Effects of the COVID-19 Pandemic on Clinical Manifestations and Therapeutic Outcomes in Acute Endophthalmitis
Abstract
:Introduction
Materials and Methods
- Study design
- Statistical analysis
Results
Discussions
Conclusions
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sadiq, M.A.; Hassan, M.; Agarwal, A.; et al. Endogenous endophthalmitis: Diagnosis, management, and prognosis. J Ophthalmic Inflamm Infect. 2015, 5, 32. [Google Scholar] [CrossRef]
- Kernt, M.; Kampik, A. Endophthalmitis: Pathogenesis, clinical presentation, management, and perspectives. Clin Ophthalmol. 2010, 4, 121–135, Published 2010 Mar 24. [Google Scholar] [CrossRef] [PubMed]
- Sheu, S.J. Endophthalmitis. Korean J Ophthalmol. 2017, 31, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Kuo, G.; Yen, C.L.; Lu, Y.A.; et al. Clinical and visual outcomes following endogenous endophthalmitis: 175 consecutive cases from a tertiary referral center in Taiwan. J Microbiol Immunol Infect. 2022, 55, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Gajdzis, M.; Figuła, K.; Kamińska, J.; Kaczmarek, R. Endogenous Endophthalmitis-The Clinical Significance of the Primary Source of Infection. J Clin Med. 2022, 11, 1183, Published 2022 Feb 23. [Google Scholar] [CrossRef]
- Fortes, B.H.; Tailor, P.D.; Xu, T.T.; Churchill, R.A.; Starr, M.R. Clinical Characteristics and Outcomes of Endophthalmitis Before and During the COVID-19 Pandemic. J Ophthalmic Vis Res. 2023, 18, 289–296, Published 2023 Jul 28. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, M.F.S.O.; Elshafei, A.M.K.; Nassar, M.M.; Abu Elela, M.A.; Abdallah, R.M.A. Combined endophthalmitis and orbital cellulitis in patients with corona virus disease (COVID-19). J Ophthalmic Inflamm Infect. 2021, 11, 27, Published 2021 Sep 15. [Google Scholar] [CrossRef]
- Khatwani, P.R.; Goel, N.P.; Trivedi, K.Y.; Aggarwal, S.V. Unveiling endophthalmitis post COVID-19—A case series. Indian J Ophthalmol. 2021, 69, 2869–2871. [Google Scholar] [CrossRef]
- Chaudhary, S.; Sitaula, R.K.; Karki, P.; Joshi, S.N. Endogenous endophthalmitis in post-COVID-19 patients: A case report. Ann Med Surg (Lond). 2023, 85, 4137–4141, Published 2023 Jul 8. [Google Scholar] [CrossRef]
- Markan, A.; Dogra, M.; Katoch, D.; Tomar, M.; Mittal, H.; Singh, R. Endogenous Endophthalmitis in COVID-19 Patients: A Case Series and Literature Review. Ocul Immunol Inflamm. 2023, 31, 1191–1197. [Google Scholar] [CrossRef]
- Riazi, M. The Impact of COVID-19 on Ophthalmology Practice: Changes and Controversies in Endophthalmitis Risk. J Ophthalmic Vis Res. 2023, 18, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Endophthalmitis Vitrectomy Study Group. Arch Ophthalmol. 1995, 113, 1479–1496. [Google Scholar] [CrossRef]
- Serban, D.; Socea, B.; Badiu, C.D.; et al. Acute surgical abdomen during the COVID-19 pandemic: Clinical and therapeutic challenges. Exp Ther Med. 2021, 21, 519. [Google Scholar] [CrossRef]
- Dascalu, A.M.; Tudosie, M.S.; Smarandache, G.C.; Serban, D. Impact of the COVID-19 pandemic upon the ophthalmological clinical practice. Rom J Leg Med. 2020, 28, 96–100. [Google Scholar] [CrossRef]
- Wen, J.C.; McCannel, C.A.; et al. Bacterial dispersal associated with speech in the setting of intravitreous injections. Arch Ophthalmol. 2011, 129, 1551–54. [Google Scholar] [CrossRef]
- Karimi, S.; Nikkhah, H.; Mohammadzadeh, A.; et al. Intravitreal Injections and Face Masks: Endophthalmitis Risk Before and During the COVID-19 Pandemic. J Ophthalmic Vis Res. 2023, 18, 283–288, Published 2023 Jul 28. [Google Scholar] [CrossRef]
- Patel, S.N.; Hsu, J.; Sivalingam, M.D.; et al. The Impact of Physician Face Mask Use on Endophthalmitis After Intravitreal Anti-Vascular Endothelial Growth Factor Injections. Am J Ophthalmol. 2021, 222, 194–201. [Google Scholar] [CrossRef]
- Serban, D.; Branescu, C.M.; Smarandache, G.C.; Tudor, C.; Tănăsescu, C.; Tudosie, M.S.; Stana, D.; Costea, D.O.; Dascălu, A.M.; Spătaru, R.I. Safe surgery in day care centers: Focus on preventing medical legal issues. Rom J Leg Med. 2021, 29, 60–64. [Google Scholar] [CrossRef]
- Agi, N.; Zarbin, M.A.; Bhagat, N. Klebsiella Endogenous Endophthalmitis During the COVID-19 Pandemic. J Vitreoretin Dis. 2023, 7, 316–321. [Google Scholar] [CrossRef]
- Shroff, D.; Narula, R.; Atri, N.; Chakravarti, A.; et al. Endogenous fungal endophthalmitis following intensive corticosteroid therapy in severe COVID-19 disease. Indian J Ophthalmol. 2021, 69, 1909–1914. [Google Scholar] [CrossRef]
- Fekri, S.; Khorshidifar, M.; Esfahanian, F.; Veisi, A.; Nouri, H.; Abtahi, S.H. Endogenous Fungal Endophthalmitis following COVID-19 Hospitalization: Evidence from a Year-Long Observational Study. J Fr Ophtalmol. 2023, 46, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Samanta, R.; Jayaraj, S.; Mittal, S.K.; et al. Post-COVID-19 endogenous endophthalmitis case series and review of literature. Indian J Ophthalmol. 2023, 71, 3677–3683. [Google Scholar] [CrossRef] [PubMed]
- Grecescu, M.L.; Grecescu, M.; Smarandache, A.M.; et al. Klebsiella pneumoniae cryptogenic liver abscess and endophthalmitis—a case report and review of literature. J Mind Med Sci. 2021, 8, 330–335. [Google Scholar] [CrossRef]
- Brănescu, C.; Serban, D.; Dascălu, A.M.; et al. Interleukin 6 and lipopolysaccharide binding protein—Markers of inflammation in acute appendicitis. Chirurgia (Bucur). 2013, 108, 206–214. [Google Scholar] [PubMed]
- Chung, C.Y.; Wong, E.S.; Liu, C.C.H.; Wong, M.O.M.; Li, K.K.W. Clinical features and prognostic factors of Klebsiella endophthalmitis-10-year experience in an endemic region. Eye (Lond). 2017, 31, 1569–1575. [Google Scholar] [CrossRef]
- Vaziri, K.; Schwartz, S.G.; Kishor, K.; Flynn, H.W., Jr. Endophthalmitis: State of the art. Clin Ophthalmol. 2015, 9, 95–108, Published 2015 Jan 8. [Google Scholar] [CrossRef]
- Ham, Y.R.; Na, K.R. Endogenous Bacterial Endopthalmitis from Long-term Use of a Tunneled Cuffed Hemodialysis Catheter Infection. Chonnam Med J. 2018, 54, 129–130. [Google Scholar] [CrossRef]
- Noditi, A.; Caragheorghe, G.; Stoleru, S.; Blidaru, A.; Bordea, C.I. Contralateral Prophylactic Mastectomy in Patients with Breast Cancer. Chirurgia (Bucur). 2021, 116, 73–83. [Google Scholar] [CrossRef]
- Kuo, G.; Lu, Y.A.; Sun, W.C.; et al. Epidemiology and outcomes of Endophthalmitis in chronic dialysis patients: A 13-year experience in a tertiary referral center in Taiwan. BMC Nephrol. 2017, 18, 270, Published 2017 Aug 16. [Google Scholar] [CrossRef]
- Fometescu, S.G.; Costache, M.; Coveney, A.; Oprescu, S.M.; Serban, D.; Savlovschi, C. Peritoneal fibrinolytic activity and adhesiogenesis. Chirurgia (Bucur). 2013, 108, 331–340. [Google Scholar]
- Altalhi, R.; Pechlivani, N.; Ajjan, R.A. PAI-1 in Diabetes: Pathophysiology and Role as a Therapeutic Target. Int J Mol Sci. 2021, 22, 3170, Published 2021 Mar 20. [Google Scholar] [CrossRef]
- Suceveanu, A.I.; Mazilu, L.; Katsiki, N.; et al. NLRP3 Inflammasome Biomarker-Could Be the New Tool for Improved Cardiometabolic Syndrome Outcome. Metabolites. 2020, 10, 448, Published 2020 Nov 6. [Google Scholar] [CrossRef] [PubMed]
- Dascalu, A.M.; Anghelache, A.; Stana, D.; Costea, A.C.; Nicolae, V.A.; et al. Serum levels of copper and zinc in diabetic retinopathy: Potential new therapeutic targets (Review). Exp Ther Med. 2022, 23, 324. [Google Scholar] [CrossRef] [PubMed]
- Coburn, P.S.; Wiskur, B.J.; Christy, E.; Callegan, M.C. The diabetic ocular environment facilitates the development of endogenous bacterial endophthalmitis. Invest Ophthalmol Vis Sci. 2012, 53, 7426–7431, Published 2012 Nov 1. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.Y.Y.; Harris, S.; Krawchenko, I.; et al. Impact of the COVID-19 Pandemic on Adults With Type 2 Diabetes Care and Clinical Parameters in a Primary Care Setting in Ontario, Canada: A Cross-sectional Study. Can J Diabetes. 2023, 47, 345–351. [Google Scholar] [CrossRef]
- Weng, T.H.; Chang, H.C.; Chung, C.H.; et al. Epidemiology and Mortality-Related Prognostic Factors in Endophthalmitis. Invest Ophthalmol Vis Sci. 2018, 59, 2487–2494. [Google Scholar] [CrossRef]
- Grigore, A.; Vatasescu-Balcan, A.; Stoleru, S.; Zugravu, A.; Poenaru, E.; Engi, M.; Coman, O.A.; Fulga, I. Experimental Research on the Influence of Ion Channels on the Healing of Skin Wounds in Rats. Processes. 2024, 12, 109. [Google Scholar] [CrossRef]
- Grigore, A.; Stoleru, S.; Zugravu, A.; et al. Experimental evaluation of the influence of amiodarone on wound healing. Farmacia 2024, 72, 234–242. [Google Scholar] [CrossRef]
- Khunti, K.; Aroda, V.R.; Aschner, P.; et al. The impact of the COVID-19 pandemic on diabetes services: Planning for a global recovery. Lancet Diabetes Endocrinol. 2022, 10, 890–900. [Google Scholar] [CrossRef]
- Groß, R.; Kleger, A. COVID-19 and diabetes—Where are we now? Nat Metab. 2022, 4, 1611–1613. [Google Scholar] [CrossRef]
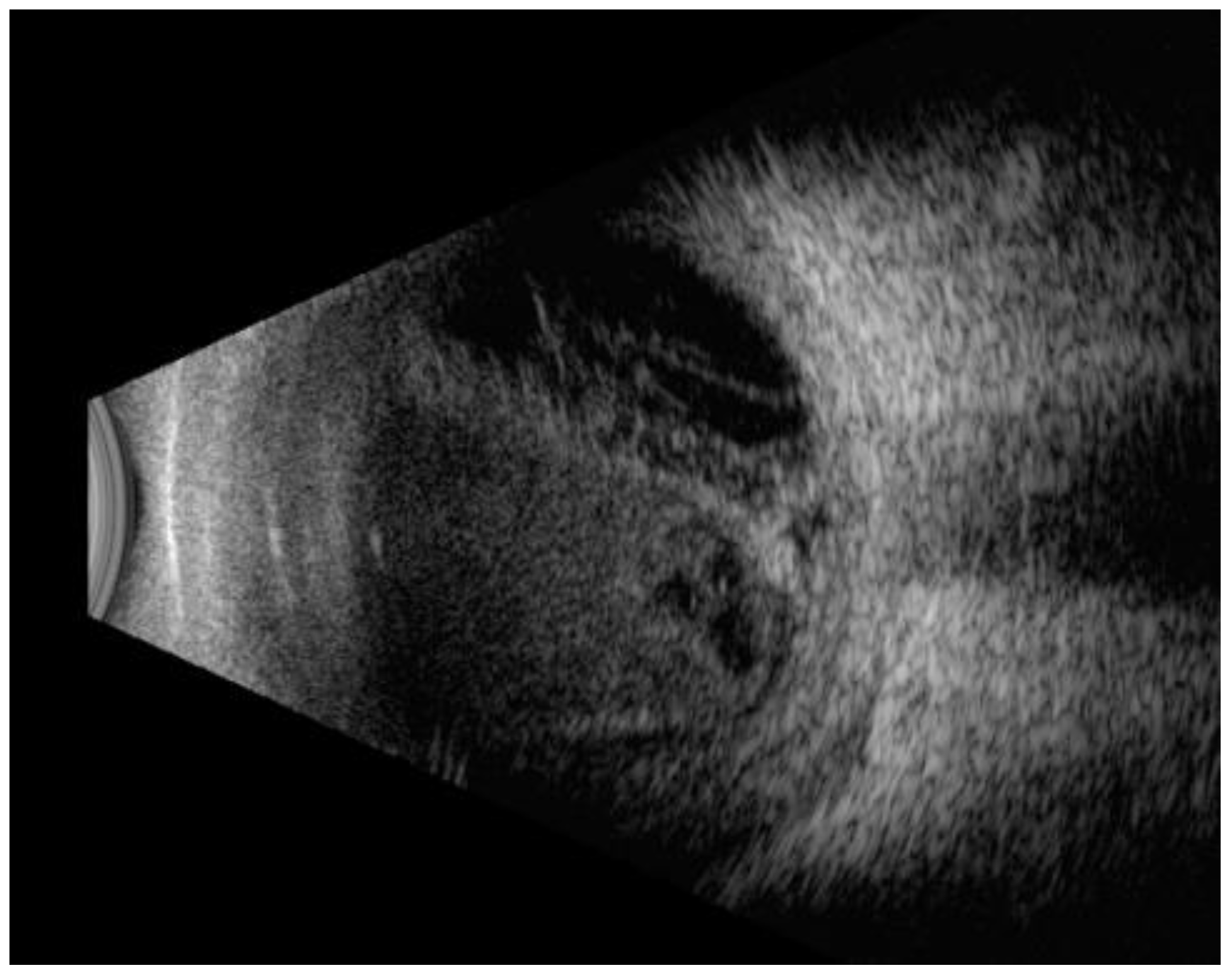
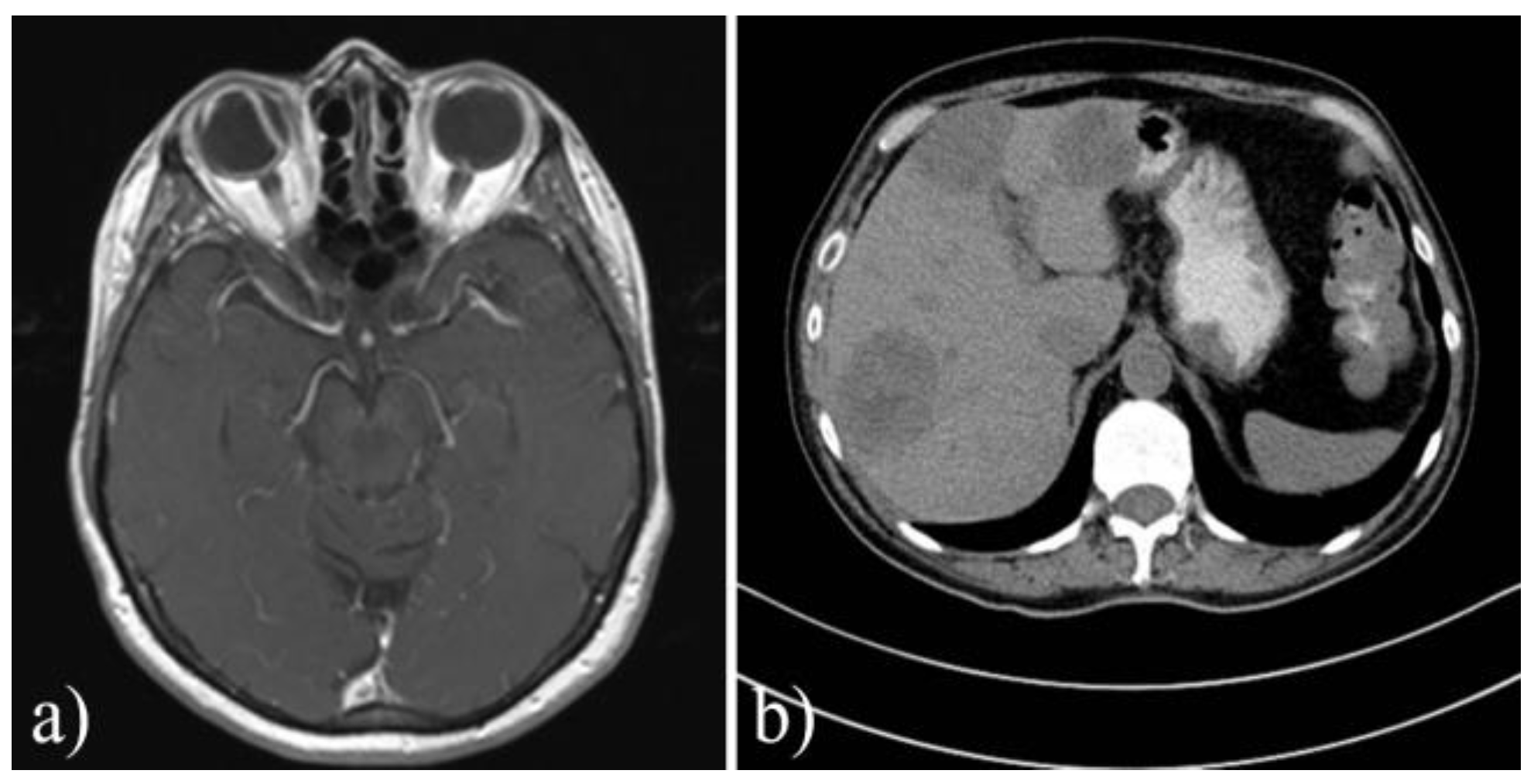
| Parameter | Total group (n=56) | Pre-pandemic group (n=24) | Covid-19 Pandemic group (n=32) | p-value |
|---|---|---|---|---|
| Age | 65.4±13.2 | 64.17 (± 17.98) | 66.38 (± 8.86) | 0.87 |
| Gender (male, n; %) | 26 (46.4%) | 10 (41.6%) | 16 (50%) | 0.72 |
| Rural area | 36 (64.2%) | 18 (75.0%) | 18 (56.25%) | 0.17 |
| Diabetes | 8 (14.3%) | 2 (8.33%) | 6 (18.75%) | 0.44 |
| Neoplasm (chemotherapy catheter) | 4 (7.1%) | 0 (0%) | 4 (12.5%) | 0.17 |
| Chronic renal disease (with dialysis) | 6 (10.7%) | 4 (16.67%) | 2 (6.25%) | 0.32 |
| History of recent ocular surgery (including intravitreal injections) | 22 (39.2%) | 10 (41.67%) | 12 (37.5%) | 0.78 |
| History of recent ocular trauma | 6 (10.7%) | 4 (16.67%) | 2 (6.25%) | 0.32 |
| Recent history of COVID-19 (<30 days) | 2 (3.5%) | 0 (0%) | 2 (6.25%) | 0.28 |
| Time lapsed from onset to presentation | 6.43 (±7.76) | 3.42 (± 2.41) | 8.69 (± 9.6) | 0.02* |
Location:
| 4 (7.1%) 32 (57.1%) 20 (35.7%) | 0 (0.0%) 14 (58.33%) 10 (41.67%) | 4 (12.5%) 18 (56.25%) 10 (31.25%) | 0.19 |
Cause:
| 21 (37.5%) 35 (33.5%) | 5 (26.3%) 19 (73.7%) | 16 (50%) 16 (50%) | 0.025* |
| Associated orbital cellulitis at admission | 10 (17.8%) | 2 (8.33%) | 8 (25.0%) | 0.16 |
| Fever (at admission) | 11 (19.6%) | 1 (4.1%) | 10 (33.3%) | 0.008* |
| Exophthalmia | 12 (21.4%) | 2 (8.33%) | 10 (31.25%) | 0.05* |
| Impaired ocular motility | 14 (25%) | 2 (8.33%) | 12 (40.0%) | 0.012* |
| Footnote: * statistically significant | ||||
| Parameter | Total group (n=56) | Pre-pandemic group (n=24) | Covid-19 Pandemic group (n=32) | p-value |
|---|---|---|---|---|
BCVA at admission:
| 6 (10.7%) 16 (28.5%) 18 (32.3%) 16 (28.5%) | 2 (8.33%) 8 (33.3%) 8 (33.3%) 6 (25%) | 4 (12.5%) 8 (25%) 10 (31.2%) 10 (31.2%) | 0.118 |
IOP at admission
| 24 (42.8%) 27 (48.3%) 5 (8.9%) | 10 (41.6%) 11 (45.8%) 3 (12.5%) | 14 (46.67%) 16 (50%) 2 (6.65%) | 0.117 |
| Chemosis | 20 (35.7%) | 6 (25.0%) | 14 (43.75%) | 0.171 |
| Keratic precipitates | 24 (42.8%) | 10 (41.67%) | 14 (43.75%) | >0.99 |
| Hypopyon | 38 (69.6%) | 18 (75%) | 20 (62.5%) | 0.47 |
| Seclusio pupillae | 38 (67.8%) | 16 (66.6%) | 22 (68.5%) | 0.32 |
Ocular fundus
| 54 (96.4%) 2(3.6%) | 24 (100%) | 30 (93.7%) 2 (6.3%) | 0.5 |
Ocular ultrasound exam (B-mode)
| 53 (94.6%) 12 (21.4%) 3 (5.3%) | 23 (95.8%) 4 (16.6%) 1 (4.1%) | 30 (93.7%) 8 (25%) 2 (6.25%) | 0.08 |
Culture for pathogen identification:
| 36 (64.2%) 14 (25%) 6 (10.7%) 2 (3.57%) 3 (5.35%) 2 (3.57%) | 14 (58.33%) 8 (33.33%) 2 (8.33%) 0 0 0 | 22 (68.75%) 6 (18.75%) 4 (12.5%) 2 (6.25%) 3 (9.3%) 2 (6.25%) | 0.52 |
Pathogen:
| 40 (71.4%) 2 (3.5%) 4 (7%) 4 (7%) 4 (7%) | 16 (66.67%) 2 (8.33%) 4 (16.67%) 0 (0.0%) 2 (8.33%) | 24 (80.0%) 0 (0.0%) 1 (3.1%) 4 (13.33%) 2 (6.67%) | 0.01* |
| WBC (cells/mmc; mean±SD) | 11.11 (±4.81) | 10.66 (± 5.5) | 12.15 (± 4.36) | 0.138 |
| WBC>10000 cells/mmc (n, %) | 25 (44.6%) | 7 (29.1%) | 18 (56.2%) | 0.03* |
| Fibrinogen (mg/dl; mean±SD) | 413.78 (±169.78) | 349.42 (± 138.0) | 454.0 (± 179.82) | 0.01* |
| Fibrinogen>400 mg/dl (n, %) | 20 (35.7%) | 4 (16.6%) | 16 (50%) | 0.009* |
| CRP (mg/dl; mean±SD) | 3.46 ± 4.39) | 2.02 (± 1.93) | 4.36 (± 5.28) | 0.1 |
| CRP>0.5 mg/dl (n, %) | 42 (75%) | 14 (50%) | 28 (87.5%) | <0.001* |
| Glycemia (mg/dl; mean±SD | 119.74 (±34.83) | 104.0 (± 26.33) | 130.56 (± 36.71) | 0.006* |
| Glycemia >110 mg/dl | 23 (41%) | 4 (16.6%) | 19 (59.3%) | 0.001* |
| CT/IRM for involved orbit | 10 (17.8%) | 2 (8.33%) | 8 (25%) | 0.1 |
| Footnote: BCVA: best corrected visual acuity; CF: counting fingers; HM: hand movement; LP: light perception;WBC: white blood cells; CRP: C-reactive protein; * statistically significant (p<0.05) | ||||
| Outcomes | Total group (n=56) | COVID-19 pandemic group (n=32) | Pre-pandemic group (n=24) | p-value |
|---|---|---|---|---|
| Evisceration/Enucleation | 10 (17.8%) | 4 (13.33%) | 6 (25%) | 0.17 |
| Final BCVA ≥0.1 CF HM LP No LP | 8 (14.2%) 11 (19.6%) 5 (8.9%) 7 (12.5%) 15 (26.7%) | 2 (6.25%) 7 (21.8%) 3 (9.3%) 4 (12.5%) 12 (37.5%) | 6 (25%) 4 (16.6%) 2 (8.3%) 3 (12.5%) 3 (12.5%) | 0.001* |
| Death | 1 (1.7%) | 1 (3.1%) | 0 | 0.67 |
| Hospital stays | 13.1 (± 4.62) | 14.0 (± 5.88) | 11.0 (± 5.11) | 0.06 |
| Footnote: * statistically significant | ||||
© 2024 by the authors. 2024 Ana Maria Dascalu, Sanda Jurja, Carmen Luminita Mocanu, Cristina Alexandrescu, Daniela Stana, Madalina Totir, Ece Ergin, Corneliu Tudor, Catalin Cicerone Grigorescu, Dragos Serban, Laurentiu Simion, Dan Dumitrescu, Andrei Marin, Catalin Teodor Constantinescu, Bogdan Mihai Cristea1.
Share and Cite
Dascalu, A.M.; Jurja, S.; Mocanu, C.L.; Alexandrescu, C.; Stana, D.; Totir, M.; Ergin, E.; Tudor, C.; Grigorescu, C.C.; Serban, D.; et al. Effects of the COVID-19 Pandemic on Clinical Manifestations and Therapeutic Outcomes in Acute Endophthalmitis. J. Mind Med. Sci. 2024, 11, 475-481. https://doi.org/10.22543/2392-7674.1554
Dascalu AM, Jurja S, Mocanu CL, Alexandrescu C, Stana D, Totir M, Ergin E, Tudor C, Grigorescu CC, Serban D, et al. Effects of the COVID-19 Pandemic on Clinical Manifestations and Therapeutic Outcomes in Acute Endophthalmitis. Journal of Mind and Medical Sciences. 2024; 11(2):475-481. https://doi.org/10.22543/2392-7674.1554
Chicago/Turabian StyleDascalu, Ana Maria, Sanda Jurja, Carmen Luminita Mocanu, Cristina Alexandrescu, Daniela Stana, Madalina Totir, Ece Ergin, Corneliu Tudor, Catalin Cicerone Grigorescu, Dragos Serban, and et al. 2024. "Effects of the COVID-19 Pandemic on Clinical Manifestations and Therapeutic Outcomes in Acute Endophthalmitis" Journal of Mind and Medical Sciences 11, no. 2: 475-481. https://doi.org/10.22543/2392-7674.1554
APA StyleDascalu, A. M., Jurja, S., Mocanu, C. L., Alexandrescu, C., Stana, D., Totir, M., Ergin, E., Tudor, C., Grigorescu, C. C., Serban, D., Simion, L., Dumitrescu, D., Marin, A., Constantinescu, C. T., & Cristea, B. M. (2024). Effects of the COVID-19 Pandemic on Clinical Manifestations and Therapeutic Outcomes in Acute Endophthalmitis. Journal of Mind and Medical Sciences, 11(2), 475-481. https://doi.org/10.22543/2392-7674.1554



