Low Anterior Rectal Resection; The Impact of Anastomotic Fistula on Incidence and Severity of Low Anterior Resection Syndrome
Abstract
Highlights
- ✓
- Despite its higher frequency, anastomotic leakage does not appear to substantially increase the risk of LARS.
- ✓
- Conversely, the main factors influencing the occurrence of LARS included neoadjuvant chemotherapy and biological sex.
Abstract
Introduction
Materials and Methods
Study Design
Statistical Analysis
Ethics Committee
Diagnosis and Staging
Preoperative Preparation
Technique
Postoperative Follow-Up
LARS Questionnaire
Anastomotic Leak
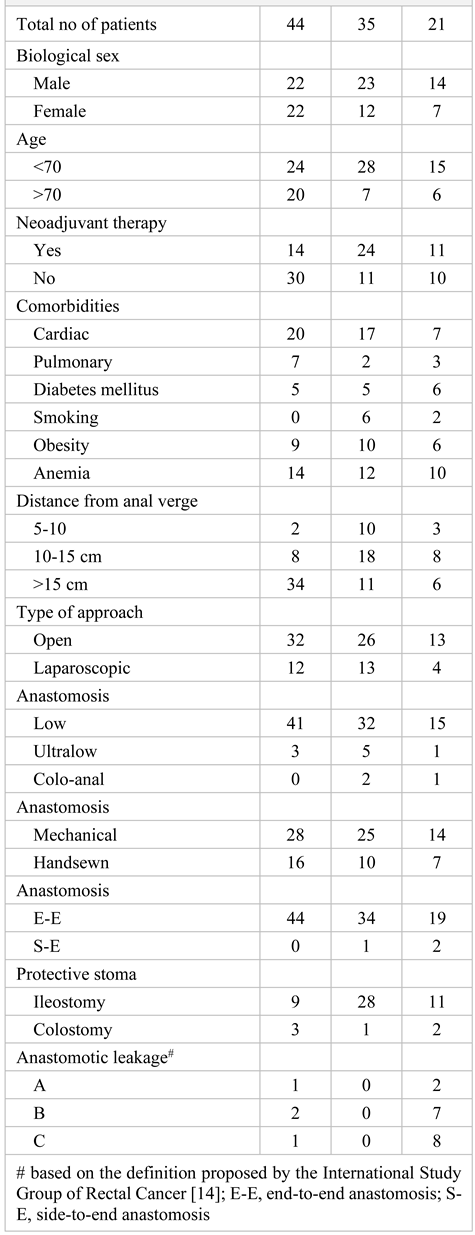
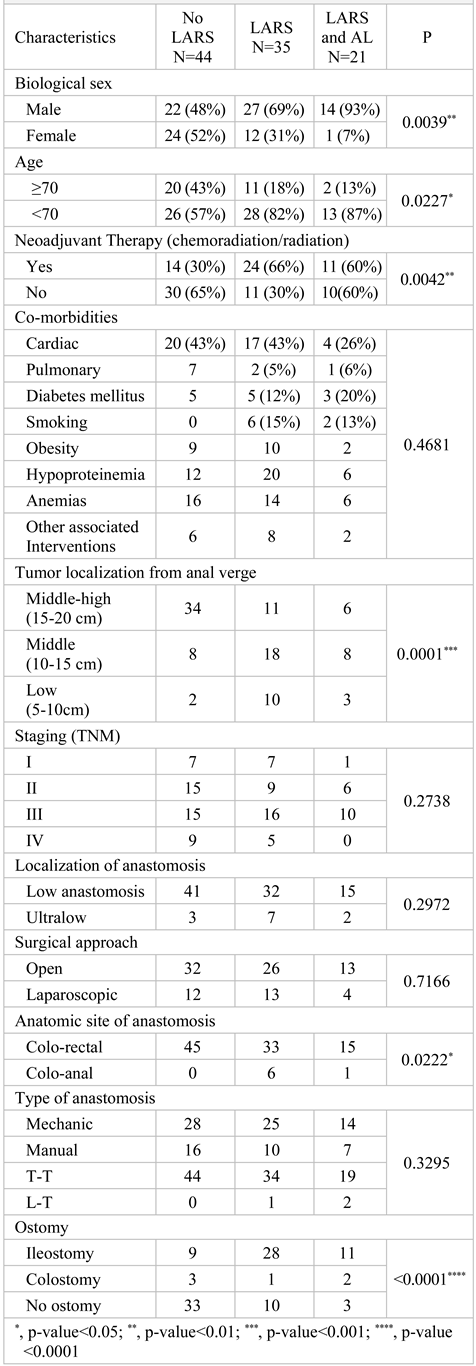
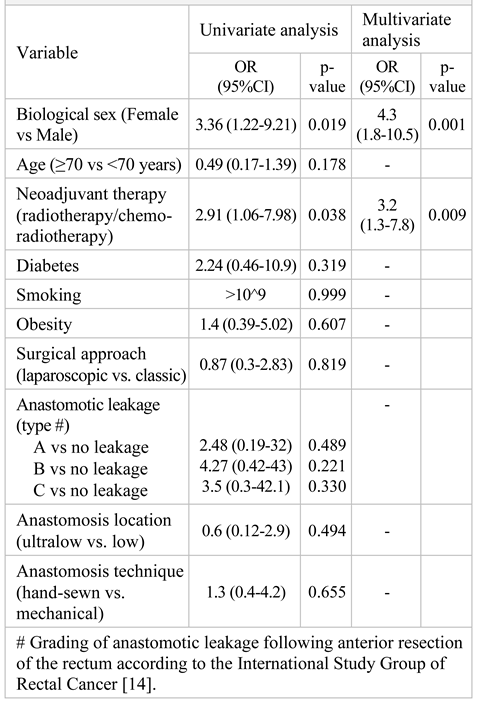
Results
Discussions
Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviations
| LAR | : | low anterior resection |
| LARS | : | Low Anterior Resection Syndrome |
| AL | : | Anastomotic Leakage |
| LMWH | : | Low Molecular Weight Heparins |
| E-E | : | End-to-End anastomosis |
| S-E | : | Side-to-End anastomosis |
| TME | : | Total Mesorectal Excision |
| CT | : | Computed Tomography |
References
- Bryant, C.L.; Lunniss, P.J.; Knowles, C.H.; A Thaha, M.; Chan, C.L. Anterior resection syndrome. Lancet Oncol. 2012, 13, e403–e408. [Google Scholar] [CrossRef] [PubMed]
- Sarcher, T.; Dupont, B.; Alves, A.; Menahem, B. Anterior resection syndrome: What should we tell practitioners and patients in 2018? J. Visc. Surg. 2018, 155, 383–391. [Google Scholar] [CrossRef]
- Emmertsen, K.J.; Laurberg, S.; Jess, P.; Madsen, M.R.; Nielsen, H.J.; Ovesen, A.U.; Salomon, S.; Nielsen, K.T.; Vilandt, J. Impact of bowel dysfunction on quality of life after sphincter-preserving resection for rectal cancer. Br. J. Surg. 2013, 100, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Emmertsen, K.J.; Laurberg, S. Low Anterior Resection Syndrome Score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann. Surg. 2012, 255, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Martellucci, J.; Sturiale, A.; Bergamini, C.; Boni, L.; Cianchi, F.; Coratti, A.; Valeri, A. Role of transanal irrigation in the treatment of anterior resection syndrome. Tech. Coloproctology 2018, 22, 519–527. [Google Scholar] [CrossRef]
- Chen, T.Y.-T.; Wiltink, L.M.; Nout, R.A.; Kranenbarg, E.M.-K.; Laurberg, S.; Marijnen, C.A.; van de Velde, C.J. Bowel Function 14 Years After Preoperative Short-Course Radiotherapy and Total Mesorectal Excision for Rectal Cancer: Report of a Multicenter Randomized Trial. Clin. Color. Cancer 2015, 14, 106–114. [Google Scholar] [CrossRef]
- Sturiale, A.; Martellucci, J.; Zurli, L.; Vaccaro, C.; Brusciano, L.; Limongelli, P.; Docimo, L.; Valeri, A. Long-term functional follow-up after anterior rectal resection for cancer. Int. J. Color. Dis. 2016, 32, 83–88. [Google Scholar] [CrossRef]
- Dulskas, A.; Smolskas, E.; Kildusiene, I.; Samalavicius, N.E. Treatment possibilities for low anterior resection syndrome: a review of the literature. Int. J. Color. Dis. 2018, 33, 251–260. [Google Scholar] [CrossRef]
- Jannasch, O.; Klinge, T.; Otto, R.; Chiapponi, C.; Udelnow, A.; Lippert, H.; Bruns, C.J.; Mroczkowski, P. Risk factors, short and long term outcome of anastomotic leaks in rectal cancer. Oncotarget 2015, 6, 36884–36893. [Google Scholar] [CrossRef]
- Zhang, W.; Lou, Z.; Liu, Q.; Meng, R.; Gong, H.; Hao, L.; Liu, P.; Sun, G.; Ma, J. Multicenter analysis of risk factors for anastomotic leakage after middle and low rectal cancer resection without diverting stoma: a retrospective study of 319 consecutive patients. Int. J. Color. Dis. 2017, 32, 1431–1437. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, Y.S.; Park, S.C.; Sohn, D.K.; Kim, D.Y.; Chang, H.J.; Oh, J.H. Risk factors for permanent stoma after rectal cancer surgery with temporary ileostomy. Surgery 2016, 159, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Choi, G.-S.; Kim, S.H.; Kim, H.R.; Kim, N.K.; Lee, K.Y.; Kang, S.B.; Kim, J.Y.; Lee, K.Y.; Kim, B.C.; et al. Multicenter Analysis of Risk Factors for Anastomotic Leakage After Laparoscopic Rectal Cancer Excision: the Korean laparoscopic colorectal surgery study group. Ann. Surg. 2013, 257, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Cronin, M.; Dengler, N.; Krauss, E.S.; Segal, A.; Wei, N.; Daly, M.; Mota, F.; Caprini, J.A. Completion of the Updated Caprini Risk Assessment Model (2013 Version). Clin. Appl. Thromb Hemost. 2019, 25, 1076029619838052. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Weitz, J.; Hohenberger, W.; Heald, R.J.; Moran, B.; Ulrich, A.; Holm, T.; Wong, W.D.; Tiret, E.; Moriya, Y.; et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: A proposal by the International Study Group of Rectal Cancer. Surgery 2010, 147, 339–351. [Google Scholar] [CrossRef]
- Wells, C.I.; Vather, R.; Chu, M.J.J.; Robertson, J.P.; Bissett, I.P. Anterior Resection Syndrome—A Risk Factor Analysis. J. Gastrointest. Surg. 2015, 19, 350–359. [Google Scholar] [CrossRef]
- Battersby, N.J.; Juul, T.; Christensen, P.; Janjua, A.Z.; Branagan, G.; Emmertsen, K.J.; Norton, C.; Hughes, R.; Laurberg, S.; Moran, B.J. Predicting the Risk of Bowel-Related Quality-of-Life Impairment After Restorative Resection for Rectal Cancer: A Multicenter Cross-Sectional Study. Dis. Colon Rectum 2016, 59, 270–280. [Google Scholar] [CrossRef]
- Daams, F.; Monkhorst, K.; Broek, J.v.D.; Slieker, J.; Jeekel, J.; Lange, J. Local Ischaemia Does Not Influence Anastomotic Healing: An Experimental Study. Eur. Surg. Res. 2013, 50, 24–31. [Google Scholar] [CrossRef]
- Hallböök, O.; Sjödahl, R. Anastomotic leakage and functional outcome after anterior resection of the rectum. Br. J. Surg. 1996, 83, 60–62. [Google Scholar] [CrossRef]
- Nesbakken, A.; Nygaard, K.; Lunde, O.C. Outcome and late functional results after anastomotic leakage following mesorectal excision for rectal cancer. Br. J. Surg. 2001, 88, 400–404. [Google Scholar] [CrossRef]
- Ahmad, N.Z.; Abbas, M.H.; Khan, S.U.; Parvaiz, A. A meta-analysis of the role of diverting ileostomy after rectal cancer surgery. Int. J. Color. Dis. 2021, 36, 445–455. [Google Scholar] [CrossRef]
- Degiuli, M.; Elmore, U.; De Luca, R.; De Nardi, P.; Tomatis, M.; Biondi, A.; Persiani, R.; Solaini, L.; Rizzo, G.; Soriero, D.; et al. Risk factors for anastomotic leakage after anterior resection for rectal cancer (RALAR study): A nationwide retrospective study of the Italian Society of Surgical Oncology Colorectal Cancer Network Collaborative Group. Color. Dis. 2022, 24, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Podda, M.; Coccolini, F.; Gerardi, C.; Castellini, G.; Wilson, M.S.J.; Sartelli, M.; Pacella, D.; Catena, F.; Peltrini, R.; Bracale, U.; et al. Early versus delayed defunctioning ileostomy closure after low anterior resection for rectal cancer: a meta-analysis and trial sequential analysis of safety and functional outcomes. Int. J. Color. Dis. 2022, 37, 737–756. [Google Scholar] [CrossRef]
- Carr, N.D.; Pullen, B.R.; Hasleton, P.S.; Schofield, P.F. Microvascular studies in human radiation bowel disease. Gut 1984, 25, 448–454. [Google Scholar] [CrossRef]
- Varma, J.S.; Smith, A.N.; Busuttil, A. Function of the anal sphincters after chronic radiation injury. Gut 1986, 27, 528–533. [Google Scholar] [CrossRef]
- Beppu, N.; Kimura, H.; Matsubara, N.; Tomita, N.; Yanagi, H.; Yamanaka, N. Long-Term Functional Outcomes of Total Mesorectal Excision Following Chemoradiotherapy for Lower Rectal Cancer: Stapled Anastomosis versus Intersphincteric Resection. Dig. Surg. 2016, 33, 33–42. [Google Scholar] [CrossRef]
- Savlovschi, C.; Serban, D.; Trotea, T.; Borcan, R.; Dumitrescu, D. Post-surgery morbidity and mortality in colorectal cancer in elderly subjects. Chirurgia (Bucur) 2013, 108, 177–9. [Google Scholar]
- Pappou, E.P.; Temple, L.K.; Patil, S.; Smith, J.J.; Wei, I.H.; Nash, G.M.; Guillem, J.G.; Widmar, M.; Weiser, M.R.; Paty, P.B.; et al. Quality of life and function after rectal cancer surgery with and without sphincter preservation. Front. Oncol. 2022, 12, 944843. [Google Scholar] [CrossRef]
- Şavlovschi, C.; Comandaşu, M.; Şerban, D. Specifics of diagnosis and treatment in synchronous colorectal cancers (SCC). Chirurgia (Bucur) 2013, 108, 43–5. [Google Scholar]
- Machado, M.; Nygren, J.; Goldman, S.; Ljungqvist, O. Similar Outcome After Colonic Pouch and Side-to-End Anastomosis in Low Anterior Resection for Rectal Cancer: a prospective randomized trial. Ann. Surg. 2003, 238, 214–220. [Google Scholar] [CrossRef]
- Wallner, C.; Lange, M.M.; Bonsing, B.A.; Maas, C.P.; Wallace, C.N.; Dabhoiwala, N.F.; Rutten, H.J.; Lamers, W.H.; DeRuiter, M.C.; van de Velde, C.J. Causes of Fecal and Urinary Incontinence After Total Mesorectal Excision for Rectal Cancer Based on Cadaveric Surgery: A Study From the Cooperative Clinical Investigators of the Dutch Total Mesorectal Excision Trial. J. Clin. Oncol. 2008, 26, 4466–4472. [Google Scholar] [CrossRef]
- Ashburn, J.H.; Stocchi, L.; Kiran, R.P.; Dietz, D.W.; Remzi, F.H. Consequences of Anastomotic Leak After Restorative Proctectomy for Cancer: effect on long-term function and quality of life. Dis. Colon Rectum 2013, 56, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Hultberg, D.K.; Svensson, J.; Jutesten, H.; Rutegård, J.M.; Matthiessen, P.M.; Lydrup, M.-L.M.; Rutegård, M.M. The Impact of Anastomotic Leakage on Long-term Function After Anterior Resection for Rectal Cancer. Dis. Colon Rectum 2020, 63, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Savlovschi, C.; Serban, D.; Andreescu, C.; Dascalu, A.; Pantu, H. Economic analysis of medical management applied for left colostomy. Chirurgia (Bucur) 2013, 108, 666–9. [Google Scholar]
- Pătraşcu, Ş.; Cercelaru, L.; Graure, G.M.; Firuţ, M.A.; Rotaru, I.; Cârţu, D.; Marinescu, D.; Pătraşcu, A.M.; Radu, R.I.; Mitroi, G.; et al. The histopathological features and their prognostic impact in the postoperative follow-up of colorectal cancer patients. Romanian J. Morphol. Embryol. 2022, 63, 555–561. [Google Scholar] [CrossRef]
- Ri, H.; Kang, H.; Xu, Z.; Kim, K.; Ren, Y.; Gong, Z.; Chen, X. The risk factors of low anterior resection syndrome after colorectal cancer surgery: A retrospective study of 566 patients in a single institution in China. Front. Surg. 2022, 9, 990702. [Google Scholar] [CrossRef]
 |
 |
 |
© 2024 by the authors. 2024 Giorgiana Coțofană Graure, Cecil Mirea, Silviu Daniel Preda, Stefan Patrascu, Tiberiu Stefanita Tenea-Cojan, Adina Turcu-Stiolica, Alexandru Munteanu, Vlad Padureanu, Dragos Margaritescu, Sandu Ramboiu, Dan Cartu, Valeriu Șurlin, Petre Radu.
Share and Cite
Coțofană Graure, G.; Mirea, C.; Preda, S.D.; Patrascu, S.; Tenea-Cojan, T.S.; Turcu-Stiolica, A.; Munteanu, A.; Padureanu, V.; Margaritescu, D.; Ramboiu, S.; et al. Low Anterior Rectal Resection; The Impact of Anastomotic Fistula on Incidence and Severity of Low Anterior Resection Syndrome. J. Mind Med. Sci. 2024, 11, 139-145. https://doi.org/10.22543/2392-7674.1465
Coțofană Graure G, Mirea C, Preda SD, Patrascu S, Tenea-Cojan TS, Turcu-Stiolica A, Munteanu A, Padureanu V, Margaritescu D, Ramboiu S, et al. Low Anterior Rectal Resection; The Impact of Anastomotic Fistula on Incidence and Severity of Low Anterior Resection Syndrome. Journal of Mind and Medical Sciences. 2024; 11(1):139-145. https://doi.org/10.22543/2392-7674.1465
Chicago/Turabian StyleCoțofană Graure, Giorgiana, Cecil Mirea, Silviu Daniel Preda, Stefan Patrascu, Tiberiu Stefanita Tenea-Cojan, Adina Turcu-Stiolica, Alexandru Munteanu, Vlad Padureanu, Dragos Margaritescu, Sandu Ramboiu, and et al. 2024. "Low Anterior Rectal Resection; The Impact of Anastomotic Fistula on Incidence and Severity of Low Anterior Resection Syndrome" Journal of Mind and Medical Sciences 11, no. 1: 139-145. https://doi.org/10.22543/2392-7674.1465
APA StyleCoțofană Graure, G., Mirea, C., Preda, S. D., Patrascu, S., Tenea-Cojan, T. S., Turcu-Stiolica, A., Munteanu, A., Padureanu, V., Margaritescu, D., Ramboiu, S., Cartu, D., Surlin, V., & Radu, P. (2024). Low Anterior Rectal Resection; The Impact of Anastomotic Fistula on Incidence and Severity of Low Anterior Resection Syndrome. Journal of Mind and Medical Sciences, 11(1), 139-145. https://doi.org/10.22543/2392-7674.1465



