HIV, syphilis, hepatitis B, and hepatitis C, were negative.

Table 1.
Patient’s deviant laboratory data at presentation (hospital archive).
Table 1.
Patient’s deviant laboratory data at presentation (hospital archive).
| Parameter | Value | Normal value |
|---|---|---|
| Chlorine | 94 | 101-109 mmol/L |
| Sodium | 124 | 136-146 mmol/L |
| Calcium | 7.36 | 8.2-10.7 mg/dL |
| APTT | 53.8 | 22-36 sec |
| Albumin | 2.9 | 3.4-5.2 g/dL |
| Direct bilirubin | 0.54 | 0-0.3 mg/dL |
| Total bilirubin | 1.40 | 0-1.2 mg/dL |
| C-reactive protein | 129.15 | 0-5 mg/L |
| Alkaline phosphatase | 320 | 40-136 U/L |
| LDH | 359 | 125-220 U/L |
| Gamma-glutamil transpherase | 366 | 5-85 U/L |
| Urea | 83.3 | 17-43 mg/dL |
| Creatinine | 2.5 | 0.7-1.2 mg/dL |
Cerebral imaging revealed no discernible lesions. Further imaging studies identified a laterocervical tumor mass measuring 56/43 mm, exhibiting peripheral iodine uptake and a parafluid center, with necrotic areas affecting the sternocleidomastoid muscle and parotid gland. Additionally, bilateral submandibular and mediastinal adenopathies were observed, alongside pulmonary metastases.
Furthermore, an angiography of the upper limb indicated the absence of vascularization at the level of bilateral proximal phalanges. The brachial, radial, and ulnar arteries were found to be patent, exhibiting a normal appearance.
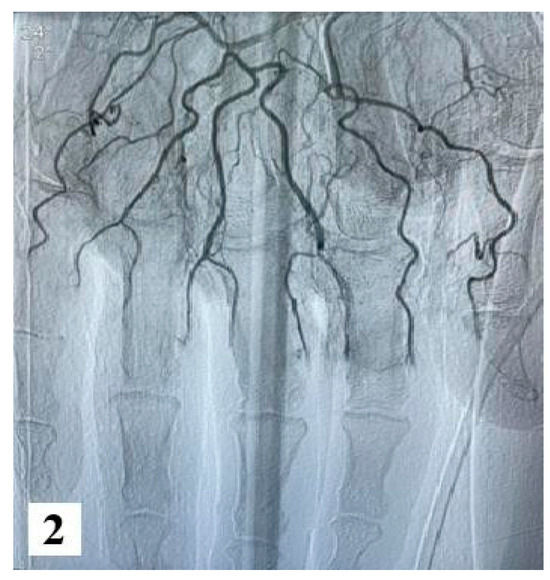
Figure 2.
Angiography images (from hospital archive) (part 1).
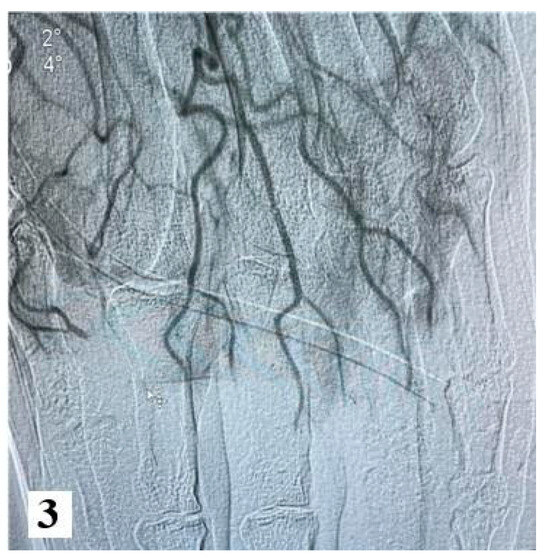
Figure 3.
Angiography images (from hospital archive) (part 2).
Following a multidisciplinary evaluation involving specialists in vascular, surgery plastic surgery, otorhinolaryngology, and oncology, vasodilator therapy with Ilomedin (Iloprost) was initiated, along with palliative care due to the advanced stage of the disease. The patient received a titrated dose of iloprost intravenously for six hours per day, over a total of five days, and he exhibited a partial response to pain management. Throughout the hospitalization, the patient also received anticoagulants (low molecular weight heparin), analgesics, vasodilators (pentoxifylline, nifedipine). However, despite medical interventions, the clinical course was unfavorable, marked by local deterioration and the development of dry gangrene in the upper limb phalanges, which progressed to more extensive tissue necrosis, along with the onset of cyanosis in the lower limbs after one week. Subsequently, the patient developed hemodynamic instability, which ultimately led to death on March 22nd.
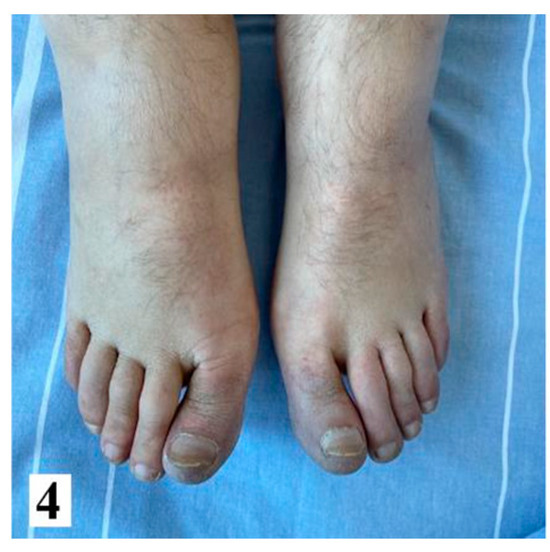
Figure 4.
Fingers of the patient after one week-upper and lower limbs (from hospital archive) (part 1).
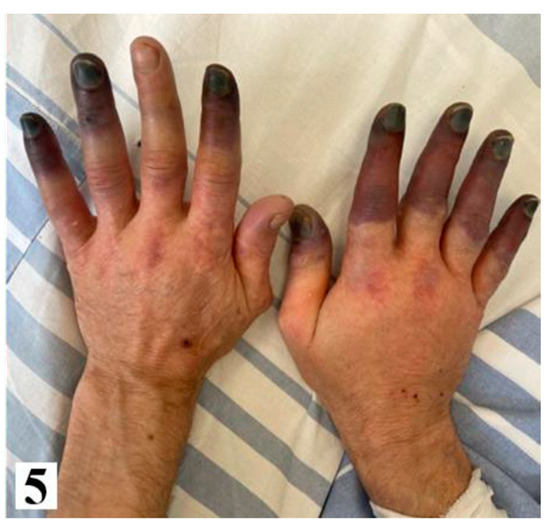
Figure 5.
Fingers of the patient after one week-upper and lower limbs (from hospital archive) (part 2).
Discussions
We chose to discuss this case due to the rarity of this clinical presentation and the difficulty in establishing a diagnosis. Thus, the initial symptomatology was consistent with Raynaud's syndrome, however, the rapid progression to ischemia and subsequent dry gangrene, along with the patient’s history of oropharyngeal squamous cell carcinoma with pulmonary metastases, suggested alternative etiologies. It is important to mention that digital ischemia’s differential diagnosis includes: Raynaud's phenomenon, Buerger's disease (thromboangiitis obliterans), autoimmune connective tissue diseases, vasculitis, and Hammer syndrome [3].
Although the literature on PAVS is scarce, insights from several seminal studies provide information on its epidemiology, clinical features, and association with malignancy. The correlation between cancer types and acral vascular syndrome is described in one article [1], with adenocarcinoma emerging as the predominant malignancy, notably at the level of gastrointestinal, gynecological, and pulmonary systems. Intriguingly, a slight female predominance was noted, typically manifesting symptoms around the age of 40. Le Besnerais et al. [3] further explored the temporal relationship between PAVS and cancer diagnosis, revealing variability in symptom onset relative to cancer detection. This emphasizes the critical need for vigilant monitoring of cancer patients to enable prompt intervention.
Despite comprehensive investigation, the precise pathophysiology of PAVS remains elusive. Proposed mechanisms suggest tumor-induced vasospasm, microemboli, arteritis stemming from tumor antigen-antibody complexes, and hypercoagulability due to tumor products [4,5]. While some studies have offered valuable insights into these mechanisms, further research is warranted to delineate their precise roles.
Treatment strategies for PAVS encompass a spectrum of measures, ranging from general interventions such as smoking cessation to vasodilator therapy. Vasodilators, including calcium channel blockers, angiotensin receptor blockers (ARBs), angiotensin-converting enzyme inhibitors (ACEIs), alpha-blockers, nitrates, prostacyclin analogs, and phosphodiesterase-5 inhibitors, have demonstrated efficacy. Additionally, antiplatelet and anticoagulant therapy may confer benefits, while severe cases may necessitate surgical interventions, such as sympathectomy or debridement [6]. Crucially, addressing the underlying cancer is paramount for alleviating symptoms associated with PAVS. The efficacy of chemotherapy or other cancer-directed therapies, and surgical resection in resolving vascular manifestations highlights the importance of addressing the underlying malignancy [2].
Furthermore, there have been reported associations between COVID-19 and acral lesions, suggesting a potential link between viral infections and vascular pathology. This underscores the necessity for a comprehensive differential diagnosis, particularly in the context of epidemics or pandemics [7].
Besides PAVS, a series of paraneoplastic syndromes in oropharyngeal carcinoma captured attention as far back as 50 years ago, prompting ongoing interest and investigation. Next, we will review these syndromes to show the importance of the multidisciplinary approach to these cases.
Bazex Syndrome (Acrokeratosis Neoplastica)
Acrokeratosis paraneoplastica, commonly referred to as Bazex syndrome, is an uncommon paraneoplastic skin condition characterized by erythematous, violaceous, scaly plaques primarily appearing on the hands and feet, as well as other acral areas, such as the nose and ears. It is associated with various malignancies, and often appears prior to the cancer diagnosis. It is hypothesized that immunological mechanism, along with deficiencies in vitamin A or zinc and increased secretion of growth factors, may play a role in this phenomenon [8].
While in most cases, skin lesions resolve entirely with tumor treatment, there is an exception worth mentioning. In a unique instance [9], a male patient diagnosed with oropharyngeal cancer did not experience the disappearance of the lesions following anti-tumoral therapy. This situation raises the possibility of an additional underlying squamous cell carcinoma.

Table 2.
Neoplasms presenting with PAVS or analogous clinical features (Raynaud's phenomenon, Bazex, and digital ischemia) which may suggest the potential presence of an underlying malignancy.
Table 2.
Neoplasms presenting with PAVS or analogous clinical features (Raynaud's phenomenon, Bazex, and digital ischemia) which may suggest the potential presence of an underlying malignancy.
| PAVS | LYMPH NODES | 2021, AlRasbi [10] |
| SKIN | 2017, Gambichler [11] | |
| PANCREAS | 2018, Maharaj [12] | |
| LUNG | 2020, Jud [13] | |
| LIVER | 2020, Olivito [14] | |
| ASTROCYTE | 2020, Bilancini [15] | |
| UTERUS | 2022, Yoshizaki [16] | |
| RAYNAUD’S PHENOMENON | LUNG | 2010, Schildman [17]2012, Scheinfeld [18]2012, Madabhavi [19]2016, Wang [20]2021, Lokineni [21] |
| THYMUS | 2015, Gong [22] | |
| OVARY | 2020, Lai [23] | |
| DIGITAL ISCHAEMIA | COLON AND RECTUM | 2017, Schattner [24] |
| LYMPH NODES | 2018, Villano [25] | |
| LUNG | 2010, Moulakakis [26] | |
| TONSIL | 2016, Warrier [27] | |
| PROSTATE | 2021, Ilie [28] | |
| ACROKERATOSIS PARANEOPLASTICA | LUNG | 2016, Amano [29]2011, Zarzour [30] |
| OVARY | 2015, Hempen [31] | |
| LIP | 2022, Gaurav [32] | |
| PANCREAS | 1995, Halpern [33]2020, Horton [34] | |
| ESOPHAGUS | 2013, Rodrigues [35]2008, Medenica [36] | |
| COLON | 2008, Baek [37] | |
| T-CELL | 2019, McClatchey [38] | |
| CERVIX | 2016, Squires [39] | |
| LIVER | 2022, Holzgruber [40] |
Hypercalcemia
Oropharyngeal cancer has the potential to induce pseudo-hyperparathyroidism or ectopic PTH syndrome due to the production of PTH-like substances by the tumor. Consequently, affected individuals may manifest hypercalcemia as a paraneoplastic syndrome. In a study conducted in the early 1970s, Terz et al. investigated the prevalence of hypercalcemia associated with this type of cancer [41]. Among 240 patients, 20 were identified with hypercalcemia, and 5 of them were selected for detailed analysis. Notably, none of the 5 patients exhibited bone metastases, and the hypercalcemia resolved following the treatment of the tumor, such as radiotherapy. This secretion of PTH-like substances is also reported in a case report of squamous carcinoma of the tonsil [42].
Paraneoplastic Neurological Syndromes (PNS)
PNS encompasses a range of neurological disorders arising from an aberrant immune response directed at the nervous system, often triggered by a distant tumor. These disorders often present themselves before identifying the associated underlying neoplasm. The three case reports below outline some unique disorders that find their roots in oropharyngeal carcinoma.
In a 2011 study [43], researchers explored the potential occurrence of paraneoplastic encephalomyelitis. While not definitively established, the authors suggested that it could be a manifestation linked to either oropharyngeal or lung cancer. The patient under investigation presented with hypoesthesia to cold and hot stimuli in the right limb. Despite corticotherapy, symptom improvement did not occur, leading to the conclusion that it might be a PNS. Subsequent diagnosis revealed the presence of both oropharyngeal and lung cancer. The recurrence of the pulmonary neoplasm a year later raised the possibility that the paraneoplastic encephalomyelitis originated from the previous malignancy, but other investigations suggest the possibility of an oropharyngeal origin.
In a case report detailed by Yong et. al [44], a patient suffering from oropharyngeal cancer presented at the hospital with complaints of memory issues, orientation difficulties, handwriting problems, mood swings, and disequilibrium. After oncologic treatment, the patient experienced partial recovery of these cognitive functions, though some residual effects persisted. It should be noted that the sooner a patient presents to the hospital, the greater the likelihood of achieving complete recovery.

Table 3.
Summary of the paraneoplastic manifestations of oropharyngeal cancer.
Table 3.
Summary of the paraneoplastic manifestations of oropharyngeal cancer.
| Condition | Suggested etiology | Source |
|---|---|---|
| Bazex | Immunologic mechanisms Tumor secretion of GFLack of vitamin A or zinc | [8,9] |
| Hypercalcemia | Pseudo-PTH | [41,42] |
| PNS (dizziness, PCD-LEM,encephalomyelitis) | Aberrant immune response directed at the nervous system | [43, 44, 46] |
A distinct syndrome identified through extensive research in scientific databases is the combination of Paraneoplastic Cerebellar Degeneration and LambertEaton Myasthenic Syndrome (PCD-LEM). While these syndromes are commonly associated with small-cell lung carcinoma, investigations have revealed instances where oropharyngeal carcinoma can also trigger their manifestation. A 78-year-old man presented with dysphagia, dysarthria, and muscle fatigue was found suffering from small-cell neuroendocrine carcinoma of the oropharynx [45]. After further investigations, the absence of deep tendon reflexes and a calculated SARA score of 13.5 was reported. Scientific evidence supports the notion that anti-tumor treatments have demonstrated efficacy in repairing certain neurological damage associated with PCD-LAM. There is a belief among researchers that, had the therapy been initiated at an earlier stage, a complete recovery of neurological functions might have been achievable.
Conclusions
The presented case underscores the intricate nature of managing advanced-stage oropharyngeal squamous cell carcinoma with distant metastases, highlighting the necessity of multidisciplinary care and palliative interventions to effectively address symptoms and enhance the quality of life in afflicted individuals. The diagnostic and therapeutic complexities associated with Paraneoplastic Acral Vascular Syndrome (PAVS) further emphasize the importance of a collaborative, multidisciplinary approach for optimal management. Given the ongoing challenges in understanding its pathogenesis and refining treatment modalities, rigorous investigation is essential to elucidate the underlying mechanisms driving this condition and to develop effective therapeutic strategies. Ultimately, collective efforts aimed at enhancing our understanding of PAVS and optimizing its management hold significant promise for improving outcomes and alleviating the burden of this condition on affected patients.
Institutional Review Board Statement
Any aspect of the work covered in this manuscript has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript. Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
There are no known conflicts of interest in the publication of this article. The manuscript was read and approved by all authors.
References
- Poszepczynska-Guigné E, Viguier M, Chosidow O, et al. Paraneoplastic acral vascular syndrome: epidemiologic features, clinical manifestations, and disease sequelae. J Am Acad Dermatol. 2002, 47, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Chow SF, McKenna CH. Ovarian cancer and gangrene of the digits: case report and review of the literature. Mayo Clin Proc. 1996, 71, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Le Besnerais M, Miranda S, Cailleux N, et al. Digital ischemia associated with cancer: results from a cohort study. Medicine (Baltimore). 2014, 93, e47. [Google Scholar] [CrossRef] [PubMed]
- Khaddour K, Singh V, Shayuk M. Acral vascular necrosis associated with immune-check point inhibitors: case report with literature review. BMC Cancer. 2019, 19, 449. [Google Scholar] [CrossRef]
- Savlovschi C, Serban D, Andreescu C, Dascalu A, Pantu H. Economic analysis of medical management applied for left colostomy. Chirurgia (Bucur). 2013, 108, 666–669. [Google Scholar]
- Noble JP, Boisnic S, Branchet-Gumila MC, Poisson M. Palmar erythema: cutaneous marker of neoplasms. Dermatology. 2002, 204, 209–213. [Google Scholar] [CrossRef]
- Constantin VD, Silaghi A, Rebegea LF, Paunica S, et al. Barrett's esophagus as a premalignant condition; medical and surgical therapeutic management. J Mind Med Sci. 2023, 10, 178–190. [Google Scholar] [CrossRef]
- Räßler F, Goetze S, Elsner P. Acrokeratosis paraneoplastica (Bazex syndrome) - a systematic review on risk factors, diagnosis, prognosis and management. J Eur Acad Dermatol Venereol. 2017, 31, 1119–1136. [Google Scholar] [CrossRef]
- Fasanmade A, Farrell K, Perkins CS. Bazex syndrome (acrokeratosis paraneoplastica): persistence of cutaneous lesions after successful treatment of an associated oropharyngeal neoplasm. Br J Oral Maxillofac Surg. 2009, 47, 138–139. [Google Scholar] [CrossRef]
- AlRasbi S, Al-Badi AH, Al Alawi AM. Paraneoplastic acral vascular syndrome: case presentation and literature review. BMJ Case Rep. 2021, 14, e237258. [Google Scholar] [CrossRef]
- Gambichler T, Strutzmann S, Tannapfel A, Susok L. Paraneoplastic acral vascular syndrome in a patient with metastatic melanoma under immune checkpoint blockade. BMC Cancer. 2017, 17, 327. [Google Scholar] [CrossRef]
- Maharaj S, Chang S, Seegobin K, Isache C. Paraneoplastic acral vascular syndrome. Cleve Clin J Med. 2018, 85, 101–102. [Google Scholar] [CrossRef] [PubMed]
- Jud P, Raggam RB, Hafner F. Paraneoplastic acral vascular syndrome. CMAJ. 2020, 192, E1470. [Google Scholar] [CrossRef] [PubMed]
- Oliveros Olivito AL, Herranz Martinez S, et al. Paraneoplastic acral vascular syndrome in a nonagenarian patient with disseminated hepatic cystadenocarcinoma. Rev Esp Geriatr Gerontol. 2020, 55, 367–368. [Google Scholar] [CrossRef]
- Bilancini S, Lucchi M, Lucchi G, et al. Paraneoplastic Raynaud's phenomenon associated to astrocytoma. J Med Vasc. 2020, 45, 161–164. [Google Scholar] [CrossRef]
- Yoshizaki A, Ibusuki A, Baba N, et al. Paraneoplastic acral vascular syndrome in a patient with uterine cancer. J Dermatol. 2022, 49, e274–e275. [Google Scholar] [CrossRef]
- Schildmann EK, Davies AN. Paraneoplastic Raynaud's phenomenon-good palliation after a multidisciplinary approach. J Pain Symptom Manage. 2010, 39, 779–783. [Google Scholar] [CrossRef]
- Scheinfeld, N. A review of the cutaneous paraneoplastic associations and metastatic presentations of ovarian carcinoma. Clin Exp Dermatol. 2008, 33, 10–15. [Google Scholar] [CrossRef]
- Madabhavi I, Revannasiddaiah S, Rastogi M, Gupta MK. Paraneoplastic Raynaud's phenomenon manifesting before the diagnosis of lung cancer. BMJ Case Rep. 2012, 2012, bcr0320125985. [Google Scholar] [CrossRef]
- Wang L, Lei QS, Liu YY, Song GJ, Song CL. A case report of the beneficial effects of botulinum toxin type A on Raynaud phenomenon in a patient with lung cancer. Medicine (Baltimore). 2016, 95, e5092. [Google Scholar] [CrossRef]
- Lokineni S, Nepal M, Mohamed A. Paraneoplastic Raynaud's Phenomenon as an Initial Manifestation of Lung Cancer? Eur J Case Rep Intern Med. 2021, 8, 002690. [Google Scholar] [CrossRef]
- Gong L, Zhang P, Liu XY, Fang M. A rare thymoma case with seven paraneoplastic syndromes. Int J Clin Exp Med. 2015, 8, 19517–19523. [Google Scholar]
- Lai TS, Shim MR, Shin D, Zakhour M. Paraneoplastic Raynaud phenomenon associated with metastatic ovarian cancer: A case report and review of the literature. Gynecol Oncol Rep. 2020, 33, 100575. [Google Scholar] [CrossRef]
- Schattner, A. Out of the blue finger ischaemia and occult colorectal cancer. BMJ Case Rep. 2017, 2017, bcr2016218779. [Google Scholar] [CrossRef] [PubMed]
- Villano F, Peixoto A, Riva E, Di Matteo C, Díaz L. Digital Ischemia as an Unusual Manifestation of Hodgkin's Lymphoma. Case Rep Hematol. 2018, 2018, 1980749. [Google Scholar] [CrossRef]
- Moulakakis K, Bessias N, Maras D, Andrikopoulos V. "Digital ischemia as a paraneoplastic manifestation of lung cancer" clinical image. Intern Med. 2010, 49, 199–200. [Google Scholar] [CrossRef]
- Warrier V, Ahmad A, Alshatti Y, Jafar A. Digital necrosis with squamous cell carcinoma of the tonsil. Int Med Case Rep J. 2016, 9, 159–162. [Google Scholar] [CrossRef]
- Ilie DS, Mitroi G, Păun I, et al. Pathological and immunohistochemical study of colon cancer. Evaluation of markers for colon cancer stem cells. Rom J Morphol Embryol. 2021, 62, 117–124. [Google Scholar] [CrossRef]
- Amano M, Hanafusa T, Chikazawa S, et al. Bazex Syndrome in Lung Squamous Cell Carcinoma: High Expression of Epidermal Growth Factor Receptor in Lesional Keratinocytes with Th2 Immune Shift. Case Rep Dermatol. 2016, 8, 358–362. [Google Scholar] [CrossRef]
- Zarzour JG, Singh S, Andea A, Cafardi JA. Acrokeratosis paraneoplastica (Bazex syndrome): report of a case associated with small cell lung carcinoma and review of the literature. J Radiol Case Rep. 2011, 5, 1–6. [Google Scholar] [CrossRef]
- Hempen A, Samartzis EP, Kamarachev J, Fink D, Dedes KJ. Acrokeratosis paraneoplastica in serous ovarian carcinoma: case report. BMC Cancer. 2015, 15, 507. [Google Scholar] [CrossRef]
- Gaurav V, Grover C. Bazex Syndrome Associated with Squamous Cell Carcinoma of the Lip: A Rare Paraneoplastic Acrokeratosis with Nail Dystrophy. Skin Appendage Disord. 2022, 8, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Halpern SM, O'Donnell LJ, Makunura CN. Acrokeratosis paraneoplastica of Bazex in association with a metastatic neuroendocrine tumour. J R Soc Med. 1995, 88, 353P–354P. [Google Scholar]
- Horton L, Bedford LM, Daveluy S. Acrokeratosis paraneoplastica (Bazex syndrome) as the presenting sign of pancreatic adenocarcinoma. BMJ Case Rep. 2020, 13, e236514. [Google Scholar] [CrossRef]
- Rodrigues IA Jr, Gresta LT, Cruz RC, Carvalho GG, Moreira MH. Bazex syndrome. An Bras Dermatol. 2013, 88 (Suppl. 1), 209–211. [Google Scholar] [CrossRef]
- Medenica L, Gajić-Veljić M, Skiljević D, Pesko P. Acrokeratosis paraneoplastica Bazex syndrome associated with esophageal squamocellular carcinoma. Vojnosanit Pregl. 2008, 65, 485–487. [Google Scholar] [CrossRef]
- Baek JO, Lee HY, Lee JR, Roh JY. Acrokeratosis Paraneoplastica with Adenocarcinoma of the Colon Treated with Topical Tretinoin. Ann Dermatol. 2008, 20, 216–220. [Google Scholar] [CrossRef]
- McClatchey TM, Haynes D, Korcheva VB, Keller J. Acrokeratosis paraneoplastica (Bazex syndrome) associated with peripheral T-cell lymphoma. JAAD Case Rep. 2018, 5, 86–88. [Google Scholar] [CrossRef]
- Squires B, Daveluy SD, Joiner MC, Hurst N, Bishop M, Miller SR. Acrokeratosis Paraneoplastica Associated with Cervical Squamous Cell Carcinoma. Case Rep Dermatol Med. 2016, 2016, 7137691. [Google Scholar] [CrossRef]
- Holzgruber J, Oberneder-Popper J, Guenova E, Hötzenecker W. Acrokeratosis Paraneoplastica (Bazex Syndrome): A Case Report. Case Rep Dermatol. 2022, 14, 307–312. [Google Scholar] [CrossRef]
- Terz JJ, Estep H, Bright R, et al. Proceedings: Primary oropharyngeal cancer and hypercalcemia. Cancer 1974, 33, 334–339. [Google Scholar] [CrossRef]
- Samaan NA, Ordonez NG, Ibanez ML, Goepfert H, Smith K. Ectopic parathyroid hormone production by a squamous carcinoma of the tonsil. Arch Otolaryngol. 1983, 109, 91–94. [Google Scholar] [CrossRef]
- Moyano MS, Gutiérrez-Gutiérrez G, Gómez-Raposo C, et al. Paraneoplastic encephalomyelitis: Is it an oropharyngeal or a lung cancer complication? Oncol Lett. 2011, 2, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Yong L, Asimakopoulos P, Mumford C, Fragkandrea Nixon I. When dizziness becomes sinister: oropharyngeal carcinoma presenting as a paraneoplastic neurological disorder. BMJ Case Rep. 2017, 2017, bcr2016216151. [Google Scholar] [CrossRef]
- Takasugi J, Shimamura M, Koda T, et al. Paraneoplastic Cerebellar Degeneration and Lambert-Eaton Myasthenic Syndrome Associated with Neuroendocrine Carcinoma of the Oropharynx. Intern Med. 2018, 57, 587–590. [Google Scholar] [CrossRef]
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).