Abstract
Background and Objectives. Hernia recurrence is still a great challenge for surgeons regarding the optimal surgical technique, the best alloplastic material and the management of risk factors (advanced age, female sex, body mass index, smoking, diabetes, the presence of connective tissue disorders, chronic cough, etc.). The present study attempts to assess the impact of these factors in hernia recurrence, as well as the integration of the prosthetic material at the tissue level, in order to reduce possible postoperative complications. Material and Methods. A retrospective study was performed on 108 patients operated (between January 2012 and December 2022) for recurrence of inguinal, umbilical and incisional hernias. Demographic data and comorbidities were analyzed in relation to hernia recurrence. Fragments of unintegrated and well-fitted mesh were sampled and examined microscopically to assess tissue-level implications. Results. The strongest factors associated with hernia recurrence were obesity (p = 0.001), diabetes mellitus (p = 0.003), high blood pressure (p = 0.003) and atrial fibrillation (p = 0.044). Microscopic analysis of unintegrated mesh fragments revealed the presence of foreign body granulomas and predominance of thin fibrillar type 3 collagen, whereas well-integrated material showed thick type I collagen fibers and low inflammatory infiltrate. Conclusions. Insufficient oxygen supply, an altered inflammatory response, and diminished proliferative capacity during the wound healing stages resulted in abnormalities in the development of mature granulation tissue. Therefore, to reduce the risk of hernia recurrence, it is essential to have a surgical treatment that must manage all these possible factors.
Introduction
Primary hernia repair and incisional hernia is the most common abdominal surgery performed by general surgeons, as this pathology is the consequence of multiple medical, surgical or traumatic injuries to the abdominal wall [1]. Hernia recurrence is a distress for the patient and still represents a great challenge for surgeons in terms of optimal surgical technique and the ideal alloplastic material used to prevent further complications. Based on anatomical distribution, abdominal hernias are catalogued by the World Society of Emergency Surgery (WSES) into ventral hernias and groin hernias, each with corresponding subcategories [2]. The prevalence of ventral abdominal hernia is increasing, accounting for about 1 in 5 adults in Australia [3], an estimated 34,000 patients in France [4] and over 500,000 cases per year in the USA [5]. Groin hernias occur even more frequently, with more than 20 million cases worldwide of surgically treated inguinal hernias reported annually by the HerniaSurge Group [6]. The estimated costs for both procedures of hernia repair in the USA are about 10 billion $ per year, an amount that increases with hernia recurrence and therefore the need for reintervention [7]. The estimated recurrence rate after incisional hernia repair is ranged between 15 to 20% [8], whereas relapse of groin hernia repair is generally lower, ranging from 1% to 15% [9].
Incisional hernia is a frequent complication following laparotomies, most likely due to relaxation of suture tension over time and altered collagen metabolism [1]. Multiple risk factors have been incriminated in the occurrence of hernia recurrences, which can be categorized into preoperative (patient-related), intraoperative (surgical technique) and postoperative (complications) indicators [10]. Preoperative predictors involve clinical and demographic characteristics of the patient such as older age, female gender, body mass index (BMI) > 30 kg/m2, smoking, diabetes mellitus, presence of connective tissue disorders, chronic cough, steroid use and previous chemotherapeutic treatment [10,11]. Intraoperative variables refer to the surgical procedure itself. The choice of surgical approach (open or laparoscopic), the specific technique used for mesh placement, selected type of mesh and the surgeon’s experience and skill can influence the recurrence rate. Postoperative complications may arise during the recovery period after surgery. Surgical site infections, the formation of seromas or hematomas, poor wound healing, chronic pain and excessive physical strain on the abdominal muscles can all contribute to an increased risk of hernia relapse [10,11]. It is important to remember that the presence of these predictors does not guarantee the recurrence of the hernia, but only increases its probability. Therefore, to minimize the risk of relapse, it is crucial to have proper patient selection and management, a skilled surgical team, appropriate operating technique and careful postoperative care.
Since patient-related factors are hardly influenced, attention has been focused on the surgical technique, the type of mesh used and its method of fixation in order to reduce the risk of recurrence. The main objective of using a mesh in hernia repair is to create a supporting structure that facilitates the colonization of native tissue through fibrotic reactions [12]. Currently, there are three primary types of meshes available for hernia repair on the market (synthetic, biological and composite), each with benefits and drawbacks in providing reinforcement and promoting tissue ingrowth [1]. Open or laparoscopic approach in accordance with mesh placement are also important considering the pressure exerted in different parts of the abdominal wall or stress developed on certain organs. Sanders et al. defined 5 essential criteria for an ideal implant: biocompatibility, reduced risk of infection, easy and low-cost manufacture, handling convenience and longevity [13]. Although the mesh materials are inert from a physical and chemical standpoint, it is important to consider the potential foreign body reaction and its consecutive inflammatory response [14]. In the early stages of wound healing, during the inflammatory and proliferative phases, collagen type III is produced and deposited providing temporary structural support. During the remodeling phase, immature collagen is gradually replaced with type I collagen leading to an organized, strong and stable new matrix [15]. In individuals with parietal defects the healing process is disrupted, resulting in an imbalance in the ratio of collagen type 1 to collagen type 3 within the abdominal wall, leading to mesh instability [16]. Poor postoperative management can result in wound complications which not only extend the recovery period, but can compromise the alloplastic material, which may require reintervention.
Materials and Methods
The present study attempts to evaluate the implications of multiple risk factors (patients’ comorbidities) on hernia recurrence and to analyze how the prosthetic material is integrated at the tissue level.
After obtaining Institutional Board Ethics Approval, a retrospective study was performed including 108 patients 18 years and older operated for recurrence of inguinal, umbilical and incisional hernias between January 2012 and December 2022 at Dr. Carol Davila Hospital, Department of Surgery and Clinical Nephrology Hospital, Bucharest, as presented in Figure 1. Our hospital is not an exclusive herniorrhaphy center and performs an average of 120 interventions for parietal defects per year. All cases were surgically treated according to the `tension-free` principle with placement of alloplastic synthetic material. Relevant exclusion criteria were clinical follow-up of less than 1 year, emergency surgery with strangulated herniated organs, parastomal hernias and patients with positive signs of malignancy after 5-year oncologic surgical and adjuvant treatment.
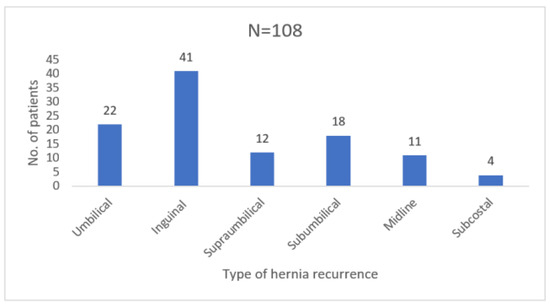
Figure 1.
Type of hernia recurrences operated in our clinic between 2012 and 2022.
The demographic data (gender, age) and comorbidities (BMI, smoking, high blood pressure, diabetes mellitus, atrial fibrillation, dyslipidemia, benign prostate hyperplasia and previous oncologic therapy) were analyzed in relation to hernia recurrence. Other relevant information registered were the location of the abdominal wall defect, type of initial surgery and postoperative complications. Patients had undergone an average of 3 previous abdominal surgical procedures (range 1 to 5) and 16.66% had at least one intervention for neoplasia associated with adjuvant oncologic treatment.
The selection of surgical technique and type of alloplastic material used was chosen according to the patient’s associated diseases and primary hernia repair intervention. Surgeries were not performed by the same operating team; therefore, certain types of surgical procedures were selected according to the physician’s experience and skills.
Intraoperative biopsies were taken from 28 patients for microscopic exploration consisting of postoperative scar tissue and fragments of non-integrated mesh, as well as properly fitted alloplastic material, which were paraffin-embedded and subsequently stained with hematoxylin-eosin. The primary focus result was surgical reoperation for postoperative incisional hernia and hernia recurrence (umbilical and inguinal) defined by the International Classification of Diseases, 11th–Revision (ICD-11). Data were collected and processed using Microsoft Excel version 15 and statistical analysis was performed using Minitab Software version 21. The normal distribution of variables was assessed using the Anderson-Darling test (similar to Shapiro-Wilk test). Categorical variables were compared using Chi-Square test and statistical significance was set at a value of p < 0.05.
Results
Patient age ranged from 26 to 88 years with a mean of 63.38 years, while the male to female ratio was 1.2:1. Rate of obese patients in the study group was 52.78% (40 patients with BMI > 30 kg/m2 and 17 patients with BMI > 40 kg/m2). From all the patients treated, only 26.85% (N = 29) had their first parietal defect repair surgery at our clinic. Relevant data concerning previous surgery and complications for the others were collected from their discharge papers. 78 patients were operated under general anesthesia with orotracheal intubation, whereas 30 procedures in the subumbilical region were performed under spinal anesthesia. Most patients admitted for recurrence were those with inguinal hernias (37.96%), umbilical hernias (20.37%) and subumbilical incisional hernias (16.66%) as presented in Figure 1.
Regarding gender distribution in relation to the type of hernia, we observed an increased prevalence of inguinal hernia relapse in men (M:F ratio was 12.6:1), compared to recurrence of umbilical and incisional hernias which were more frequent in women (M:F ratio was 1:3.4, respectively 1:6.5) (Figure 2).
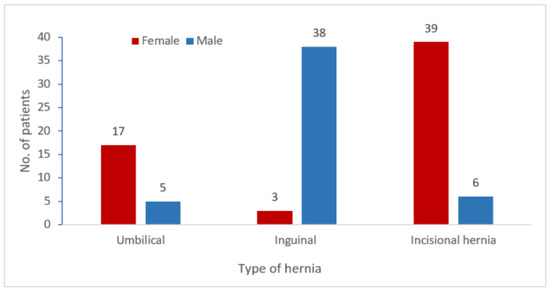
Figure 2.
Gender distribution of hernia and incisional hernia recurrence (N = 108).
97.22% of the patients included in the study were initially operated for anterior abdominal wall incisional hernia relapse by open repair technique and only 3 patients underwent laparoscopic surgery for inguinal hernia (Figure 3). Among the first mentioned, 57.14% underwent surgery for hernia repair with supraaponeurotic mesh placement, 6.66% were operated by open technique with secondary issue suture (umbilical hernia) and 36.20% had Lichtenstein procedure with retrofunicular mesh fitting (inguinal hernia).

Figure 3.
Type of surgery performed—first surgery versus second surgery.
When admitted to our clinic for secondary repair, laparoscopic approach was chosen for 24.07% of patients, with intraperitoneal dual-mesh placement for incisional hernias (13 cases) and preperitoneal polypropylene mesh fitting for inguinal hernias (TAPP technique—13 cases), both with AbsorbaTack fixation (Figure 3). For the rest of the patients with ventral hernia undergoing surgery via the open technique the decision was made to position the supraaponeurotic mesh and secure it with separate wires. There were 2 cases for whom laparoscopic technique was attempted, but due to visceral adhesions they were converted to laparotomies and Rives procedure was subsequently performed.
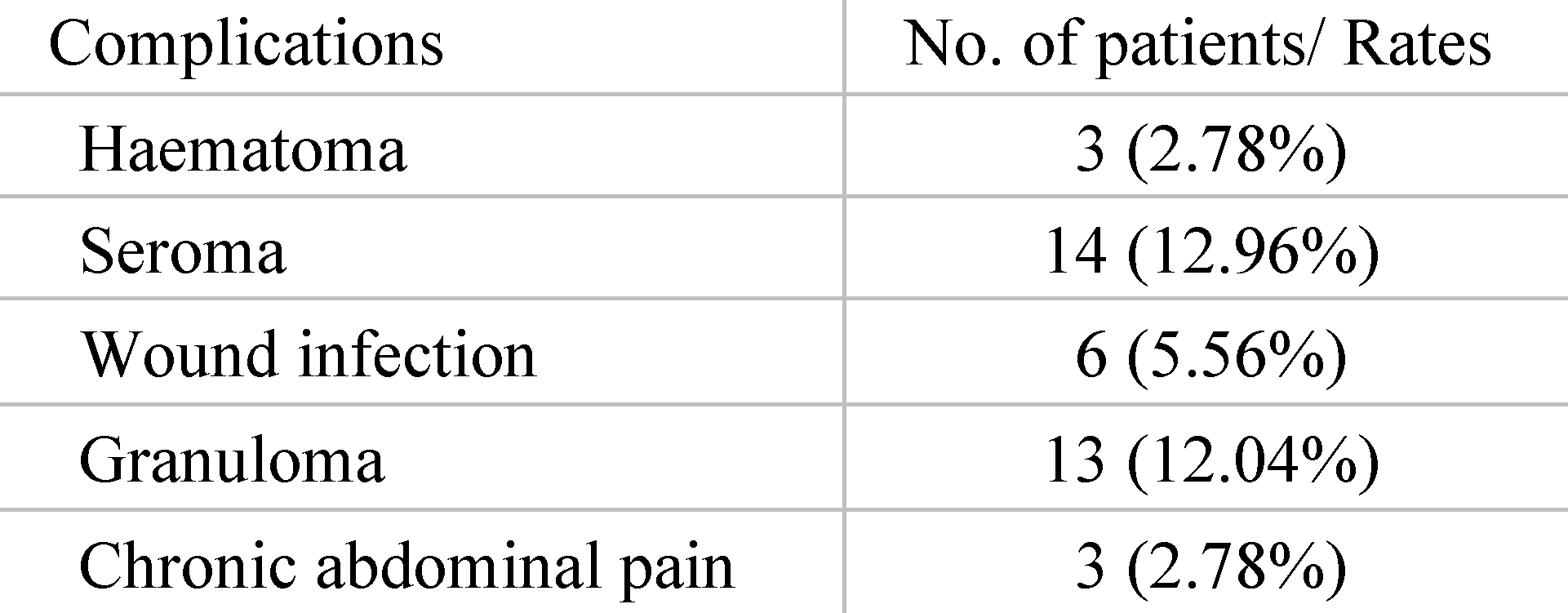
Prior surgical complications recorded were seroma, hematoma, granuloma, superficial wound infection, enterocutaneous fistula and chronic abdominal pain. In this regard, 33.34% of the patients treated developed postoperative wound complications, the most common being seroma (12.96%) and granuloma (12.04%), as presented in Table 1. Postoperative bleeding occurred in 3 cases with development of wound hematoma and required reintervention for in situ hemostasis. Also, 6 patients presented wound infection and required drain tube placement and prolonged local antibiotic lavage until symptoms resolved.

Table 1.
Complications after first surgery.
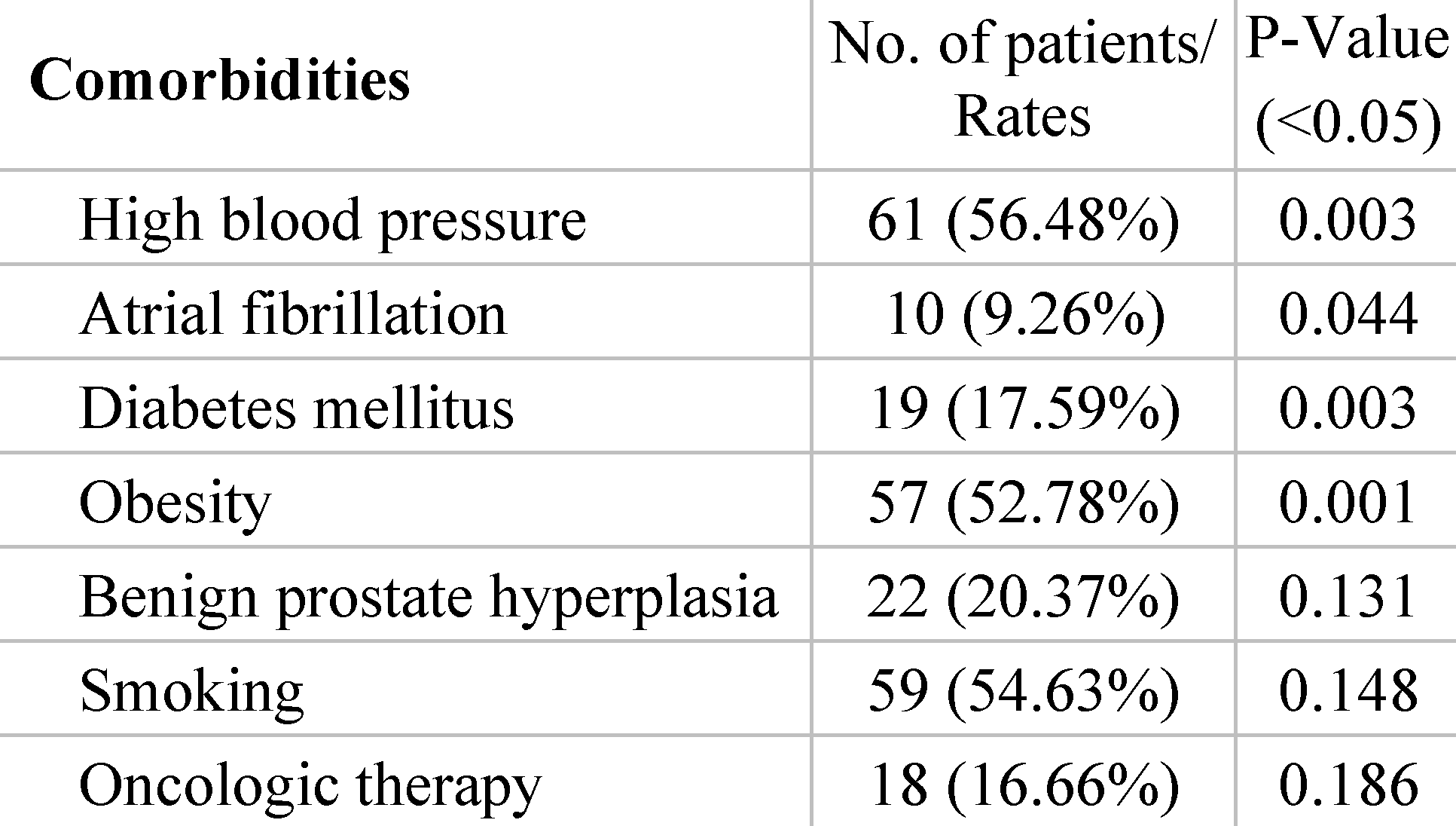
In correlation with the occurrence of local complications are patients’ associated disorders, metabolic imbalances and related chronic treatment, which influence the post-operative evolution. Several comorbidities were analyzed in order to identify the risk factors with the greatest impact on the development of complications. The most prevalent comorbidities included hypertension (56.48%), obesity (52.78%) and history of smoking or active smoker (54.63%). The strongest patient factors associated with hernia recurrence proved to be obesity (p = 0.001), diabetes mellitus (p = 0.003) and high blood pressure (p = 0.003). Atrial fibrillation also has a significant correlation (p = 0.044), most likely through the need for anticoagulant treatment and alteration of the clotting process (Table 2).

Table 2.
Risk factors analysed in association with recurrence of hernia.
Despite the fact that more than half of the patients were smokers, chronic tobacco use was not identified as a significant influencing factor (p = 0.148). Patients with benign prostate hyperplasia represented 48% of male participants, but in relation to the overall sample size, this risk factor was not deemed significant for hernia recurrence (p = 0.131). Eighteen patients had prior surgery for neoplastic conditions and subsequently received adjuvant oncological treatment which they completed at least 2 years before surgery for hernia relapse. Our analysis additionally indicated that radio-chemotherapy treatment does not emerge as a significant factor in recurrence of hernia (p = 0.186). All patients tolerated the surgery well and no pulmonary complications, cardiovascular events or deaths were recorded. Subsequently, there were no cases of incisional hernia recurrence diagnosed at 12 months follow-up.
In 44 cases, during the open surgical reintervention we were able to explore intraoperatively the status of the alloplastic material used at primary surgery for hernia repair. 9 patients with subumbilical parietal defects, 15 patients with umbilical hernia recurrence, 4 patients with midline abdominal defects, 3 cases of supraumbilical hernia and respectively 13 patients diagnosed with inguinal hernia relapse showed either mesh integration deficiency or alloplastic migration most likely due to poor fitting or non-compliance with postoperative recommendations. Fragments of both unintegrated mesh and well-attached mesh were sampled from 28 patients in order to microscopically examine for comparative analysis of tissue processes (Figure 4, Figure 5 and Figure 6).
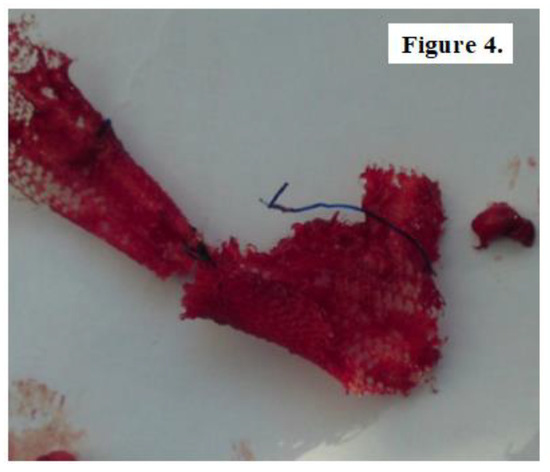
Figure 4.
Fragment of unintegrated mesh sampled from a patient with subumbilical hernia repair.
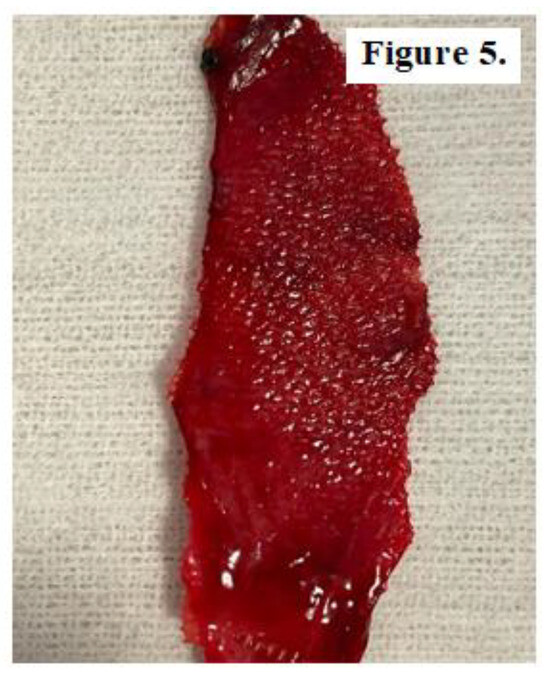
Figure 5.
Fragment of well-integrated but migrated mesh obtained from a patient with umbilical hernia recurrence.
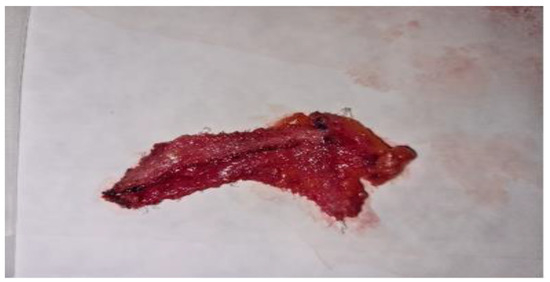
Figure 6.
Inappropriate integrated mesh fragment in a patient with inguinal hernia relapse.
The removed samples were paraffin-embedded, subsequently stained with hematoxylin-eosin and were microscopically analyzed. Fragments of unintegrated mesh revealed adipose connective tissue areas with remnants of synthetic material, with development of small sites of foreign body granuloma in the process of organization through hyaline fibrosis process. The material also presents thin fibrillar structures suggestive for immature type III collagen (Figure 7).
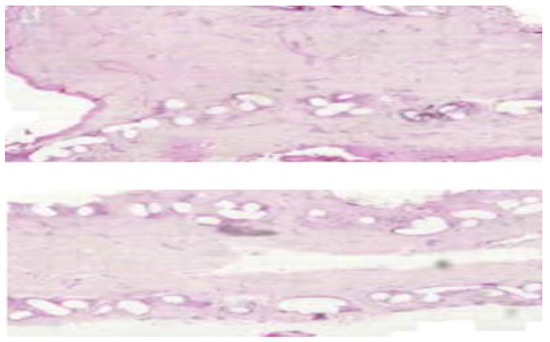
Figure 7.
Synthetic material surrounded by adipose and hyaline tissue, with type III collagen structures. (H and E, ×10).
In cases of poorly integrated meshes images of pronounced inflammatory infiltrate were highlighted, with an increased amount of collagen and fiber width. Properly incorporated areas showed superior connective tissue with a higher amount of type I collagen (Figure 8).
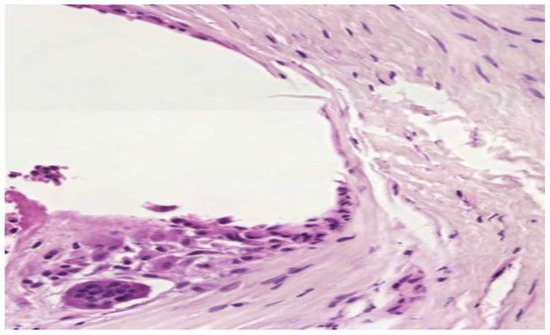
Figure 8.
Important inflammatory infiltrate in the vicinity of the mesh with thicker connective tissue increased amount of type I collagen. (H and E, ×40).
Fragments of well fitted but migrated alloplastic material presented fewer inflammatory cells, collagen fibers with increased diameter and a higher type I to type III collagen ratio (Figure 9).
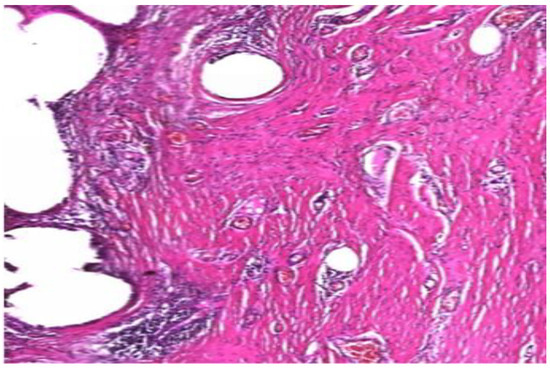
Figure 9.
Low inflammatory infiltrate. Mature type I collagen with increased diameter. (H and E, ×10).
Discussions
The concept of using mesh in hernia repairs dates back to the 1950s, when Dr. Francis Usher developed a synthetic woven prosthesis after concluding that tissue growth through its interstices gives stability and reinforcement to the abdominal wall [17]. Three decades later the concept of `tension-free` prosthetic hernia repair became the standard of care for the majority of patients [18]. Currently, the focus lies in identifying a hernia mesh that possesses sufficient strength and durability to prevent recurrence while minimizing potential side effects such as discomfort, pain or impaired wound healing [19]. To meet these criteria, previous studies have demonstrated that the alloplastic material needs to have certain qualities such as light weight, large pore size and biocompatibility [1]. In order to avoid local postoperative complications, some authors promoted the implementation of biologic meshes due to their potential to remodel into native fascia [20,21,22] The use of these collagen-based prostheses was particularly recommended for cases with complicated incisional hernias such as synthetic mesh contamination, enterocutaneous fistulae or poorly healing wounds [20]. The most commonly used biologic mesh was a processed nonimmunogenic skin harvested from human cadavers known as ADM (acellular dermal matrix). Unfortunately, apart from its high costs, the use of ADM proved effective only in early postoperative stages, with a hernia recurrence rate of 30% at 9 months [21,22] to 80% over 1-year follow-up [20].
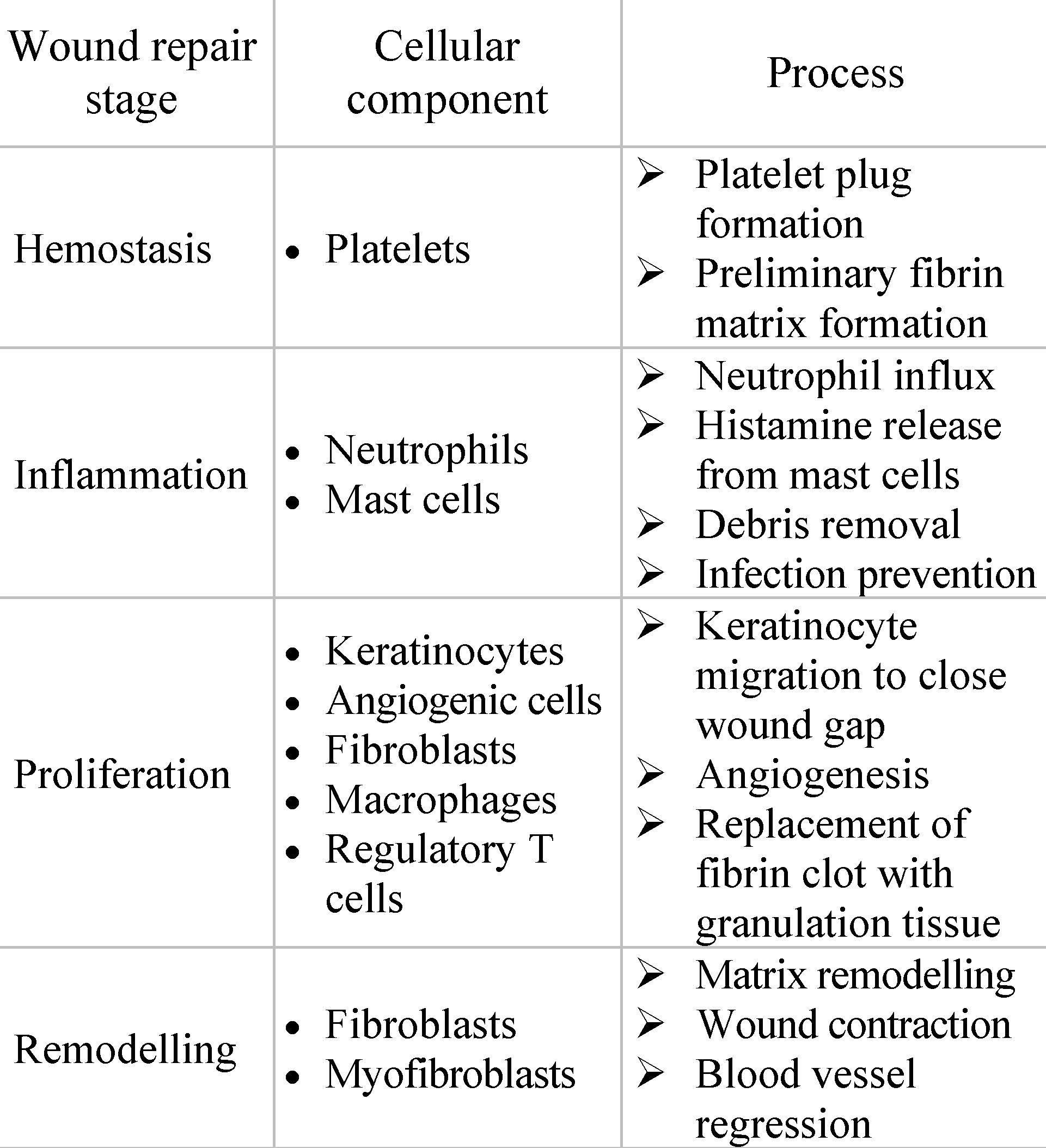
It is important to acknowledge that meshes cannot fully replicate the properties of the local tissue and as a result, their presence may trigger a foreign body reaction. Thus, it disrupts the natural regenerative capacity of the and tissue, leading to a healing response characterized by the formation of scar tissue that fills the hernia defect [23]. Wound repair implies four sequential phases: hemostasis, inflammation, proliferation, and dermal remodeling, each playing a crucial role in the process of restoring both the structural and functional integrity of the damaged tissue [24]. The stages of wound repair and their major cellular components are summarized in Table 3.

Table 3.
Stages of wound repair, revised from reference [24].
Granulation tissue synthetized by fibroblasts during the proliferative phase comprises of 30% immature type III collagen and approximately 10% type I collagen [25]. During the healing process, collagen type III is gradually substituted by collagen type I, leading to a direct enhancement in the tensile strength of the developing scar [26]. Even so, the structural integrity and organization of the scar’s extracellular matrix never fully recovers to match that of the uninjured skin (80% type I collagen and only 10% type III collagen) [25].
The majority of patients present multiple risk factors involved in hernia recurrence, and their cumulative effect leads to alterations in different phases of the healing process and the implicit integration of alloplastic material. According to study of Donovan et al. on 979 patients with relapsed umbilical hernia, individuals with a single risk factor exhibit a recurrence rate three times higher than patients with no risk factors and presence of more than 2 risk factors lead to an increased chance of relapse up to 7 times [27].
Demographic aspects are invariable, but previous meta- analysis studies showed that female sex is a risk factor for recurrence after inguinal [11], umbilical [27] or ventral hernia surgery [10]. Our results are consistent with data of previous studies, with the exception of inguinal hernia where 92% of patients treated were male. Older age is an independent risk factor for hernia relapse due to several factors that affect the body’s ability to heal and recover after parietal defect repair such as reduced inflammatory and proliferative response with altered quality collagen synthesized during the final phase of remodeling [28]. These aspects lead to diminished elasticity of tissues and increased susceptibility to wound infection, most likely due to associated underlying health conditions. In a study including patients with groin hernia repair treated at >600 hospitals in the United States, Murphy reported an increased odds ratio for recurrence progressing with age: 2.37 for age 45–54 years, 2.57 for age 55–64 years, 2.86 for individuals ranging 65 to 74 years and 3.33 for age over 75 years [29]. In our study patient age ranged from 26 to 88 years with a mean of 63.38 years, and more than 80% were in the 55–75 age group.
The incriminated preoperative factors are multiple and sometimes not therapeutically controllable or adjustable. The available literature outlines physiological indicators supporting the relationship of inadequate wound healing and the excess of adipose tissue [30]. The reduced vascularization of fat tissue diminishes its capacity to fend off infections as lower oxygen levels hinder neutrophils from effectively performing bacteria phagocytosis. In addition, surgery performed on individuals with obesity tend to have a prolonged duration, leading to extended period of wound exposure to potential contamination [31]. Another mechanism involved in the occurrence of hernia recurrence for obese patients is attributed to increased tension in the aponeurotic margins during and after wound closure [30]. Additional stress in the suture line is generated by fluid accumulation in the subcutaneous tissue or between the fascia and the mesh [32]. This occurs due to various factors associated with adipose tissue’s properties and functions within the body. Adipocytes influence the permeability of blood vessels and lymphatic system by exerting both mechanical pressure and hormonal influence, which affect fluid balance and dynamics. The presence of seroma requires evacuation of the fluid as it can lead to bacterial contamination and resulting in wound infection and mesh impairment. Our study’s results are consistent with previous reports, yielding significant higher odds of recurrence for obese patients (0.001), but it should be taken into account that >50% of individuals included have BMI > 30 kg/m2.
Diabetes mellitus is another important risk factor for hernia relapse due to the disease’s vascular, neuropathic, immune system and biochemical abnormalities which collectively contribute to altered tissue repair [33]. Diabetes exerts negative effects on the majority of cellular processes in wound healing, especially during the inflammatory phase, leading to a delay in the development of fully matured granulation tissue [34]. Diabetic wounds show decreased number and function of neutrophils and macrophages, structural alterations of fibroblasts with reduced proliferative capacity and keratinocytes with abnormal receptor localization for growth factors [33]. In addition, fibroblasts lose their ability to provide angiogenic functions thus altering the supply of oxygen and nutrients to the wound [35]. Neuropeptide dysregulation is also incriminated in poor wound healing due to their role during the inflammatory and proliferative phases through mechanisms involved in cytokine secretion, growth factor signal transmission and immune cell movement [36] (Figure 10). Although only 17,59% of patients included in our study were diabetic, this disorder proved to be a strong related factor to hernia recurrence (p < 0.003).
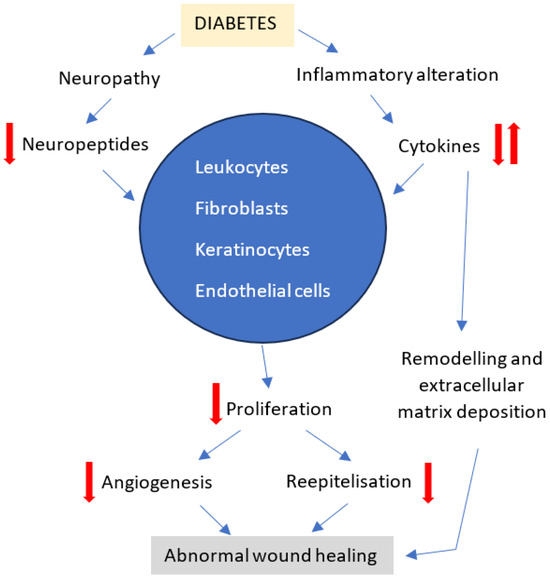
Figure 10.
Effect of diabetic neuropathy and inflammatory dysregulation on wound healing, revised from reference [36].
Cigarette smoking exerts detrimental effects on the process of wound healing through multiple mechanisms. Evidence-based guidelines for treating behavioral risks to poor healing, such as tobacco dependence, should be included in treatment plans as needed [37]. Despite doctors’ recommendations for patients to abstain from smoking prior to scheduled surgery and the potential benefits associated with such abstinence, there are reports suggesting that short-term smoking cessation may not effectively reduce the risks and complications during the post-surgery wound healing process [38]. Furthermore, studies show that thirdhand smoke, which results from the persistent accumulation of tobacco toxins on various surfaces such as curtains, carpets, bedding and walls within buildings, as well as in vehicles and even on smokers’ hair, clothing, and skin, becomes increasingly toxic and potentially more detrimental than freshly generated secondhand smoke over time [39]. It has been estimated that cigarette smoke contains over 4000 toxic substances, the primary culprits associated with hindering the healing process being nicotine, carbon monoxide, and hydrogen cyanide gases. While the exact mechanisms behind this delay in healing are not fully understood, all three have been demonstrated to disrupt the supply of oxygen to tissues [37,40] (Figure 11). Since various stages of the wound healing process such as cell replication, angiogenesis, collagen deposition and epithelization are oxygen dependent, the adverse impacts of smoking are validated.
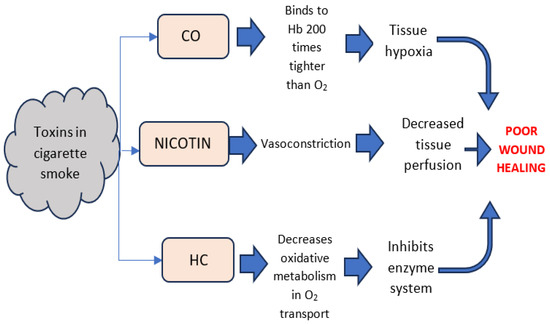
Figure 11.
Physiological effects of toxins in cigarette smoke, revised from reference [40].
At the cellular level, cigarette smoke has been correlated to a diminished rate of proliferation of red blood cells, white blood cells, and fibroblasts [41]. A decrease in the erythrocyte count will subsequently result in a reduced oxygen supply, further intensifying cellular hypoxia. There is a theory suggesting that smoking also heightens platelet adhesion and reduces fibrinolytic activity resulting in increased blood viscosity and the formation of microclots [42]. Consequently, this reduces microperfusion, increases microvascular blockage due to thrombosis, and ultimately worsens tissue ischemia [43]. In a study performed on 8 individuals, Jensen et al. reported that after smoking, subcutaneous wound tissue oxygenation decreases rapidly, persisting at a low level for up to 50 min before gradually returning to a level near the baseline within one hour [44]. In addition, nicotine stimulates the sympathetic nervous system, which triggers the release of adrenal and peripheral catecholamines. These compounds induce peripheral vasoconstriction, an elevation in heart rate, blood pressure, and oxygen demand, thus intensifying tissue hypoxia caused by smoking [45]. Although more than half of the patients surgically treated in our clinic were smokers, the results of the study showed no significant correlation of tobacco usage with hernia recurrence (p = 0.148).
The routine application of adjuvant oncologic therapy after surgery has become a standard practice in treating various neoplasms. This practice prompts inquiries regarding its impact on the actively dividing cells within both recently formed and developing surgical wounds [46]. Radiation exerts both immediate and delayed effects on the organism’s healing capacity due to the damage resulting from the ionization of atoms [47]. Achieving accurate radiation delivery to a tumor site requires allocating a percentage of the dosage to the adjacent normal skin and subcutaneous tissue. Unfortunately, the injury to these structures is irreversible and contingent on the dose administered [48].
The early effect of radiation is apoptotic cell death resulting from either direct damage to cellular DNA or indirect damage caused by the generation of free radicals [47]. The rapid activation of the coagulation cascade ensues from the free-radical inactivation of anticoagulatory factors following radiation injury. Endothelial cell apoptosis and slow regenerative proliferation contribute to heightened vascular permeability, therefore vessels lacking endothelium become susceptible to thrombosis, intimal proliferation and eventual obliteration [49]. Physical trauma triggers the initiation of an inflammatory response through stress-sensitive kinases and transcription factors. Synthesis of pro-inflammatory cytokines, including interleukin-1 (IL-1), IL-8, interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α) occurs as a result [50]. Dissolution of the inflammatory response is attributed to the brief half-life of these cytokines and the presence of anti-inflammatory cytokines like IL-4, IL-10, IL-13 and transforming growth factor-beta (TGF-β) [49]. Following radiation injury, inflammation fails to resolve adequately due to the excessive production of pro-inflammatory cytokines. This leads to disrupted cell-cell and cell-matrix interactions, uncontrolled matrix accumulation and the development of fibrosis [51]. Wounds generate Nitric Oxide (NO) through the activity of macrophages and fibroblasts, facilitating wound healing by stimulating collagen deposition [52]. NO levels are diminished in wounds that have undergone irradiation similarly to wounds in diabetic patients [53]. Radiation-induced fibroblast dysfunction results in poor wound healing due to insufficient collagen synthesis or the collagen produced does not mature quickly enough to meet the demands of regeneration [47].
Various classes of chemotherapy also have a detrimental effect on surgical wound healing due to their alteration at the DNA level [47]. Compared to radiation therapy, the effects of cytoreductive agents seem to be transient and dependent on dosage and postoperative interval of administration [54]. The most significant adverse impact is noted when the agent is administered within the two weeks preceding surgery or within one week after the intervention. Incisions made a few weeks after chemotherapy appear to heal with nearly normal tissue tensile strength [55]. Neutropenia induced by systemic oncologic treatments significantly hampers the patient’s healing capacity, given that neutrophils and monocytes are crucial cellular elements in the wound healing cascade. Clinically, it has been acknowledged that an absolute neutrophil count below 500 has a clearly adverse impact on both wound healing and the subsequent strength and stability of the wound [56]. Moreover, it shouldn’t be ignored the fact that these substances present significant morbidity and toxicity of their own unrelated to the wound- healing process. Our research showed no significant correlation between adjuvant oncologic treatment and hernia relapse (p = 0.186), most likely due to the fact that we included only patients who have completed treatment at least 2 years before admittance.
Cardiovascular disease and especially its poor therapeutic management also negatively influence the outcome of surgery. A compromised microcirculation poses a threat to the healing process since ensuring proper perfusion and oxygenation of the wound site is crucial for optimal healing [57]. Furthermore, systemic vascular disorders elevate the likelihood of hemorrhage and cause local complications in the postoperative wound. A retrospective, case-control study on 5944 patients operated for inguinal hernia revealed after a univariate analysis that several variables were significantly more prevalent in individuals who experienced postoperative hematoma. These factors included cardiac valvular disease, atrial fibrillation, hematologic abnormalities, vascular disease and previous bleeding episodes [58]. Previous studies concluded that cardiac valvular disease and atrial fibrillation were considered more likely to reflect the perioperative use of warfarin rather than being independent risk factors [58,59]. Research on wound healing has suggested that both unfractionated heparin and low molecular weight heparin exhibit anti-proliferative effects on fibroblasts, endothelial cells and angiogenesis [60]. Moreover, extended sero-hemorrhagic drainage can alter the composition of wound fluid, thereby affecting thrombin formation. The reduction in thrombin and fibrin clot formation has a detrimental impact on the initial stages of the healing process [61]. In accordance with prior studies, our results proved that high blood pressure (p = 0.003) and atrial fibrillation (p = 0.044) are important risk factors for reoccurring hernia.
Benign prostate hyperplasia is a common disease in the aging male population, with an increasing prevalence after the 4th decade [62]. Research findings indicate that irrespective of prostate size, men experiencing preoperative lower urinary tract symptoms (LUTS) with an International Prostate Symptom Score of 15 or higher should be advised about the potential requirement for inguinal hernia repair. The incriminated mechanism involves the necessity for individuals with LUTS to strain during voiding [63]. Repetitive urinary straining has the tendency to push the viscera into weak parietal areas, directly impacting the abdominal wall and thus contributing to hernia relapse. 48% of men included in the study have been diagnosed with the benign prostate hyperplasia, yet this condition did not prove to significantly impact hernia recurrence (p = 0.131).
Conclusions
Most patients exhibit multiple risk factors associated with hernia recurrence, and the combined impact of these elements results in modifications throughout various stages of the healing process and the inherent integration of alloplastic material. There are numerous preoperative aspects implicated and, in some cases, they are not amenable to therapeutic control or adjustment.
Demographic characteristics are invariable; however, the research indicates a higher occurrence of hernia recurrence among females and individuals aged 55-75, aligning with findings from prior studies. Comorbidities impact the molecular-level healing process, causing alterations in the structural integrity and organization of the scar’s extracellular matrix. The strongest patient-related factors associated with hernia recurrence proved to be obesity (p = 0.001), diabetes mellitus (p = 0.003), high blood pressure (p = 0.003), as well as atrial fibrillation (p = 0.044). Insufficient oxygen supply, an altered inflammatory response and diminished proliferative capacity during the progressive stages of wound healing led to abnormalities in the development of fully matured granulation tissue. This is reflected in disturbances in the remodeling phase, reversing the type III to type I collagen ratio, with a predominance of thin, immature and unstable collagen fibers in the tissue.
It is important to emphasize that the existence of such predictors does not guarantee a recurrence of the hernia, but it can increase the probability of its recurrence. Consequently, to mitigate the risk, it is essential to engage in proper patient selection and management, enlist a proficient surgical team, employ suitable operating techniques, and ensure attentive postoperative care.
Informed Consent Statement
Any aspect of the work covered in this manuscript has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript. Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
There are no known conflicts of interest in the publication of this article. The manuscript was read and approved by all authors.
References
- Frommer, M.; Faderani, R.; Kanapathy, M.; Pérusseau-Lambert, A.; Shankar, A.; Malhotra, A.; Zaban, M.K.; Floyd, D.; Butler, P.; Ghali, S. Preoperative CT imaging as a tool to predict incisional hernia outcomes following abdominal wall reconstruction: A retrospective cohort analysis. J. Plast. Reconstr. Aesthetic Surg. 2023, 88, 369–377. [Google Scholar] [CrossRef]
- Birindelli, A.; Sartelli, M.; Di Saverio, S.; Coccolini, F.; Ansaloni, L.; van Ramshorst, G.H.; Campanelli, G.; Khokha, V.; Moore, E.E.; Peitzman, A.; et al. 2017 update of the WSES guidelines for emergency repair of complicated abdominal wall hernias. World J. Emerg. Surg. 2017, 12, 1–16. [Google Scholar] [CrossRef]
- Gillies, M.; Anthony, L.; Al-Roubaie, A.; Rockliff, A.; Phong, J. Trends in Incisional and Ventral Hernia Repair: A Population Analysis From 2001 to 2021. Cureus 2023, 15, e35744. [Google Scholar] [CrossRef] [PubMed]
- Nho, R.L.H.; Mege, D.; Ouaïssi, M.; Sielezneff, I.; Sastre, B. Incidence and prevention of ventral incisional hernia. J. Visc. Surg. 2012, 149, e3–e14. [Google Scholar] [CrossRef] [PubMed]
- Baig, S.; Sheth, H.; Viswanath, N.G.; Madhok, B.; GENESIS Collaborative Group. Patient-reported outcomes in inguinal hernia surgery—Results from the GENESIS study: A multinational multicenter study. World J. Surg. 2024, 48, 1132–1138. [Google Scholar] [CrossRef]
- Hernia Surge Group. International guidelines for groin hernia management. Hernia 2018, 22, 1–165. [Google Scholar] [CrossRef]
- Shubinets, V.; Fox, J.P.; Lanni, M.A.; Tecce, M.G.; Pauli, E.M.; Hope, W.W.; Kovach, S.J.; Fischer, J.P. Incisional Hernia in the United States: Trends in Hospital Encounters and Corresponding Healthcare Charges. Am. Surg. 2018, 84, 118–125. [Google Scholar] [CrossRef]
- Gignoux, B.; Bayon, Y.; Martin, D.; Phan, R.; Augusto, V.; Darnis, B.; Sarazin, M. Incidence and risk factors for incisional hernia and recurrence: Retrospective analysis of the French national database. Color. Dis. 2021, 23, 1515–1523. [Google Scholar] [CrossRef]
- Köckerling, F. Data and outcome of inguinal hernia repair in hernia registers—A review of the literature. Innov. Surg. Sci. 2017, 2, 69–79. [Google Scholar] [CrossRef]
- Socea, B.; Carap, A.; Bratu, O.G.; Diaconu, C.C.; Dimitriu, M.; Socea, L.I.; Bobic, S.; Constantin, V.D. The Role of the Composite and Biologic Meshes in the Trocar Site Hernia Repair Following Laparoscopic Surgery. Mater. Plast. 2018, 55, 146–148. [Google Scholar] [CrossRef]
- Ghariani, W.; Dougaz, M.W.; Jerraya, H.; Khalfallah, M.; Bouasker, I.; Dziri, C. Recurrence Factors of Groin Hernia: A systematic Review. Tunis Med. 2019, 97, 619–625. [Google Scholar] [PubMed]
- Moga, D.; Serban, D.; Geavlete, B.; Serboiu, C.; Serban, B.; Dascalu, A.M.; Oprea, V. Endoscopic approach to recurrent inguinal hernia after previous open surgery. J. Mind Med. Sci. 2023, 10, 276–282. [Google Scholar] [CrossRef]
- Sanders, D.L.; Kingsnorth, A.N. Prosthetic mesh materials used in hernia surgery. Expert Rev. Med Devices 2012, 9, 159–179. [Google Scholar] [CrossRef] [PubMed]
- Socea, B.; Socea, L.I.; Bratu, O.G.; Mastalier, B.; Dimitriu, M.; Carap, A.; Constantin, V.D. Recurrence Rates and Mesh Shrinkage After Polypropylene vs. Polyester Mesh Hernia Repair in Complicated Hernias. Mater. Plast. 2018, 55, 79–81. [Google Scholar] [CrossRef]
- Guillaume, O.; Teuschl, A.H.; Gruber-Blum, S.; Fortelny, R.H.; Redl, H.; Petter-Puchner, A. Emerging Trends in Abdominal Wall Reinforcement: Bringing Bio-Functionality to Meshes. Adv. Healthc. Mater. 2015, 4, 1763–1789. [Google Scholar] [CrossRef]
- Klosterhalfen, B.; Junge, K.; Klinge, U. The lightweight and large porous mesh concept for hernia repair. Expert Rev. Med. Devices 2005, 2, 103–117. [Google Scholar] [CrossRef]
- Baylón, K.; Rodríguez-Camarillo, P.; Elías-Zúñiga, A.; Díaz-Elizondo, J.A.; Gilkerson, R.; Lozano, K. Past, Present and Future of Surgical Meshes: A Review. Membranes 2017, 7, 47. [Google Scholar] [CrossRef]
- Klinge, U.; Klosterhalfen, B.; Birkenhauer, V.; Junge, K.; Conze, J.; Schumpelick, V. Impact of Polymer Pore Size on the Interface Scar Formation in a Rat Model. J. Surg. Res. 2002, 103, 208–214. [Google Scholar] [CrossRef]
- Melkemichel, M.; Bringman, S.A.W.; Widhe, B.O.O. Long-term Comparison of Recurrence Rates Between Different Lightweight and Heavyweight Meshes in Open Anterior Mesh Inguinal Hernia Repair: A Nationwide Population-based Register Study. Ann. Surg. 2019, 273, 365–372. [Google Scholar] [CrossRef]
- Blatnik, J.; Jin, J.; Rosen, M. Abdominal hernia repair with bridging acellular dermal matrix—An expensive hernia sac. Am. J. Surg. 2008, 196, 47–50. [Google Scholar] [CrossRef]
- Diaz, J.J., Jr.; Guy, J.; Berkes, M.B.; Guillamondegui, O.; Miller, R.S. Acellular dermal allograft for ventral hernia repair in the com-promised surgical field. Am. Surg. 2006, 72, 1181–1188. [Google Scholar] [CrossRef]
- Schuster, R.; Singh, J.; Safadi, B.Y.; Wren, S.M.; Keller, D.S.; Ho, J.W.; Mercadel, A.J.; Ogola, G.O.; Steele, S.R. The use of acellular dermal matrix for contaminated abdominal wall defects: wound status predicts success. Am. J. Surg. 2006, 192, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Klinge, U.; Park, J.-K.; Klosterhalfen, B. ‘The Ideal Mesh?'. Pathobiology 2013, 80, 169–175. [Google Scholar] [CrossRef] [PubMed]
- George, B., II; Janis, J.E.; Attinger, C.E. Wound Healing: An Overview. Plast. Reconstr. Surg. 2006, 117, 1e. [Google Scholar] [CrossRef]
- Witte, M.B.; Barbul, A. General principles of wound healing. Surg. Clin. N. Am. 2005, 77, 509–528. [Google Scholar] [CrossRef]
- Diegelmann, R.F.; Evans, M.C. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef]
- Donovan, K.; Denham, M.; Kuchta, K.; Denham, W.; Linn, J.G.; Haggerty, S.P.; Carbray, J.; Ujiki, M. Predictors for recurrence after open umbilical hernia repair in 979 patients. Surgery 2019, 166, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, A.D.; Phillips, T.J.; Rogers, G.S.; Gilchrest, B.A. Wound healing and aging. Dermatol. Clin. 1993, 11, 749–757. [Google Scholar] [CrossRef]
- Murphy, B.L.; Ubl, D.S.; Zhang, J.; Habermann, E.B.; Farley, D.R.; Paley, K. Trends of inguinal hernia repairs performed for recurrence in the United States. Surgery 2018, 163, 343–350. [Google Scholar] [CrossRef]
- Turmine, J.; Florence, A.-M.; Tardivon, C.; Passot, G.; Gillion, J.-F.; Moszkowicz, D.; Jurczak, F.; Fromont, G.; Dabrowski, A.; Soler, M.; et al. Obesity increases the surgical complexity and risk of recurrence after midline primary ventral hernia repair: results on 2307 patients from the French Society of hernia surgery (SFCP-CH) registry database. Hernia 2023, 28, 1–10. [Google Scholar] [CrossRef]
- Wilson, J.A.; Clark, J.J. Obesity: impediment to postsurgical wound healing. Adv. Skin Wound Care 2004, 17, 426–435. [Google Scholar] [CrossRef]
- Sugerman, H.J.; Kellum, J.M.; Reines, H.D.; DeMaria, E.J.; Newsome, H.H.; Lowry, J.W. Greater risk of incisional hernia with morbidly obese than steroid-dependent patients and low recurrence with prefascial polypropylene mesh. Am. J. Surg. 1996, 171, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.Y.W.; Hooi, N.M.F.; Yeo, B.S.Y.; Sultana, R.; Bee, Y.M.; Bin Lee, A.R.Y.; Tay, S.M. Improving Diabetic Wound-Healing Outcomes With Topical Growth Factor Therapies: Systematic Review and Network Meta-analysis of Randomised-controlled Trials. J. Clin. Endocrinol. Metab. 2024, 109, e1642–e1651. [Google Scholar] [CrossRef]
- Acosta, J.B.; del Barco, D.G.; Vera, D.C.; Savigne, W.; Lopez-Saura, P.; Nieto, G.G.; Schultz, G.S. The pro-inflammatory environment in recalcitrant diabetic foot wounds. Int. Wound J. 2008, 5, 530–539. [Google Scholar] [CrossRef]
- Bauer, S.M.; Bauer, R.J.; Velazquez, O.C. Angiogenesis, Vasculogenesis, and Induction of Healing in Chronic Wounds. Vasc. Endovasc. Surg. 2005, 39, 293–306. [Google Scholar] [CrossRef]
- Socea, B.; Silaghi, A.; Rebegea, L.F.; Balan, D.G.; Balalau, C.; Tenea-Cojan, T.Ș.; Mihai, D.A.; Paunica, I. Diabetes mellitus: interdisciplinary medical, surgical and psychological therapeutic approach. J. Mind Med Sci. 2023, 10, 217–236. [Google Scholar] [CrossRef]
- McDaniel, J.C.; Browning, K.K. Smoking, Chronic Wound Healing, and Implications for Evidence-Based Practice. J. Wound, Ostomy Cont. Nurs. 2014, 41, 415–423. [Google Scholar] [CrossRef]
- Sørensen, L.T.; Jørgensen, T. Short-term pre-operative smoking cessation intervention does not affect postoperative complications in colorectal surgery: a randomized clinical trial. Color. Dis. 2003, 5, 347–352. [Google Scholar] [CrossRef]
- Dhall, S.; Alamat, R.; Castro, A.; Sarker, A.H.; Mao, J.-H.; Chan, A.; Hang, B.; Martins-Green, M. Tobacco toxins deposited on surfaces (third hand smoke) impair wound healing. Clin. Sci. 2016, 130, 1269–1284. [Google Scholar] [CrossRef]
- Whiteford, L. Nicotine, CO and HCN: the detrimental effects of smoking on wound healing. Br. J. Community Nurs. 2003, 8, S22–S26. [Google Scholar] [CrossRef]
- A Sherwin, M.; Gastwirth, C.M. Detrimental effects of cigarette smoking on lower extremity wound healing. J. Foot Surg. 1990, 29, 84–7. [Google Scholar]
- Aspera-Werz, R.H.; Mück, J.; Linnemann, C.; Herbst, M.; Ihle, C.; Histing, T.; Nussler, A.K.; Ehnert, S. Nicotine and Cotinine Induce Neutrophil Extracellular Trap Formation—Potential Risk for Impaired Wound Healing in Smokers. Antioxidants 2022, 11, 2424. [Google Scholar] [CrossRef]
- Towler, J. Cigarette smoking and its effects on wound healing. J. Wound Care 2000, 9, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.A.; Goodson, W.H.; Hopf, H.W.; Hunt, T.K. Cigarette Smoking Decreases Tissue Oxygen. Arch. Surg. 1991, 126, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.; Jenkins, R.A.; Kurihara, K.; Schultz, R.C. The Acute Effects of Cigarette Smoke Exposure on Experimental Skin Flaps. Plast. Reconstr. Surg. 1985, 75, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Falcone, R.; Nappi, J. Chemotherapy and Wound Healing. Surg. Clin. N. Am. 1984, 64, 779–794. [Google Scholar] [CrossRef]
- Drake, D.B.; Oishi, S.N. Wound Healing Considerations In Chemotherapy And Radiation Therapy. Clin. Plast. Surg. 1995, 22, 31–37. [Google Scholar] [CrossRef]
- Bernstein, E.F.; Sullivan, F.J.; Mitchell, J.B.; Salomon, G.D.; Glatstein, E. Biology Of Chronic Radiation Effect On Tissues And Wound Healing. Clin. Plast. Surg. 1993, 20, 435–453. [Google Scholar] [CrossRef]
- Dormand, E.; E Banwell, P.; Goodacre, T.E. Radiotherapy and wound healing. Int. Wound J. 2005, 2, 112–127. [Google Scholar] [CrossRef]
- Denham, J.W.; Hauer-Jensen, M. The radiotherapeutic injury—A complex ‘wound’. Radiother. Oncol. 2002, 63, 129–145. [Google Scholar] [CrossRef]
- Herskind, C.; Bamberg, M.; Rodemann, H.P. The role of cytokines in the development of normal-tissue reactions after radio-therapy. Strahlenther. Onkol. 1998, 174 (Suppl. 3), 12–15. [Google Scholar]
- Shi, H.P.; Most, D.; Efron, D.T.; Tantry, U.; Fischel, M.; Barbul, A. The role of iNOS in wound healing. Surgery 2001, 130, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Schäffer, M.; Weimer, W.; Wider, S.; Stülten, C.; Bongartz, M.; Budach, W.; Becker, H.-D. Differential Expression of Inflammatory Mediators in Radiation-Impaired Wound Healing. J. Surg. Res. 2002, 107, 93–100. [Google Scholar] [CrossRef] [PubMed]
- A Kolb, B.; E Buller, R.; Connor, J.P.; DiSaia, P.J.; Berman, M.L. Effects of early postoperative chemotherapy on wound healing. . 1992, 79, 988–92. [Google Scholar] [CrossRef] [PubMed]
- Shamberger, R.C.; Devereux, D.F.; Brennan, M.F. The effect of chemotherapeutic agents on wound healing. Int. Adv. Surg. Oncol. 1981, 4, 15–58. [Google Scholar]
- Bratu, D.; Mihetiu, A.; Sandu, A.; Serboiu, C.; Tudor, C.; Simion, L.; Cretoiu, D.; Bobirca, F.; Davitoiu, D.; Serban, B.; et al. Is open surgery still part of the current treatment of inguinal hernias? J. Mind Med. Sci. 2023, 10, 339–346. [Google Scholar] [CrossRef]
- Tostes, J.M.; Watanabe, A.L.; Watanabe, L.M. Effects of Hypertension on Abdominal Wall Healing: Experimental Study in Rats. Surg. Today 2007, 37, 215–219. [Google Scholar] [CrossRef]
- Smoot, R.L.; Oderich, G.S.; Taner, C.B.; Greenlee, S.M.; Larson, D.R.; Cragun, E.B.; Farley, D.R. Postoperative hematoma following inguinal herniorrhaphy: patient characteristics leading to increased risk. Hernia 2007, 12, 261–265. [Google Scholar] [CrossRef]
- Zeb, M.H.; Pandian, T.; El Khatib, M.M.; Naik, N.D.; Chandra, A.; Morris, D.S.; Smoot, R.L.; Farley, D.R. Risk factors for postoperative hematoma after inguinal hernia repair: an update. J. Surg. Res. 2016, 205, 33–37. [Google Scholar] [CrossRef]
- Durmaz, C.E.; Ozkan, A.; Senel, B.; Uyar, H.A. Comparison of effects of unfractionated heparin and low molecular weight heparin on skin wound healing of rats. Acta Cir. Bras. 2012, 27, 639–644. [Google Scholar] [CrossRef]
- Esen, E. The effect of low-molecular-weight heparin on rat tendon healing. Acta Orthop. Traumatol. Turc. 2009, 43, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Bin Lim, K. Epidemiology of clinical benign prostatic hyperplasia. Asian J. Urol. 2017, 4, 148–151. [Google Scholar] [CrossRef]
- Miyajima, A. Inseparable interaction of the prostate and inguinal hernia. Int. J. Urol. 2018, 25, 644–648. [Google Scholar] [CrossRef] [PubMed]
© 2008 by the author. 2008 Anca Tigora, Vlad Paic, Dragos Nicolae Garofil, Mircea Nicolae Bratucu, Mihai Zurzu, Daniela Nuta, Florian Popa, Valeriu Surlin, Stefan Patrascu, Victor Strambu, Petru Adrian Radu