Infertility as a Possible Multifactorial Condition; The Experience of a Single Center
Abstract
Introduction
Material and Methods
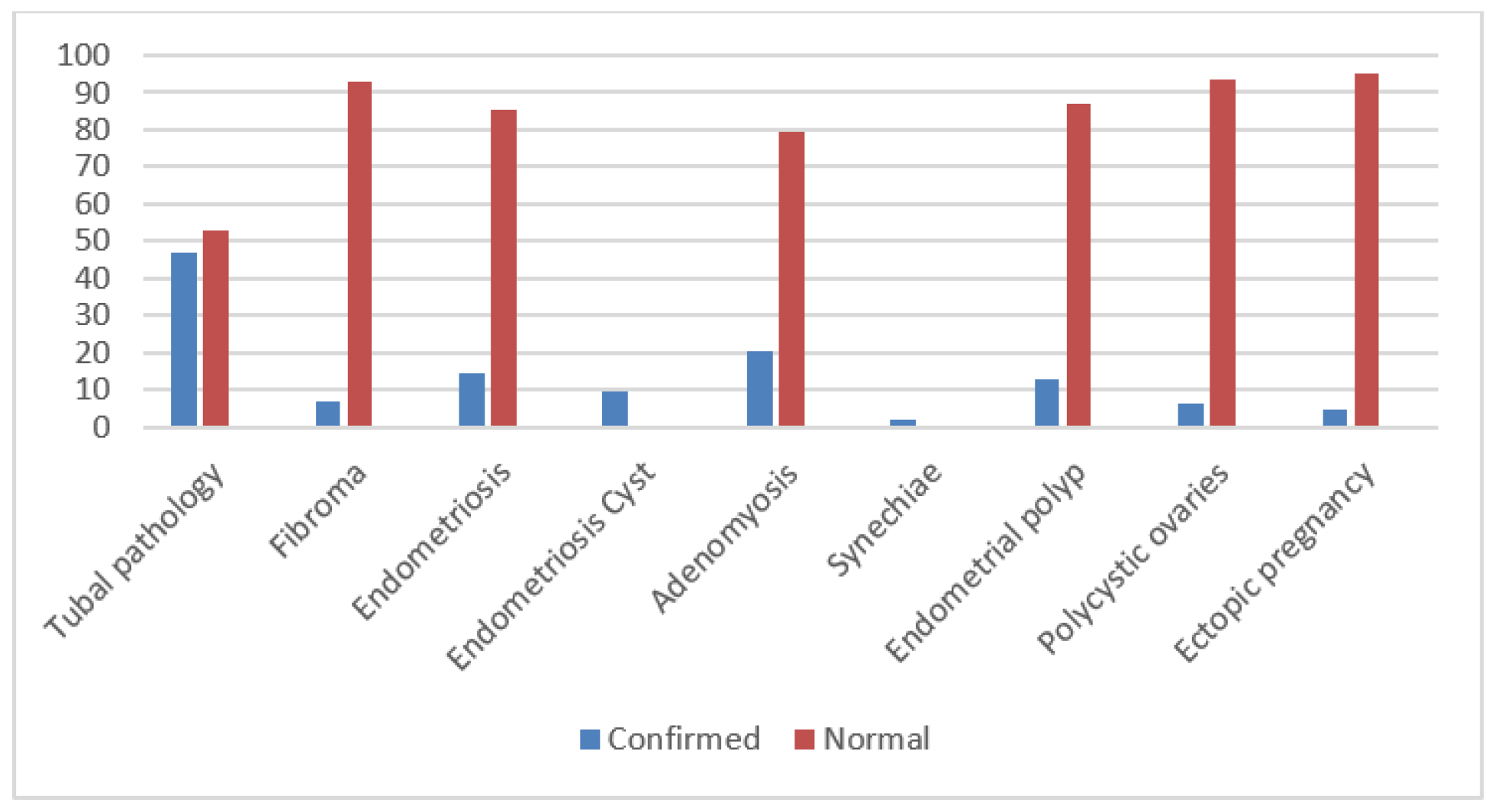
Results
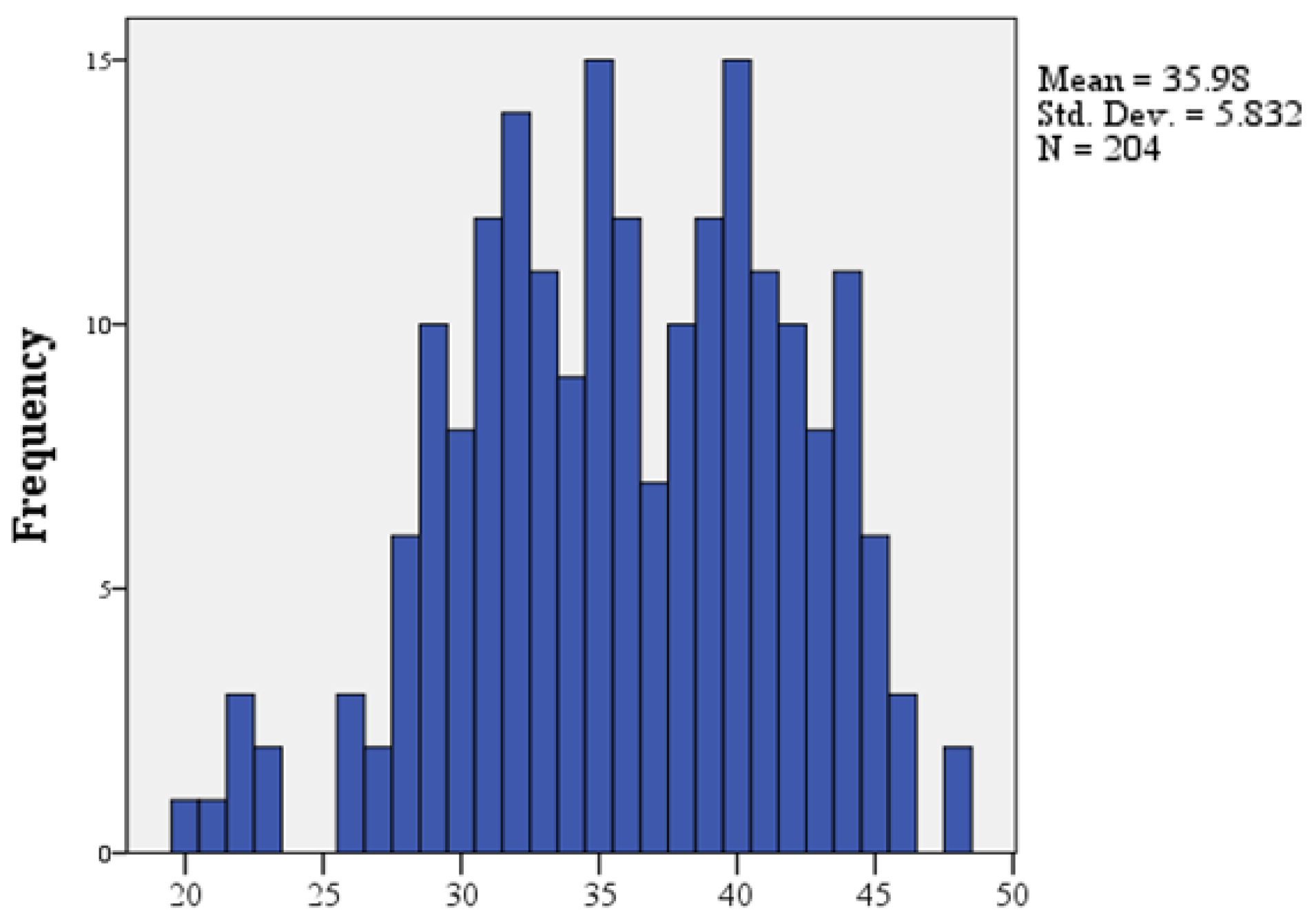

Discussions
Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Van Der Kelen, A.; Okutman, Ö.; Javey, E.; et al. A systematic review and evidence assessment of monogenic gene-disease relationships in human female infertility and differences in sex development. Hum Reprod Update. 2023, 29, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Sang, Q.; Ray, P.F.; Wang, L. Understanding the genetics of human infertility. Science. 2023 Apr 14, 380, 158–163. [Google Scholar] [CrossRef]
- Brezina, P.R.; Kutteh, W.H. Clinical applications of preimplantation genetic testing. BMJ. 2015, 350, g7611, Published 2015 Feb 19. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.T.; Kappy, M.; Forman, E.J.; Dokras, A. Genetics in reproductive endocrinology and infertility. Fertil Steril 2023, 120(3 Pt 1), 521–527. [Google Scholar] [CrossRef]
- Hwang, S.I.; Yoon, Y.J.; Sung, S.H.; Cho, S.J.; Park, J.K. Acupuncture Treatment for Emotional Problems in Women with Infertility: A Systematic Review and Meta-Analysis. Healthcare (Basel). 2023, 11, 2704. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.; Olanrewaju, R.A.; Omole, F. Infertility: Evaluation and Management. Am Fam Physician. 2023, 107, 623–630. [Google Scholar]
- Ennab, F.; Atiomo, W. Obesity and female infertility. Best Pract Res Clin Obstet Gynaecol. 2023, 89, 102336. [Google Scholar] [CrossRef]
- Gautam, D.; Purandare, N.; Maxwell, C.V.; et al. The challenges of obesity for fertility: A FIGO literature review. Int J Gynaecol Obstet 2023, 160 Suppl 1(Suppl 1), 50–55. [Google Scholar] [CrossRef]
- Haase, C.L.; Varbo, A.; Laursen, P.N.; Schnecke, V.; Balen, A.H. Association between body mass index, weight loss and the chance of pregnancy in women with polycystic ovary syndrome and overweight or obesity: A retrospective cohort study in the UK. Hum Reprod. 2023, 38, 471–481. [Google Scholar] [CrossRef]
- Popescu, C.D.; Sima, R.M.; Amza, M.; et al. Hysteroscopy for Infertility in Young Women—Our Experience. Maedica (Bucur). 2023, 18, 631–638. [Google Scholar] [CrossRef]
- Dreisler, E.; Stampe Sorensen, S.; Ibsen, P.H.; Lose, G. Prevalence of endometrial polyps and abnormal uterine bleeding in a Danish population aged 20-74 years. Ultrasound Obstet Gynecol 2009, 33, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Pinzauti, S.; Lazzeri, L.; Tosti, C.; et al. Transvaginal sonographic features of diffuse adenomyosis in 18-30-year-old nulligravid women without endometriosis: Association with symptoms. Ultrasound Obstet Gynecol. 2015, 46, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.D.; Dunson, D.B.; Hill, M.C.; et al. High cumulative incidence of uterine leiomyoma in black and white women: Ultrasound evidence. Am J Obstet Gynecol. 2003, 188, 100–107. [Google Scholar] [CrossRef]
- Pérez-Medina, T.; Bajo-Arenas, J.; Salazar, F.; et al. Endometrial polyps and their implication in the pregnancy rates of patients undergoing intrauterine insemination: A prospective, randomized study. Hum Reprod. 2005, 20, 1632–1635. [Google Scholar] [CrossRef] [PubMed]
- Bosteels, J.; Kasius, J.; Weyers, S.; Broekmans, F.J.; Mol, B.W.; D’Hooghe, T.M. Hysteroscopy for treating subfertility associated with suspected major uterine cavity abnormalities. Cochrane Database Syst Rev 2015, CD009461. [Google Scholar] [CrossRef]
- Kalampokas, T.; Tzanakaki, D.; Konidaris, S.; Iavazzo, C.; Kalampokas, E.; Gregoriou, O. Endometrial polyps and their relationship in the pregnancy rates of patients undergoing intrauterine insemination. Clin Exp Obstet Gynecol. 2012, 39, 299–302. [Google Scholar]
- Richlin, S.S.; Ramachandran, S.; Shanti, A.; Murphy, A.A.; Parthasarathy, S. Glycodelin levels in uterine flushings and in plasma of patients with leiomyomas and polyps: Implications for implantation. Hum Reprod. 2002, 17, 2742–2747. [Google Scholar] [CrossRef]
- Maia, H., Jr.; Pimentel, K.; Silva, T.M.; et al. Aromatase and cyclooxygenase-2 expression in endometrial polyps during the menstrual cycle. Gynecol Endocrinol. 2006, 22, 219–224. [Google Scholar] [CrossRef]
- Ben-Nagi, J.; Miell, J.; Yazbek, J.; Holland, T.; Jurkovic, D. The effect of hysteroscopic polypectomy on the concentrations of endometrial implantation factors in uterine flushings. Reprod Biomed Online. 2009, 19, 737–744. [Google Scholar] [CrossRef]
- Rackow, B.W.; Jorgensen, E.; Taylor, H.S. Endometrial polyps affect uterine receptivity. Fertil Steril. 2011, 95, 2690–2692. [Google Scholar] [CrossRef]
- Sunkara, S.K.; Khan, K.S. Adenomyosis and female fertility: A critical review of the evidence. J Obstet Gynaecol. 2012, 32, 113–116. [Google Scholar] [CrossRef]
- Vercellini, P.; Consonni, D.; Dridi, D.; Bracco, B.; Frattaruolo, M.P.; Somigliana, E. Uterine adenomyosis and in vitro fertilization outcome: A systematic review and meta-analysis. Hum Reprod. 2014, 29, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Miura, S.; Khan, K.N.; Kitajima, M.; et al. Differential infiltration of macrophages and prostaglandin production by different uterine leiomyomas. Hum Reprod. 2006, 21, 2545–2554. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.; Pereira, L. Pregnancy outcomes following treatment for fibroids: Uterine fibroid embolization versus laparoscopic myomectomy. Curr Opin Obstet Gynecol. 2006, 18, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Keltz, J.; Levie, M.; Chudnoff, S. Pregnancy Outcomes After Direct Uterine Myoma Thermal Ablation: Review of the Literature. J Minim Invasive Gynecol. 2017, 24, 538–545. [Google Scholar] [CrossRef]
- Jegaden, M.; Capmas, P.; Debras, E.; et al. Treatment of synechiae related to infertility. Gynecol Obstet Fertil Senol. 2021, 49, 930–935. [Google Scholar] [CrossRef]
- Carson, S.A.; Kallen, A.N. Diagnosis and Management of Infertility: A Review. JAMA. 2021, 326, 65–76. [Google Scholar] [CrossRef]
- Solecki, R.; Kortenkamp, A.; Bergman, Å.; et al. Scientific principles for the identification of endocrine-disrupting chemicals: A consensus statement. Arch Toxicol. 2017, 91, 1001–1006. [Google Scholar] [CrossRef]
- Silva, A.B.P.; Carreiró, F.; Ramos, F.; Sanches-Silva, A. The role of endocrine disruptors in female infertility. Mol Biol Rep 2023, 50, 7069–7088. [Google Scholar] [CrossRef]
- Kleinplatz, P.J.; Weindling, P. Women’s experiences of infertility after the Holocaust. Soc Sci Med. 2022, 309, 115250. [Google Scholar] [CrossRef]
- Pasternak, A.; Brooks, P.G. The long-term effects of the Holocaust on the reproductive function of female survivors. J Minim Invasive Gynecol. 2007, 14, 211–217. [Google Scholar] [CrossRef]
- Gordon, EG. A Medical Education Recommendation for Improving Sexual Health and Humanism and Professionalism. Sex Med Rev. 2021, 9, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Koroma, L.; Stewart, L. Infertility: Evaluation and initial management. J Midwifery Womens Health. 2012, 57, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Gong, T.T.; Jiang, Y.T.; Zhang, S.; Zhao, Y.H.; Wu, Q.J. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990-2017: Results from a global burden of disease study, 2017. Aging (Albany NY). 2019, 11, 10952–10991. [Google Scholar] [CrossRef]
- Snow, M.; Vranich, T.M.; Perin, J.; Trent, M. Estimates of infertility in the United States: 1995-2019. Fertil Steril. 2022, 118, 560–567. [Google Scholar] [CrossRef]
- Case, A.M. Infertility evaluation and management. Strategies for family physicians. Can Fam Physician. 2003, 49, 1465–1472. [Google Scholar]
- Poston, L.; Caleyachetty, R.; Cnattingius, S.; et al. Preconceptional and maternal obesity: Epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016, 4, 1025–1036. [Google Scholar] [CrossRef]
- Hanson, M.; Gluckman, P.; Bustreo, F. Obesity and the health of future generations. Lancet Diabetes Endocrinol. 2016, 4, 966–967. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wan, L. Associations between smoking status and infertility: A cross-sectional analysis among USA women aged 18-45 years. Front Endocrinol (Lausanne). 2023, 14, 1140739, Published 2023 Apr 19. [Google Scholar] [CrossRef]
- Macaluso, M.; Wright-Schnapp, T.J.; Chandra, A.; et al. A public health focus on infertility prevention, detection, and management. Fertil Steril. 2010, 93, 16.e1-10. [Google Scholar] [CrossRef]
- Vannucchi, G.; Persani, L.; Fugazzola, L. Thyroid pathology and female fertility: Myth or reality? Ann Endocrinol (Paris). 2022, 83, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Concepción-Zavaleta, M.J.; Coronado-Arroyo, J.C.; Quiroz-Aldave, J.E.; Concepción-Urteaga, L.A.; Paz-Ibarra, J. Thyroid dysfunction and female infertility. A comprehensive review. Diabetes Metab Syndr. 2023, 17, 102876. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: A committee opinion. Fertil Steril. 2015, 103, e9–e17. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Xu, Z.; Liu, J. Mendelian randomization study of thyroid function and anti-Müllerian hormone levels. Front Endocrinol (Lausanne). 2023, 14, 1188284. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, M.N.; Flaxman, S.R.; Boerma, T.; Vanderpoel, S.; Stevens, G.A. National, regional, and global trends in infertility prevalence since 1990: A systematic analysis of 277 health surveys. PLoS Med. 2012, 9, e1001356. [Google Scholar] [CrossRef]
- Islam, H.; Masud, J.; Islam, Y.N.; Haque, F.K.M. An update on polycystic ovary syndrome: A review of the current state of knowledge in diagnosis, genetic etiology, and emerging treatment options. Womens Health (Lond). 2022, 18, 17455057221117966. [Google Scholar] [CrossRef]
- Kicińska, A.M.; Maksym, R.B.; Zabielska-Kaczorowska, M.A.; Stachowska, A.; Babińska, A. Immunological and Metabolic Causes of Infertility in Polycystic Ovary Syndrome. Biomedicines. 2023, 11, 1567. [Google Scholar] [CrossRef]
- Koniares, K.G.; Patel, K.; Baecher-Lind, L. Evaluation and Management of Infertility for Patients Without Insurance Coverage. Clin Obstet Gynecol. 2022, 65, 739–752. [Google Scholar] [CrossRef]
- Butts, S.F. Health disparities of African Americans in reproductive medicine. Fertil Steril. 2021, 116, 287–291. [Google Scholar] [CrossRef]
- Infertility Workup for the Women’s Health Specialist: ACOG Committee Opinion, Number 781. Obstet Gynecol. 2019, 133, e377–e384. [CrossRef]
- Antonisamy, N.; Reddy, N.S.; Chinta, P.; et al. Role of Hysterosalpingography in Diagnosing Tubal Blockage—A Prospective Diagnostic Study. J Hum Reprod Sci. 2021, 14, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Citu, C.; Gorun, F.; Motoc, A.; et al. Hysteroscopy as a Primary Tool in Exploration and Treatment of Infertility: Single Center Experience in Western Romania. Diagnostics (Basel). 2021, 11, 1917, Published 2021 Oct 16. [Google Scholar] [CrossRef] [PubMed]
- Kaseso, D.; Valyananzighu, J.; Mulisya, O.; et al. Hysterosalpingographic, ultrasonographic and clinical profile of infertile women in Butembo, Eastern Democratic Republic of Congo. Pan Afr Med J. 2023, 46, 105, Published 2023 Dec 14. [Google Scholar] [CrossRef]
- Casini, M.L.; Rossi, F.; Agostini, R.; Unfer, V. Effects of the position of fibroids on fertility. Gynecol Endocrinol. 2006, 22, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Munro, M.G. Uterine polyps, adenomyosis, leiomyomas, and endometrial receptivity. Fertil Steril. 2019, 111, 629–640. [Google Scholar] [CrossRef]
- Klatsky, P.C.; Tran, N.D.; Caughey, A.B.; Fujimoto, V.Y. Fibroids and reproductive outcomes: A systematic literature review from conception to delivery. Am J Obstet Gynecol. 2008, 198, 357–366. [Google Scholar] [CrossRef]
- Giatras, K.; Berkeley, A.S.; Noyes, N.; Licciardi, F.; Lolis, D.; Grifo, J.A. Fertility after hysteroscopic resection of submucous myomas. J Am Assoc Gynecol Laparosc. 1999, 6, 155–158. [Google Scholar] [CrossRef]
- Eldar-Geva, T.; Meagher, S.; Healy, D.L.; et al. Effect of intramural, subserosal, and submucosal uterine fibroids on the outcome of assisted reproductive technology treatment. Fertil Steril. 1998, 70, 687–691. [Google Scholar] [CrossRef]


| Frequency | Percent | Valid Percent | Cumulative Percent | ||
|---|---|---|---|---|---|
| Valid | Underweight | 6 | 2.9 | 5.0 | 5.0 |
| Normal weight | 73 | 35.8 | 60.8 | 65.8 | |
| Overweight | 28 | 13.7 | 23.3 | 89.2 | |
| Obesity grade I | 11 | 5.4 | 9.2 | 98.3 | |
| Obesity grade II | 1 | .5 | .8 | 99.2 | |
| Morbid obesity | 1 | .5 | .8 | 100.0 | |
| Total | 120 | 58.8 | 100.0 | ||
| Missing | System | 84 | 41.2 | ||
| Total | 204 | 100.0 | |||
© 2024 by the authors. 2024 Cristina Diana Popescu, Bashar Haj Hamoud, Romina Marina Sima, Anca Bobirca, Oana Denisa Balalau, Mihaela Amza, Romeo Micu, Gabriel Petre Gorecki, Liana Ples.
Share and Cite
Popescu, C.D.; Hamoud, B.H.; Sima, R.M.; Bobirca, A.; Balalau, O.D.; Amza, M.; Micu, R.; Gorecki, G.P.; Ples, L. Infertility as a Possible Multifactorial Condition; The Experience of a Single Center. J. Mind Med. Sci. 2024, 11, 466-474. https://doi.org/10.22543/2392-7674.1535
Popescu CD, Hamoud BH, Sima RM, Bobirca A, Balalau OD, Amza M, Micu R, Gorecki GP, Ples L. Infertility as a Possible Multifactorial Condition; The Experience of a Single Center. Journal of Mind and Medical Sciences. 2024; 11(2):466-474. https://doi.org/10.22543/2392-7674.1535
Chicago/Turabian StylePopescu, Cristina Diana, Bashar Haj Hamoud, Romina Marina Sima, Anca Bobirca, Oana Denisa Balalau, Mihaela Amza, Romeo Micu, Gabriel Petre Gorecki, and Liana Ples. 2024. "Infertility as a Possible Multifactorial Condition; The Experience of a Single Center" Journal of Mind and Medical Sciences 11, no. 2: 466-474. https://doi.org/10.22543/2392-7674.1535
APA StylePopescu, C. D., Hamoud, B. H., Sima, R. M., Bobirca, A., Balalau, O. D., Amza, M., Micu, R., Gorecki, G. P., & Ples, L. (2024). Infertility as a Possible Multifactorial Condition; The Experience of a Single Center. Journal of Mind and Medical Sciences, 11(2), 466-474. https://doi.org/10.22543/2392-7674.1535



