The Usage of Polidocanol in Varicose Disease Treatment
Abstract
Introduction
Materials and Methods
Results
Discussions
Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Carlton, R.; Mallick, R.; Campbell, C.; Raju, A.; O’Donnell, T.; Eaddy, M. Evaluating the Expected Costs and Budget Impact of Interventional Therapies for the Treatment of Chronic Venous Disease. Am Health Drug Benefits. 2015, 8, 366–374. [Google Scholar] [CrossRef] [PubMed]
- van Eekeren, R.R.; Boersma, D.; Konijn, V.; de Vries, J.P.; Reijnen, M.M. Postoperative pain and early quality of life after radiofrequency ablation and mechanochemical endovenous ablation of incompetent great saphenous veins. J Vasc Surg. 2013, 57, 445–450. [Google Scholar] [CrossRef]
- Nicolaides, A.N.; Allegra, C.; Bergan, J.; et al. Management of chronic venous disorders of the lower limbs: Guidelines according to scientific evidence. Int Angiol. 2008, 27, 1–59. [Google Scholar] [CrossRef] [PubMed]
- Criqui, M.H.; Jamosmos, M.; Fronek, A.; et al. Chronic venous disease in an ethnically diverse population: The San Diego Population Study. Am J Epidemiol. 2003, 158, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.H.; Bjoern, L.; Lawaetz, M.; Blemings, A.; Lawaetz, B.; Eklof, B. Randomized trial comparing endovenous laser ablation of the great saphenous vein with high ligation and stripping in patients with varicose veins: Short-term results. J Vasc Surg. 2007, 46, 308–315. [Google Scholar] [CrossRef]
- Cabrera, J.; Cabrera, J.; et al. Treatment of Varicose Long Saphenous Veins with Sclerosant in Microfoam Form: Long-Term Outcomes. Phlebology. 2000, 15, 19–23. [Google Scholar] [CrossRef]
- Cavezzi, A.; Frullini, A.; Ricci, S.; Tessari, L. Treatment of Varicose Veins by Foam Sclerotherapy: Two Clinical Series. Phlebology. 2002, 17, 13–18. [Google Scholar] [CrossRef]
- Breu, F.X.; Guggenbichler, S. European Consensus Meeting on Foam Sclerotherapy, April, 4-6, 2003, Tegernsee, Germany. Dermatol Surg. 2004, 30, 709–717. [Google Scholar] [CrossRef]
- Bountouroglou, D.G.; Azzam, M.; Kakkos, S.K.; et al. Ultrasound-guided foam sclerotherapy combined with sapheno-femoral ligation compared to surgical treatment of varicose veins: Early results of a randomised controlled trial. Eur J Vasc Endovasc Surg. 2006, 31, 93–100. [Google Scholar] [CrossRef]
- Purwins, S.; Herberger, K.; Debus, E.S.; et al. Cost-of-illness of chronic leg ulcers in Germany. Int Wound J. 2010, 7, 97–102. [Google Scholar] [CrossRef]
- Margolis, D.J.; Bilker, W.; Santanna, J.; Baumgarten, M. Venous leg ulcer: Incidence and prevalence in the elderly. J Am Acad Dermatol. 2002, 46, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Nelzèn, O. Leg Ulcers: Economic Aspects. Phlebology. 2000, 15, 110–114. [Google Scholar] [CrossRef]
- Paunică-Panea, G.; Teodorescu, S.; Preda, A.; Gligor, L.E.; Silaghi, A.; Constantin, V.D. Chronic wound management; surgical therapy and complementary nursing with Manuka honey. J Mind Med Sci. 2023, 10, 139–147. [Google Scholar] [CrossRef]
- Babtan, A.M.; Ilea, A.; Feurdean, C.N.; et al. Biostimulation with low-level laser therapy and its effects on soft and hard tissue regeneration. Literature review. J Mind Med Sci. 2022, 9, 28–37. [Google Scholar] [CrossRef]
- Tatu, A.L.; Ionescu, M.A.; Nwabudike, L.C. Contact Allergy to Topical Mometasone Furoate Confirmed by Rechallenge and Patch Test. Am J Ther. 2018, 25, e497–e498. [Google Scholar] [CrossRef]
- Paius, C.T.; Constantin, V.D.; Carap, A.; Tarus, A.; Tinica, G. Revascularization impact: Quality of life enhancement in chronic limb-threatening ischemia. J Mind Med Sci. 2023, 10, 321–329. [Google Scholar] [CrossRef]
- Nwabudike, L.C.; Tatu, A.L. Magistral Prescription With Silver Nitrate and Peru Balsam in Difficult-to-Heal Diabetic Foot Ulcers. Am J Ther. 2018, 25, e679–e680. [Google Scholar] [CrossRef]
- Droahnă, A.R.; Moroianu, L.A.; Pietrosel, V.A.; Bica, C.I.; Salmen, T.; Curis, C.; Merlo, E.M.; Stoica, R.A.; Moroianu, M. Anxio-depressive disorders in a pandemic context: A comparative analysis: Year 2019 versus 2020. J Mind Med Sci. 2023, 10, 156–162. [Google Scholar] [CrossRef]
- Moroianu, M.; Moroianu, L.A.; Ciubara, A.; Matei, N.M. Persistent Depressive Disorder: The Clinical Approach of the Patient Associating Depression and Dental Pathology—Case Report and Clinical Considerations. BRAIN. Broad Research in Artificial Intelligence and Neuroscience. 2022, 13, 229–238. [Google Scholar] [CrossRef]
- BTG. FDA approves Varithena (polidocanol injectable foam) for the treatment of patients with varicose veins. Press release. November 26, 2013. https://www.prnewswire.com/news-releases/btg-plc-fda-approves-varithena-polidocanol-injectable-foam-for-the-treatment-of-patients-with-varicose-veins-233441701.html.
- Peterson, J.D.; Goldman, M.P.; Weiss, R.A.; et al. Treatment of reticular and telangiectatic leg veins: Double-blind, prospective comparative trial of polidocanol and hypertonic saline. Dermatol Surg. 2012, 38, 1322–1330. [Google Scholar] [CrossRef]
- Benigni, J.P.; Bihari, I.; Rabe, E.; et al. Venous symptoms in C0 and C1 patients: UIP consensus document. Int Angiol. 2013, 32, 261–265. [Google Scholar] [PubMed]
- Campos, W., Jr.; Torres, I.O.; da Silva, E.S.; Casella, I.B.; Puech-Leão, P. A prospective randomized study comparing polidocanol foam sclerotherapy with surgical treatment of patients with primary chronic venous insufficiency and ulcer. Ann Vasc Surg. 2015, 29, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Bertanha, M.; Sobreira, M.L.; Pinheiro Lúcio Filho, C.E.; et al. Polidocanol versus hypertonic glucose for sclerotherapy treatment of reticular veins of the lower limbs: Study protocol for a randomized controlled trial. Trials. 2014, 15, 497, Published 2014 Dec 19. [Google Scholar] [CrossRef]
- Todd, K.L., 3rd; Wright, D.I.; VANISH-2 Investigator Group. Durability of treatment effect with polidocanol endovenous microfoam on varicose vein symptoms and appearance (VANISH-2). J Vasc Surg Venous Lymphat Disord. 2015, 3, 258–264.e1. [Google Scholar] [CrossRef]
- Fometescu, S.G.; Costache, M.; Coveney, A.; Oprescu, S.M.; Serban, D.; Savlovschi, C. Peritoneal fibrinolytic activity and adhesiogenesis. Chirurgia (Bucur). 2013, 108, 331–340. [Google Scholar]
- Staniszewska, A.; Tambyraja, A.; Afolabi, E.; Bachoo, P.; Brittenden, J. The Aberdeen varicose vein questionnaire, patient factors and referral for treatment. Eur J Vasc Endovasc Surg. 2013, 46, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, M.A.; Rabe, E.; McLafferty, R.B.; et al. Revision of the venous clinical severity score: Venous outcomes consensus statement: Special communication of the American Venous Forum Ad Hoc Outcomes Working Group. J Vasc Surg. 2010, 52, 1387–1396. [Google Scholar] [CrossRef]
- Bobeica, C.; Niculet, E.; Craescu, M.; et al. Immunologic and nonimmunologic sclerodermal skin conditions—review. Front Immunol. 2023, 14, 1180221. [Google Scholar] [CrossRef]
- Tatu, A.L.; Ionescu, M.A. Multiple autoimmune syndrome type III-thyroiditis, vitiligo and alopecia areata. Acta Endocrinol (Buchar) 2017, 13, 124–125. [Google Scholar] [CrossRef]
- Happle, R.; Kluger, N. Koebner’s sheep in Wolf’s clothing: Does the isotopic response exist as a distinct phenomenon? J Eur Acad Dermatol Venereol. 2018, 32, 542–543. [Google Scholar] [CrossRef]
- Ardeleanu, V.; Dobre, M.; Georgescu, E.M. Deep Facial Wrinkle Treatment Outcome After First Injection of Reticulated Hyaluronic Acid. Rev. Chim. 2015, 66, 2129–2131, http://bch.ro/pdfRC/ARDELEANU%2012%2015.pdf. [Google Scholar]
- Ardeleanu, V.; Toma, A.; Pafili, K.; et al. Current Pharmacological Treatment of Painful Diabetic Neuropathy: A Narrative Review. Medicina (Kaunas). 2020, 56, 25, Published 2020 Jan 9. [Google Scholar] [CrossRef] [PubMed]
- Ardeleanu, V.; Sabina Radaschin, D.; Tatu, A.L. Excimer laser for psoriasis treatment: A case report and short review. Exp Ther Med. 2020, 20, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Jecan, R.C.; Nicolau, A.; Florescu, I.P.; et al. Use of trichloroacetic acid in treating facial hyperpigmentation. Mater Plast. 2017, 54, 88–90, https://revmaterialeplastice.ro/pdf/20%20JECAN%20R%20C%201%2017.pdf. [Google Scholar] [CrossRef]
- Hexsel, D.; Serra, M.; Mazzuco, R.; Dal’Forno, T.; Zechmeister, D. Phosphatidylcholine in the treatment of localized fat. J Drugs Dermatol. 2003, 2, 511–518. [Google Scholar]
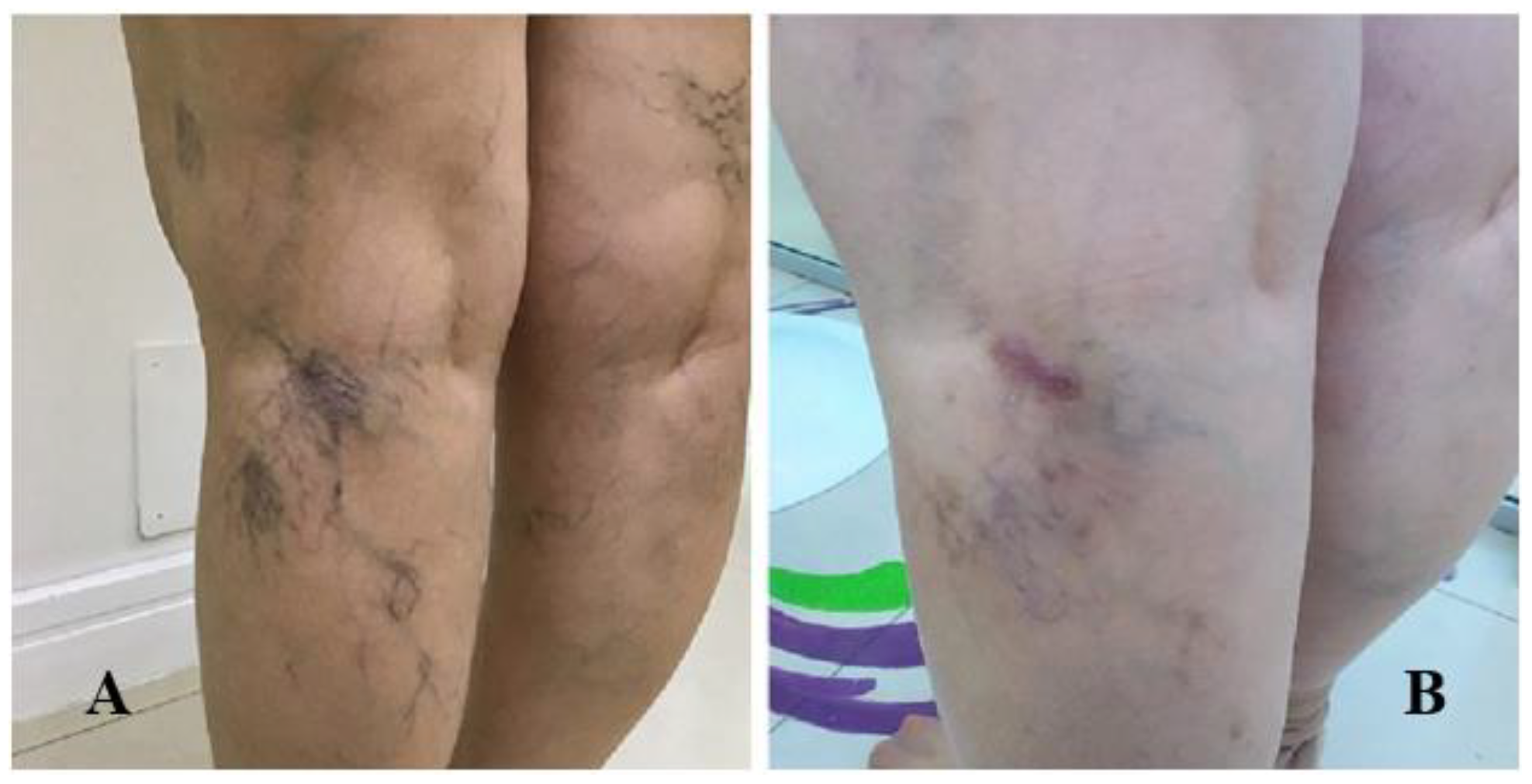
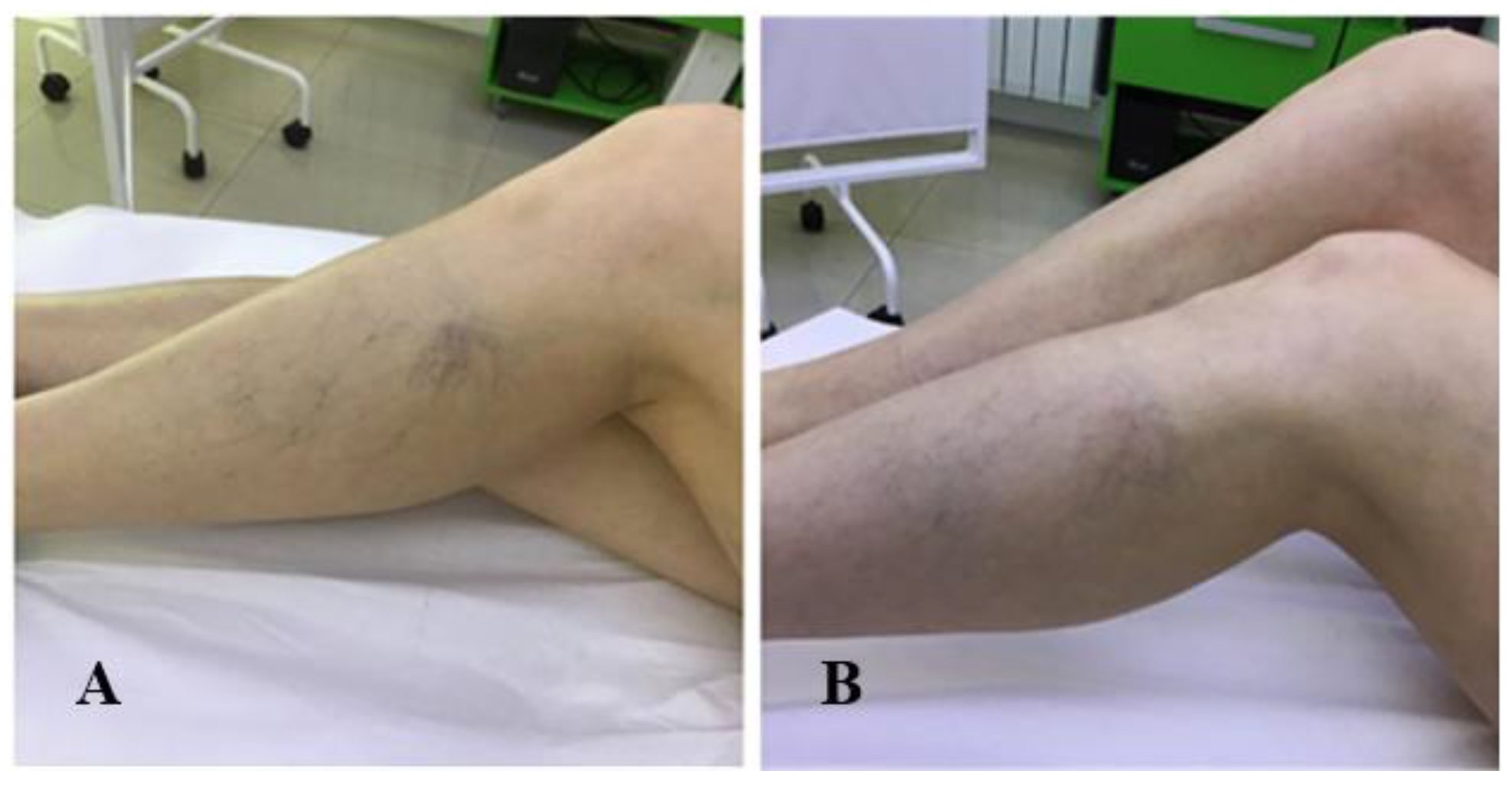
| Item | Absent (0 points) | Mild (1 point) | Moderate (2 points) | Severe (3 points) |
| Pain or other discomfort: aching, heaviness, fatigue, burning | None | Occasional pain or other discomfort | Daily pain or other discomfort without limits daily activities | Daily pain or discomfort which limits regular daily activities |
| Varicose veins (must be more 3 mm diameter to qualify in the standing position) | None | Few (isolated branch varicosities or clusters; also include ankle flare) | Confined to calf or thigh | Involves calf or thigh |
| Venous edema (presumes venous origin) | None | Limited to foot and ankle area | Extended above ankle but below ankle | Extended to knee and above |
| Skin pigmentation (does not include focal pigmentation over varicose vein or pigmentation due to other chronic disease) | None | Perimalleolar | Diffuse over lower third of calf | Wider, above lower third of calf |
| Inflammation | None | Perimalleolar | Diffuse over lower third of calf | Wider, above lower third of calf |
| Induration (includes white atrophy and lipodermatosclerosis) | None | Perimalleolar | Diffuse over lower third of calf | Wider, above lower third of calf |
| No active ulcers | None | 1 | 2 | ≥3 |
| Active size ulcers | None | <2 cm | 2-6 cm | >6 cm |
| Ulcer duration | None | <3 months | 3-12 months | >1 year |
| Compression therapy | None | Intermittent | Most days | Fully comply |
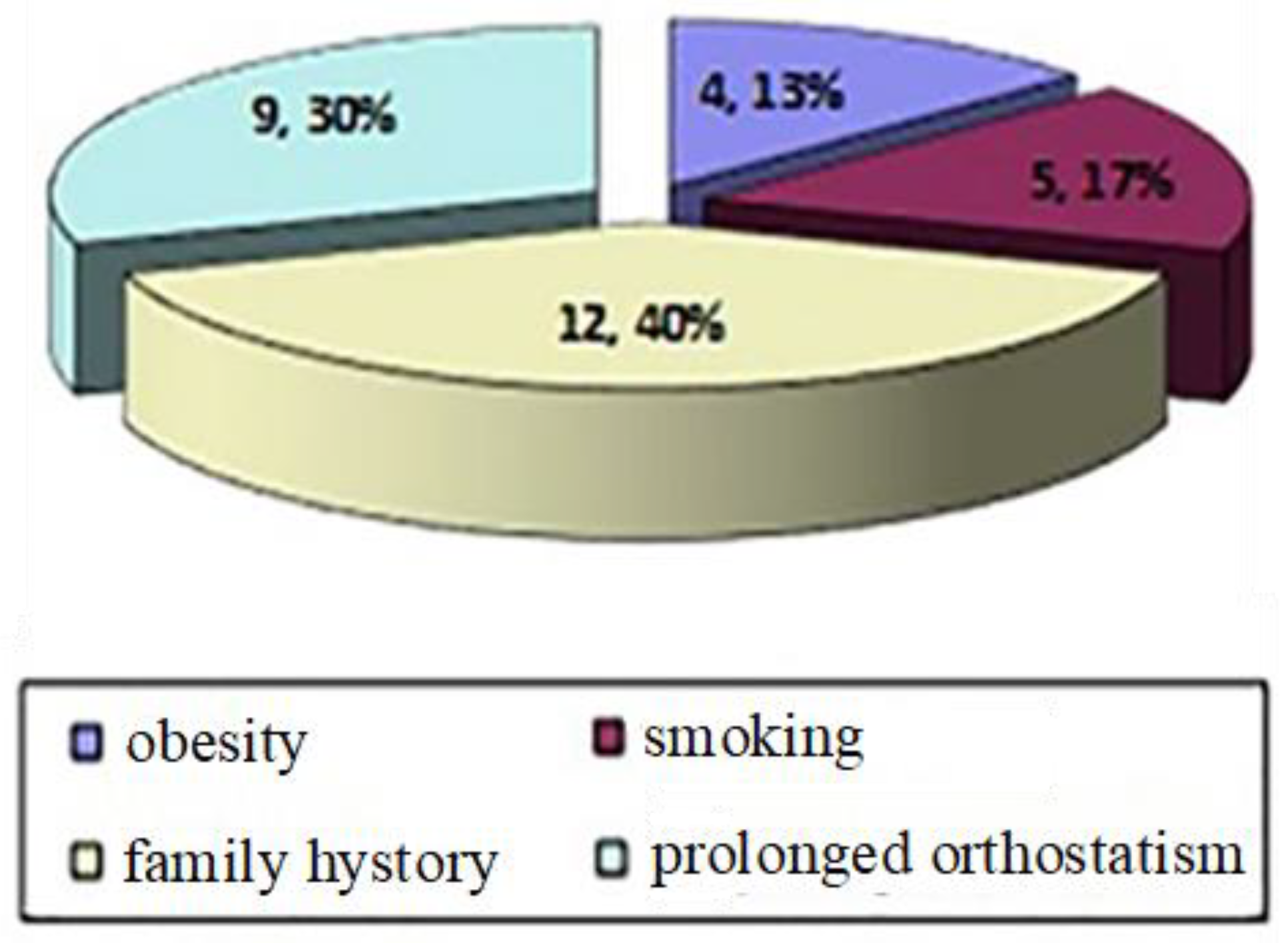
| Patient | Age (average 49.5) | Gender | Number of sessions | Favoring Factors | VCSS at the beginning of the treatment | VCSS 1 year after the treatment | Complications |
|---|---|---|---|---|---|---|---|
| 1 | 56 | F | 1 | Family history, | 3 | 0 | No |
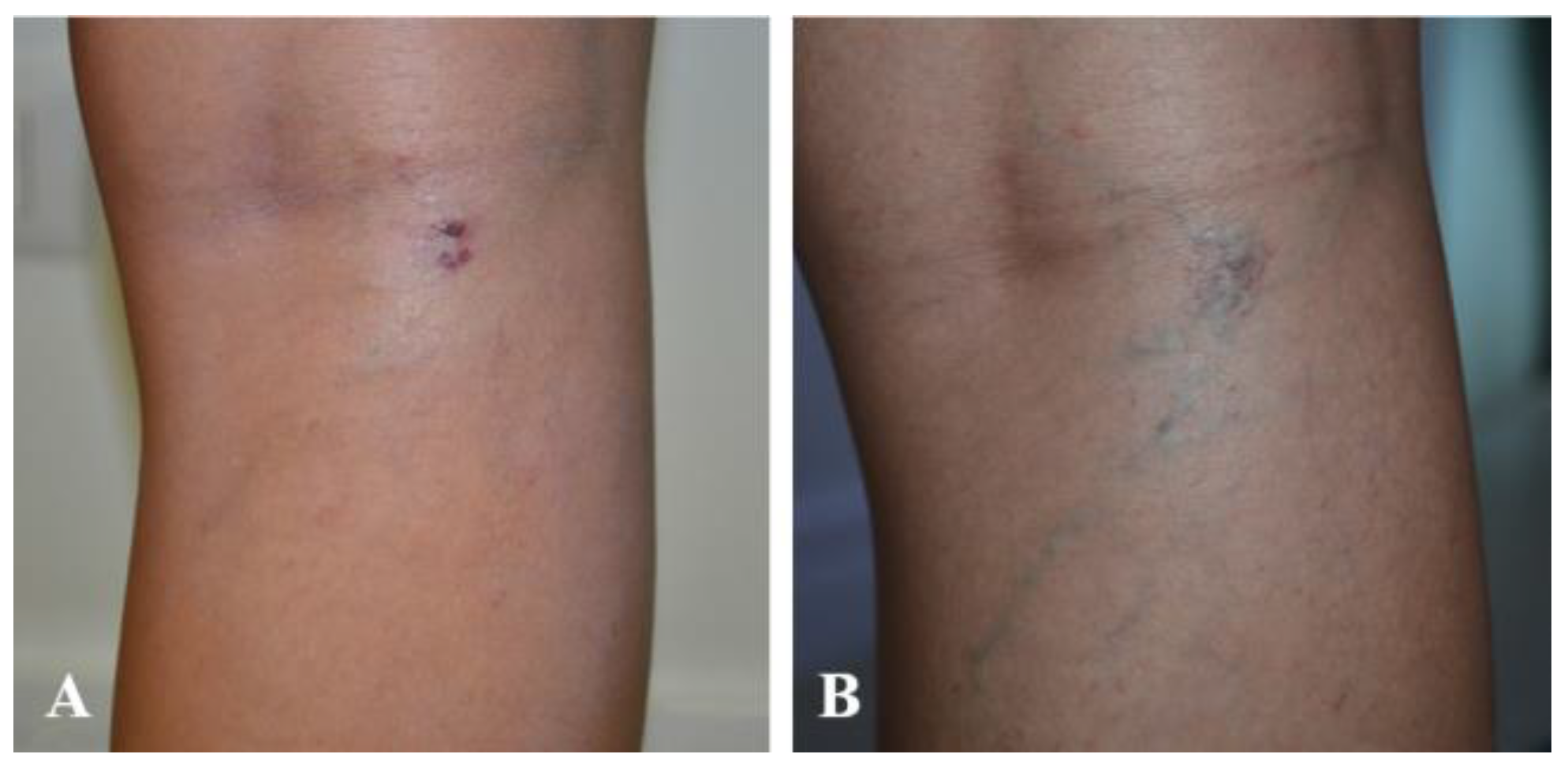
| 2 | 48 | F | 3 | Prolonged orthostatism, family history, obesity, smoking | 8 | 3 | Yes, pigmentation, hardening of the injected path |
| 3 | 52 | F | 1 | Prolonged orthostatism, family history | 4 | 1 | No |
| 4 | 31 | F | 1 | Family history, | 3 | 0 | No |
| 5 | 49 | F | 2 | Prolonged orthostatism, family history, obesity, smoking | 6 | 2 | No |
| 6 | 56 | F | 1 | Prolonged orthostatism, smoking, | 4 | 1 | No |
| 7 | 62 | F | 1 | Family history, smoking | 4 | 1 | No |
| 8 | 42 | F | 1 | Prolonged orthostatism, | 3 | 0 | No |
| 9 | 42 | F | 1 | Prolonged orthostatism, family history | 4 | 0 | No |
| 10 | 47 | B | 2 | Prolonged orthostatism, family history, obesity | 6 | 2 | No |
| 11 | 53 | F | 2 | Prolonged orthostatism, family history, smoking | 5 | 1 | No |
| 12 | 37 | F | 1 | Family history | 3 | 0 | No |
| 13 | 60 | F | 2 | Family history, obesity | 5 | 2 | Yes, pigmentation |
| 14 | 58 | F | 1 | Prolonged orthostatism, family history | 4 | 1 | No |
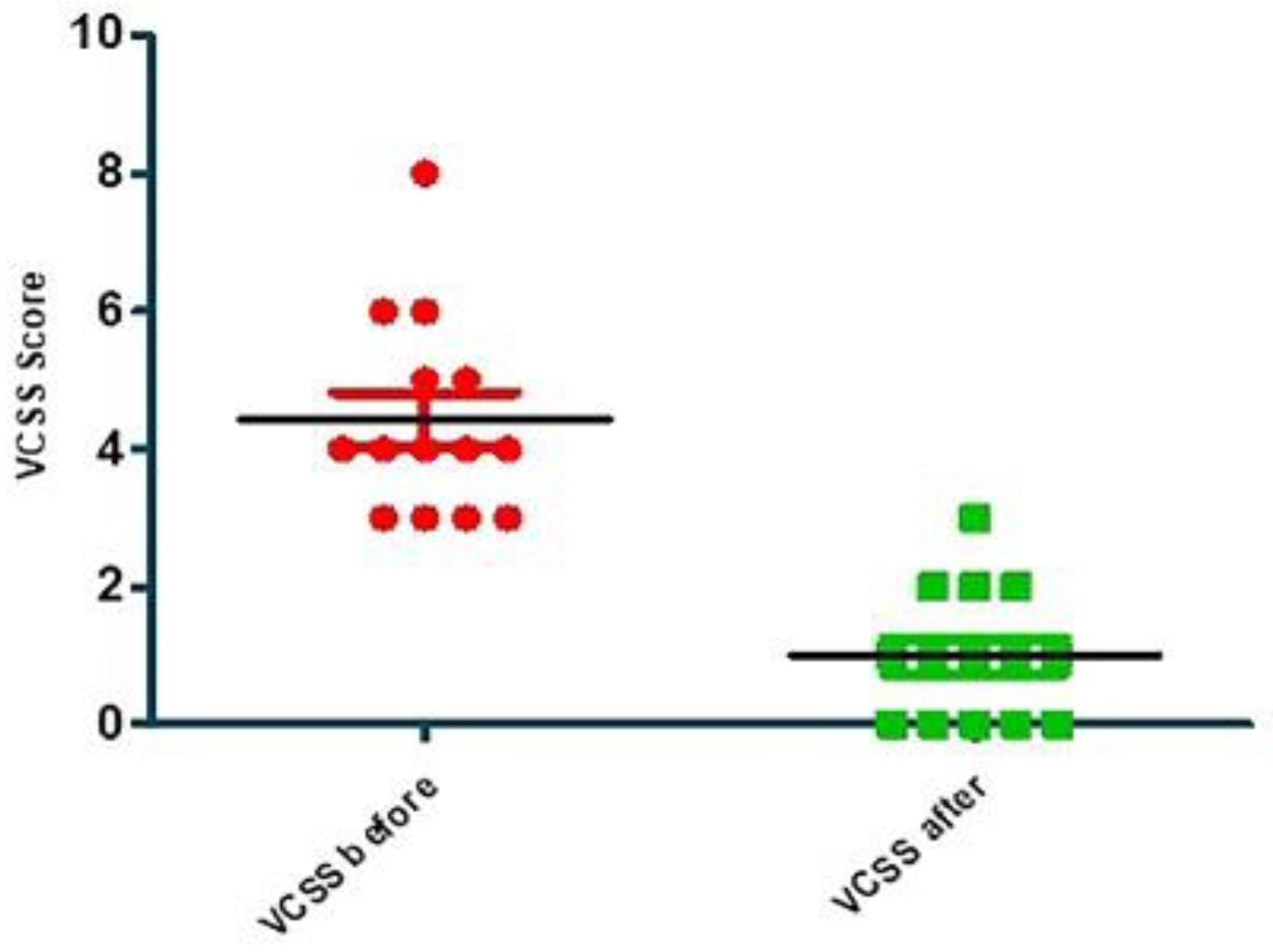
| VCSS before | VCSS after | |
|---|---|---|
| Number of values | 14 | 14 |
| Minimum | 3.000 | 0.0 |
| 25% Percentile | 3.000 | 0.0 |
| Median | 4.000 | 1.000 |
| 75% Percentile | 5.250 | 2.000 |
| Maximum | 8.000 | 3.000 |
| Mean | 4.429 | 1.000 |
| Std. Deviation | 1.453 | 0.9608 |
| Std. Error | 0.3882 | 0.2568 |
| Lower 95% CI of mean | 3.590 | 0.4453 |
| Upper 95% CI of mean | 5.267 | 1.555 |
| KS normality test | ||
| KS distance | 0.2589 | 0.2143 |
| P value | 0.0117 | 0.0808 |
| Passed normality test (alpha=0.05)? | No | Yes |
| P value summary | * | Ns |
| One sample t test | ||
| Theoretical mean | 0.0 | 0.0 |
| Actual mean | 4.429 | 1.000 |
| Discrepancy | -4.429 | -1.000 |
| 95% CI of discrepancy | 3.590 to 5.267 | 0.4454 to 1.555 |
| t, df | t=11.41 df=13 | t=3.894 df=13 |
| P value (two tailed) | < 0.0001 | 0.0018 |
| Significant (alpha=0.05)? | Yes | Yes |
| Coefficient of variation | 32.80% | 96.08% |
© 2024 by the authors. 2024 Valeriu Ardeleanu, Lavinia-Alexandra Moroianu, Cecilia Curis, Alin Laurentiu Tatu, Iasmina-Raisa Ardeleanu.
Share and Cite
Ardeleanu, V.; Moroianu, L.-A.; Curis, C.; Tatu, A.L.; Ardeleanu, I.-R. The Usage of Polidocanol in Varicose Disease Treatment. J. Mind Med. Sci. 2024, 11, 488-495. https://doi.org/10.22543/2392-7674.1478
Ardeleanu V, Moroianu L-A, Curis C, Tatu AL, Ardeleanu I-R. The Usage of Polidocanol in Varicose Disease Treatment. Journal of Mind and Medical Sciences. 2024; 11(2):488-495. https://doi.org/10.22543/2392-7674.1478
Chicago/Turabian StyleArdeleanu, Valeriu, Lavinia-Alexandra Moroianu, Cecilia Curis, Alin Laurentiu Tatu, and Iasmina-Raisa Ardeleanu. 2024. "The Usage of Polidocanol in Varicose Disease Treatment" Journal of Mind and Medical Sciences 11, no. 2: 488-495. https://doi.org/10.22543/2392-7674.1478
APA StyleArdeleanu, V., Moroianu, L.-A., Curis, C., Tatu, A. L., & Ardeleanu, I.-R. (2024). The Usage of Polidocanol in Varicose Disease Treatment. Journal of Mind and Medical Sciences, 11(2), 488-495. https://doi.org/10.22543/2392-7674.1478



