Correlation between Molecular Prognostic Factors and Bevacizumab Therapeutic Resistance in Patients with Metastatic Colorectal Cancer; The AVAMET Study
Abstract
Introduction
Materials and Methods
Participants, Data Collection and Ethical Statement
Specimen Collection
Statistical Analyses
Results
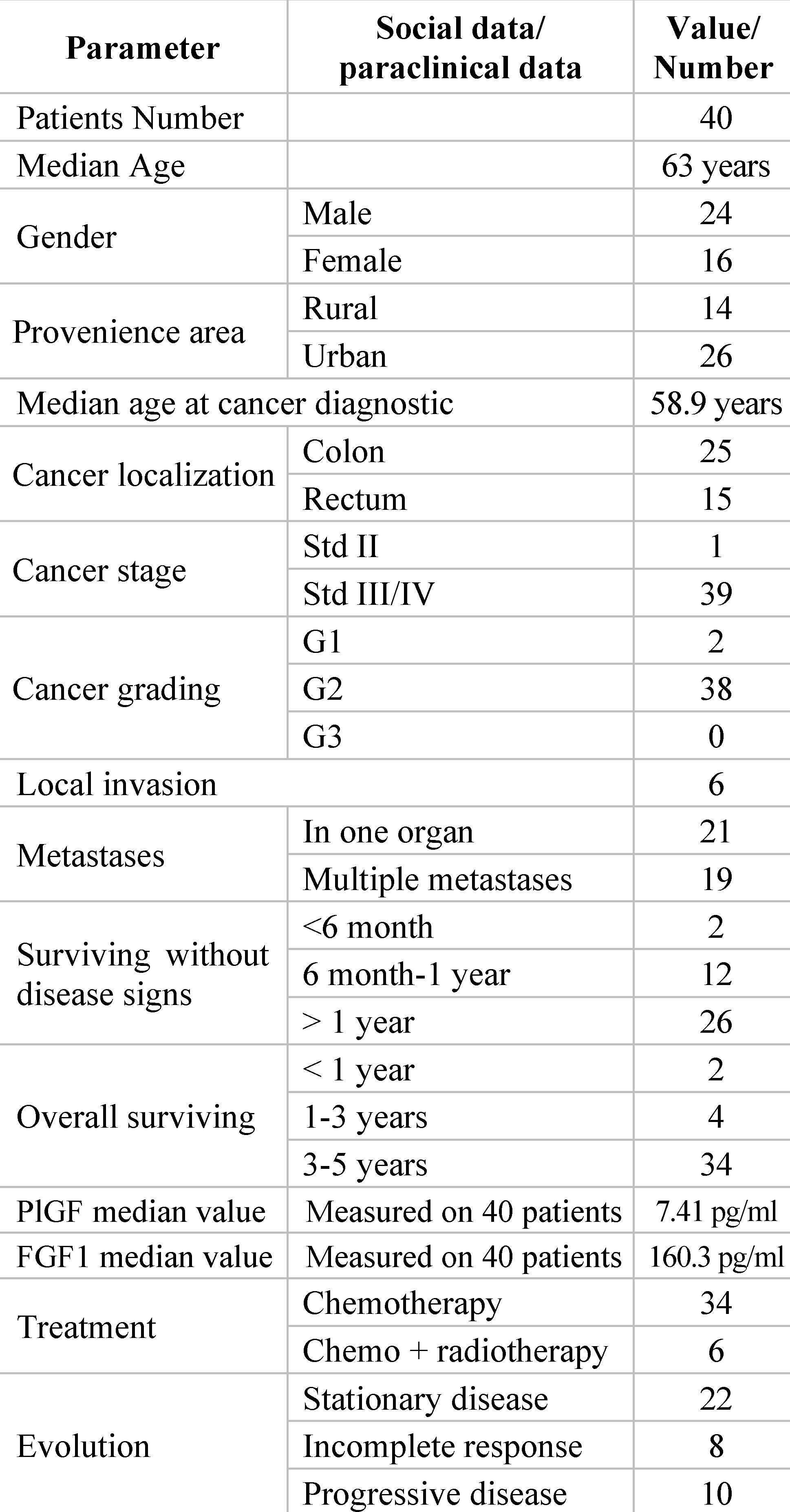
Socio-Demographic and Clinical Data of the Patients Included in the Study
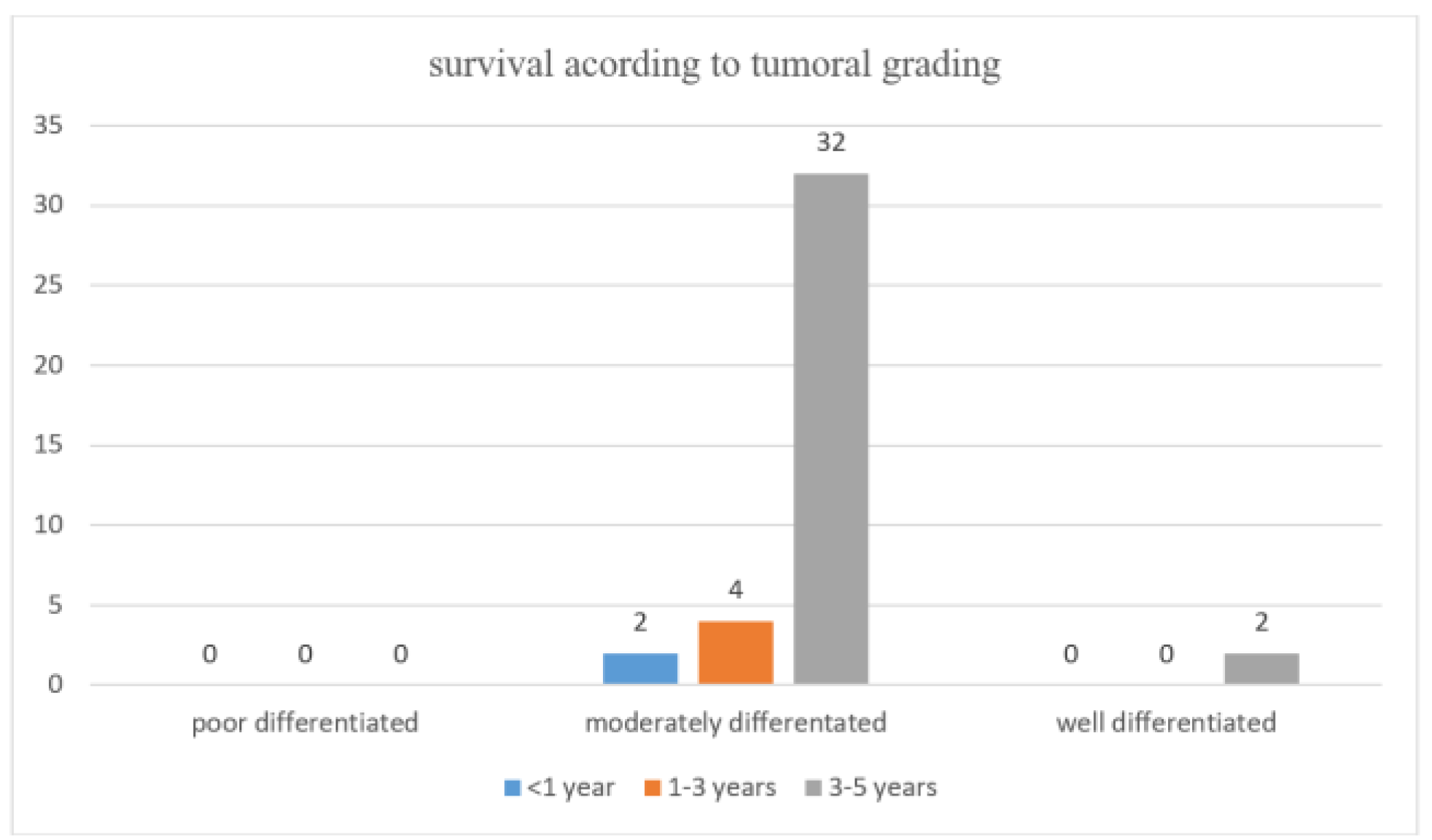
Survival Data During Anti-VEGF Treatment Overall Survival (OS) Rates
Disease-Free Survival (DFS) Rates
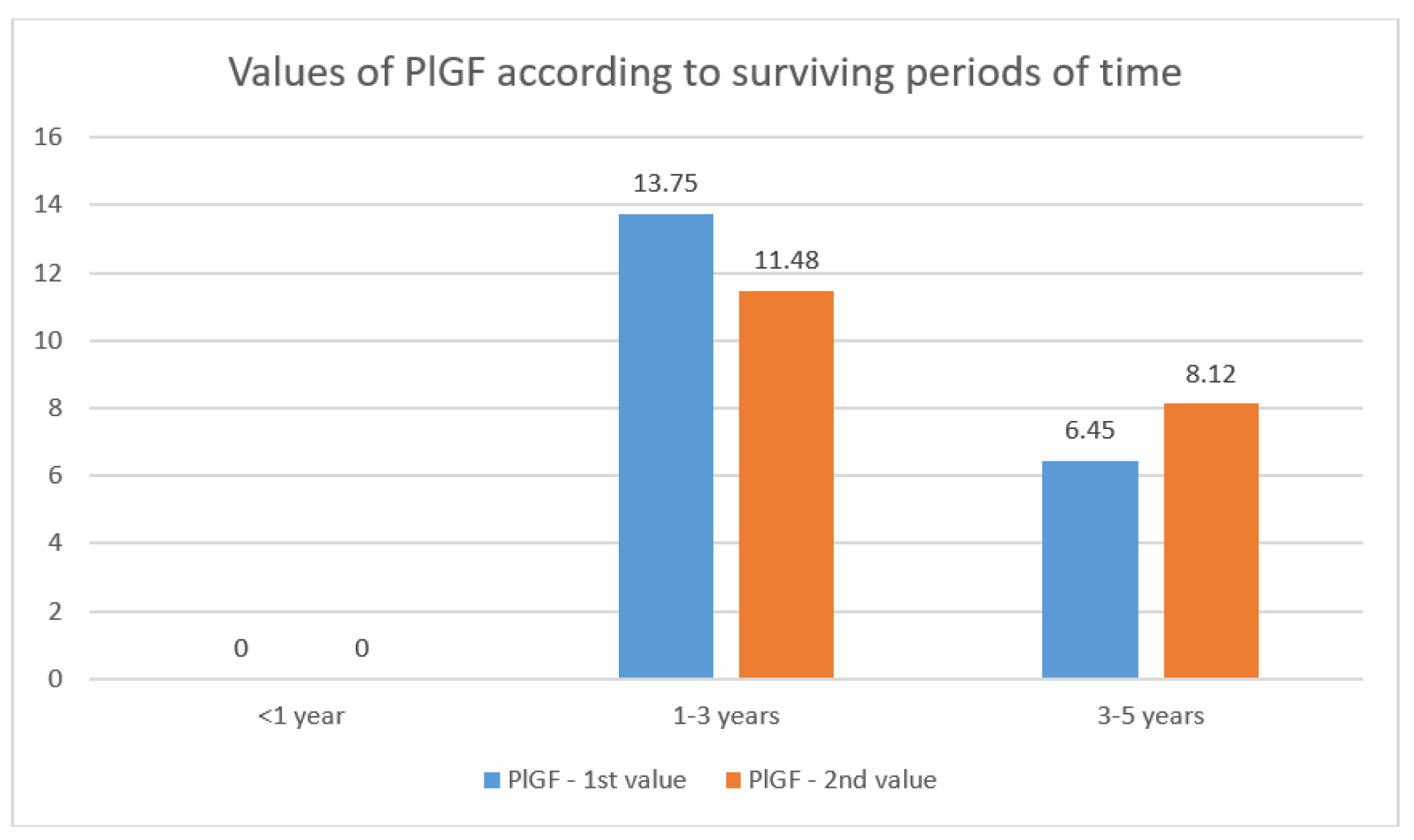
PlGF Values Data and Dynamics
Variation over Time of FGF1 Values
Discussions
Conclusions
Highlights
- ✓
- Prognostic Biomarkers in Metastatic CRC: This study focuses on the under-investigated prognostic roles of placental growth factor (PlGF) and fibroblast growth factor 1 (FGF1) identified by ELISA techniques in patients with metastatic colorectal cancer (CRC) undergoing treatment with bevacizumab.
- ✓
- Insights for Improved Outcomes: By highlighting the roles of PlGF and FGF1, the data obtained in this study provide valuable information on disease prognosis, treatment resistance and overall survival in advanced CRC. Such an exploration of new biomarkers seems to be a promising perspective for the further development of treatment strategies able to improve the therapeutic outcomes of patients with metastatic CRC.
Informed Consent Statement
Informed Consent Statement
Acknowledgments
Abbreviations
References
- Bretthauer, M.; Løberg, M.; Wieszczy, P.; Kalager, M.; Emilsson, L.; Garborg, K.; Rupinski, M.; Dekker, E.; Spaander, M.; Bugajski, M.; et al. Effect of Colonoscopy Screening on Risks of Colorectal Cancer and Related Death. N. Engl. J. Med. 2022, 387, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.C.H.; Liang, J.Q. Advances in tests for colorectal cancer screening and diagnosis. Expert Rev. Mol. Diagn. 2022, 22, 449–460. [Google Scholar] [CrossRef]
- Ohhara, Y.; Fukuda, N.; Takeuchi, S.; Honma, R.; Shimizu, Y.; Kinoshita, I.; Dosaka-Akita, H. Role of targeted therapy in metastatic colorectal cancer. World J. Gastrointest. Oncol. 2016, 8, 642–655. [Google Scholar] [CrossRef] [PubMed]
- An, S.-X.; Yu, Z.-J.; Fu, C.; Wei, M.-J.; Shen, L.-H. Biological factors driving colorectal cancer metastasis. World J. Gastrointest. Oncol. 2024, 16, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Pascual, J.; Cubillo, A. Dynamic Biomarkers of Response to Antiangiogenic Therapies in Colorectal Cancer: A Review. Curr. Pharmacogenom. Pers. Med. 2018, 15, 81–85. [Google Scholar] [CrossRef]
- Macarulla, T.; Montagut, C.; Sánchez-Martin, F.J.; Granja, M.; Verdaguer, H.; Sastre, J.; Tabernero, J. The role of PIGF blockade in the treatment of colorectal cancer: Overcoming the pitfalls. Expert Opin. Biol. Ther. 2019, 20, 15–22. [Google Scholar] [CrossRef]
- Henrich, L.M.; Greimelmaier, K.; Wessolly, M.; Klopp, N.A.; Mairinger, E.; Krause, Y.; Berger, S.; Wohlschlaeger, J.; Schildhaus, H.-U.; Baba, H.A.; et al. The Impact of Cancer-Associated Fibroblasts on the Biology and Progression of Colorectal Carcinomas. Genes 2024, 15, 209. [Google Scholar] [CrossRef]
- Saif, W. Anti-VEGF agents in metastatic colorectal cancer (mCRC): Are they all alike? Cancer Manag. Res. 2013, 5, 103–115. [Google Scholar] [CrossRef]
- Rejali, L.; Nazemalhosseini-Mojarad, E.; Valle, L.; Maghsoudloo, M.; Aghdaei, H.A.; Mohammadpoor, H.; Zali, M.R.; Khanabadi, B.; Entezari, M.; Hushmandi, K.; et al. Identification of antisense and sense RNAs of intracrine fibroblast growth factor components as novel biomarkers in colorectal cancer and in silico studies for drug and nanodrug repurposing. Environ. Res. 2023, 239, 117117. [Google Scholar] [CrossRef]
- Zawawi, S.S.A.; Azram, N.A.S.M.; Sulong, S.; Zakaria, A.D.; Lee, Y.Y.; Jalil, N.A.C.; Musa, M. Identification of AOC3 and LRRC17 as Colonic Fibroblast Activation Markers and Their Potential Roles in Colorectal Cancer Progression. Asian Pac. J. Cancer Prev. 2023, 24, 3099–3107. [Google Scholar] [CrossRef]
- Wolf, D.; Salcher, S.; Pircher, A. The multivisceral landscape of colorectal cancer metastasis: Ifmplications for targeted therapies. J. Clin. Investig. 2024, 134. [Google Scholar] [CrossRef] [PubMed]
- Motofei, I.G. Biology of cancer; from cellular and molecular mechanisms to developmental processes and adaptation. Semin. Cancer Biol. 2021, 86, 600–615. [Google Scholar] [CrossRef] [PubMed]
- Cambrea, S.C.; Aschie, M.; Resul, G.; Mitroi, A.F.; Chisoi, A.; Nicolau, A.A.; Baltatescu, G.I.; Cretu, A.M.; Lupasteanu, G.; Serbanescu, L.; et al. HPV and HIV Coinfection in Women from a Southeast Region of Romania—PICOPIV Study. Medicina 2022, 58, 760. [Google Scholar] [CrossRef] [PubMed]
- Savlovschi, C.; Serban, D.; Andreescu, C.; Dascalu, A.; Pantu, H. Economic analysis of medical management applied for left co-lostomy. Chirurgia 2013, 108, 666–669. [Google Scholar]
- Zheng, L.; Li, B.; Lei, L.; Wang, L.-J.; Zeng, Z.-P.; Yang, J.-D. Effect of screening colonoscopy frequency on colorectal cancer mortality in patients with a family history of colorectal cancer. World J. Gastrointest. Oncol. 2024, 16, 354–363. [Google Scholar] [CrossRef]
- Suceveanu, A.I.; Suceveanu, A.; Voinea, F.; Mazilu, L.; Mixici, F.; Adam, T. Introduction of cytogenetic tests in colorectal cancer screening. J. Gastrointestin. Liver Dis. 2009, 18, 33–38. [Google Scholar]
- Savlovschi, C.; Serban, D.; Trotea, T.; Borcan, R.; Dumitrescu, D. Post-surgery morbidity and mortality in colorectal cancer in elderly subjects. Chirurgia 2013, 108, 177–9. [Google Scholar]
- Mazilu, L.; Ciufu, N.; Gălan, M.; Suceveanu, A.I.; Parepa, I.R.; Tofolean, D. Postherapeutic follow-up of colorectal cancer patients treated with curative intent. Chirurgia 2012, 107, 55–58. [Google Scholar]
- Şavlovschi, C.; Comandaşu, M.; Şerban, D. Specifics of diagnosis and treatment in synchronous colorectal cancers (SCC). Chirurgia 2013, 108, 43–45. [Google Scholar]
- Ciuhu, A.N.; Rahnea-Niță, R.A.; Popescu, M.; Badiu, C.D.; Pantea Stoian, A.M.; Lupuliasa, D.; Gherghiceanu, F.; Diaconu, C.C.; Rahnea-Niță, G. Evidence of strong opioid therapy for palliation of breathlessness in cancer patients. Farmacia 2017, 65, 173–178. [Google Scholar]
- Soerjomataram, I.; Bray, F. Planning for tomorrow: global cancer incidence and the role of prevention 2020–2070. Nat. Rev. Clin. Oncol. 2021, 18, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Ginghină, O.; Negrei, C.; Hudiță, A.; Ioana-Lavric, V.; Gălățeanu, B.; Dragomir, S.; Burcea Dragomiroiu, G.T.A.; Bârcă, M.; Nițipir, C.; Diaconu, C.C.; et al. In vitro impact of some natural compounds on HT-29 colorectal adenocarcinoma cells. Farmacia 2017, 65, 947–953. [Google Scholar]
- Bray, F.; Jemal, A.; Grey, N.; Ferlay, J.; Forman, D. Global cancer transitions according to the Human Development Index (2008–2030): A population-based study. Lancet Oncol. 2012, 13, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Tulin, A.; Slavu, I.; Tulin, R.; Alecu, L.; Jecan, R.; Orlov, C.; Iaciu, C.; Stanculeanu, D.; Hainarosie, R.; Pituru, S.; et al. Does sex of the patient play a role in survival for MSI colorectal cancer? J. Mind Med Sci. 2018, 5, 101–108. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Paccard, C.; Chiron, M.; Tabernero, J. Impact of Prior Bevacizumab Treatment on VEGF-A and PlGF Levels and Outcome Following Second-Line Aflibercept Treatment: Biomarker Post Hoc Analysis of the VELOUR Trial. Clin. Cancer Res. 2020, 26, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Caraban, B.M.; Matei, E.; Cozaru, G.C.; Aşchie, M.; Deacu, M.; Enciu, M.; Bălţătescu, G.I.; Chisoi, A.; Dobrin, N.; Petcu, L.; et al. PD-L1, CD4+, and CD8+ Tumor-Infiltrating Lymphocytes (TILs) Expression Profiles in Melanoma Tumor Microenvironment Cells. J. Pers. Med. 2023, 13, 221. [Google Scholar] [CrossRef]
- Constantin, V.D.; Silaghi, A.; Epistatu, D.; Dumitriu, A.S.; Paunica, S.; Bălan, D.G.; Socea, B. Diagnosis and management of colon cancer patients presenting in advanced stages of complications. J. Mind Med. Sci. 2023, 10, 51–65. [Google Scholar] [CrossRef]
- Schölch, S.; Bogner, A.; Bork, U.; Rahbari, M.; Győrffy, B.; Schneider, M.; Reissfelder, C.; Weitz, J.; Rahbari, N.N. Serum PlGF and EGF are independent prognostic markers in non-metastatic colorectal cancer. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Wei, S.; Tsao, P.; Wang, Y.; Lin, B.; Wu, D.; Tsai, W.; Chen, J.; Wong, J. Using serum placenta growth factor could improve the sensitivity of colorectal cancer screening in fecal occult blood negative population: A multicenter with independent cohort validation study. Cancer Med. 2019, 8, 3583–3591. [Google Scholar] [CrossRef]
- Suceveanu, A.I.; Suceveanu, A.; Dumitru, E.; Alexandrescu, L.; Voinea, F. The feasibility of FOBT tests in colorectal cancer screening in Dobrogea. Rom. J. Gastroenterol. 2005, 14, 213–7. [Google Scholar]
- Tobi, M.; Antaki, F.; Rambus, M.A.; Yang, Y.-X.; Kaplan, D.; Rodriguez, R.; Maliakkal, B.; Majumdar, A.; Demian, E.; Tobi, Y.Y.; et al. The Non-Invasive Prediction of Colorectal Neoplasia (NIPCON) Study 1995–2022: A Comparison of Guaiac-Based Fecal Occult Blood Test (FOBT) and an Anti-Adenoma Antibody, Adnab-9. Int. J. Mol. Sci. 2023, 24, 17257. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Oshima, T.; Yoshihara, K.; Yamamoto, N.; Yamada, R.; Nagano, Y.; Fujii, S.; Kunisaki, C.; Shiozawa, M. Overexpression of the fibroblast growth factor receptor-1 gene correlates with liver metastasis in colorectal cancer. Oncol. Rep. 1994, 21, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Cho, H.J.; Kim, K.-M.; Lim, H.Y.; Kang, W.K.; Lee, J.; Park, Y.S.; Kim, H.C.; Kim, S.T. Comprehensive molecular analysis to predict the efficacy of chemotherapy containing bevacizumab in patients with metastatic colorectal cancer. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2023, 31, 855–866. [Google Scholar] [CrossRef]
- Serban, D.; Smarandache, A.; Cristian, D.; Tudor, C.; Duta, L.; Dascalu, A. Medical errors and patient safety culture—Shifting the healthcare paradigm in Romanian hospitals. Romanian J. Leg. Med. 2020, 28, 195–201. [Google Scholar] [CrossRef]
- Cheng, X.; Li, X.; Yang, X.; Fang, S.; Wang, Z.; Liu, T.; Zheng, M.; Zhai, M.; Yang, Z.; Shen, T.; et al. Successful Treatment of pMMR MSS IVB Colorectal Cancer Using Anti-VEGF and Anti-PD-1 Therapy in Combination of Gut Microbiota Transplantation: A Case Report. Cureus 2023, 15, e42347. [Google Scholar] [CrossRef]
- Hashimoto, T.; Otsu, S.; Hironaka, S.; Takashima, A.; Mizusawa, J.; Kataoka, T.; Fukuda, H.; Tsukamoto, S.; Hamaguchi, T.; Kanemitsu, Y. Phase II biomarker identification study of anti-VEGF agents with FOLFIRI for pretreated metastatic colorectal cancer. Futur. Oncol. 2023, 19, 1593–1600. [Google Scholar] [CrossRef]


 |
 |
 |
© 2024 by the author. 2024 Adrian Paul Suceveanu, Dragos Serban, Andreea Daniela Caloian, Georgeta Camelia Cozaru, Anca Chisoi, Anca Antonela Nicolau, Ioan Sergiu Micu, Andra Iulia Suceveanu
Share and Cite
Suceveanu, A.P.; Serban, D.; Caloian, A.D.; Cozaru, G.C.; Chisoi, A.; Nicolau, A.A.; Micu, I.S.; Suceveanu, A.I. Correlation between Molecular Prognostic Factors and Bevacizumab Therapeutic Resistance in Patients with Metastatic Colorectal Cancer; The AVAMET Study. J. Mind Med. Sci. 2024, 11, 175-182. https://doi.org/10.22543/2392-7674.1477
Suceveanu AP, Serban D, Caloian AD, Cozaru GC, Chisoi A, Nicolau AA, Micu IS, Suceveanu AI. Correlation between Molecular Prognostic Factors and Bevacizumab Therapeutic Resistance in Patients with Metastatic Colorectal Cancer; The AVAMET Study. Journal of Mind and Medical Sciences. 2024; 11(1):175-182. https://doi.org/10.22543/2392-7674.1477
Chicago/Turabian StyleSuceveanu, Adrian Paul, Dragos Serban, Andreea Daniela Caloian, Georgeta Camelia Cozaru, Anca Chisoi, Anca Antonela Nicolau, Ioan Sergiu Micu, and Andra Iulia Suceveanu. 2024. "Correlation between Molecular Prognostic Factors and Bevacizumab Therapeutic Resistance in Patients with Metastatic Colorectal Cancer; The AVAMET Study" Journal of Mind and Medical Sciences 11, no. 1: 175-182. https://doi.org/10.22543/2392-7674.1477
APA StyleSuceveanu, A. P., Serban, D., Caloian, A. D., Cozaru, G. C., Chisoi, A., Nicolau, A. A., Micu, I. S., & Suceveanu, A. I. (2024). Correlation between Molecular Prognostic Factors and Bevacizumab Therapeutic Resistance in Patients with Metastatic Colorectal Cancer; The AVAMET Study. Journal of Mind and Medical Sciences, 11(1), 175-182. https://doi.org/10.22543/2392-7674.1477



