Abstract
Although rare, subungual carcinoma is the most common malignant nail tumor. The symptomatology is not characteristic, being very similar to that of other nail and periungual diseases. For this reason, early diagnosis is usually established by performing a biopsy. With appropriate treatment, subungual carcinoma usually has a favorable prognosis. Metastatic cases are rarely encountered, but they usually have a poor therapeutic result. This article presents the case of a patient with subungual carcinoma (diagnosed by histopathological exam), initially treated by amputation of the distal and middle phalanges of the fourth finger. Two years after the operation, a recurrence in the epitrochlear nodes was diagnosed, for which epitrochlear lymphadenectomy and postoperative chemotherapy were performed. At 7 months, metastases were detected in the axillary nodes, which were treated with radiotherapy and chemotherapy. The appearance and development of the tumor at the elbow and the lower third of the arm led the patient to accept scapulohumeral disarticulation. The patient finally adapted to the physical infirmity, having a good general condition and an optimistic attitude.
Introduction
Although rare, nail bed carcinoma remains the most common tumor of the nail [1]. The nail unit has 4 anatomical structures: nail plate, nail bed, nail fold, and hyponychium. The origin of the tumor is usually at the level of the nail bed (71%) and less often (29%) at the level of the nail fold [2].
Predisposing factors involved in the etiology of subungual carcinoma include exposure to ultraviolet radiation, papillomavirus infection, arsenic and other toxic substances, smoking, local traumas, some congenital diseases (xeroderma pigmentosa and epidermodysplasia verruciformis) and immunosuppression [1,2,3]. Mutations occurring at the level of keratocytes, spontaneously or under the action of the previously mentioned etiological agents, initiate (in case of alteration of the DNA repair mechanisms) successive transformations that lead to malignancy [4]. Several mutations were involved in the pathogenesis of subungual squamous cell carcinoma (TP53, HRAS, CTNNB1, EGFR, GNA11, BRAF, TERT), most of which also play a role in the pathogenesis of other squamous forms of cancer [5]. The accumulation of genetic and epigenetic alterations leads to the development of carcinoma [4]. It usually has a local evolution and only a small proportion of cases (< 2%) accumulates new mutations (EGFR, TP53) which exhibit metastatic characteristics [4,5].
The clinical presentation is not easily distinguishable, subungual carcinoma being a great imitator of other nail and periungual diseases. The symptoms include hyperkeratosis, lateral detachment (onycholysis) of the nail, melanonychia, painless erosion of the nail bed with or without a nodule, erythronychia, partial or total destruction of the nail plate [6,7]. The diagnosis is established by biopsy, and the depth of invasion by radiography or MRI.
The treatment is surgical, but for high-risk and advanced cases, chemotherapy and radiotherapy complete the surgical course of treatment.
Case Presentation
It is presented the case of a 64-year-old patient, who presented with a chronic suppuration of the distal phalanx of the IV finger of the right hand. The patient's history includes a long period (about one year) of minor complaints (redness, swelling of the distal phalanx, pain, marginal erosion of the nail), most of which were initially neglected. Following the exacerbation of the symptoms (swelling, redness, pain) he presented himself to the emergency service, where the case was interpreted as chronic paronychia, and furthermore antibiotic treatment was instituted. After 2 weeks he came to our service because of the unsatisfactory response to the instituted treatment. Considering the long history and poor response to treatment, the previously established diagnosis was questioned and a nail bed biopsy was performed. Histopathological examination revealed a proliferation of atypical keratinocytes with an altered nuclear-cytoplasmic ratio and increased cytological and nuclear atypia with frequent typical and atypical mitosis, invading the dermis in nests and sheets, some of them related to the overlying one, consistent with diagnosis of subungual squamous cell carcinoma (Figure 1). X-ray of the hand showed invasion of the bony phalanx. Amputation of the distal and middle phalanges of the IV finger was mandatory and therefore performed on the patient.
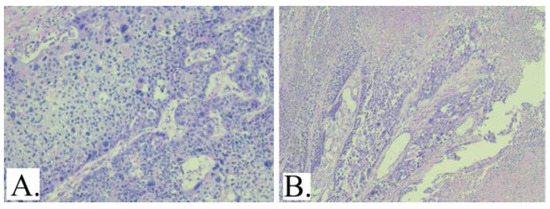
Figure 1.
A: Atypical cells with increased nucleus- cytoplasm ratio, marked nuclear and cytological atypia and frequent mitosis, some atypical (HE20X). B: Recurrence with the histopathological appearance, but with significant tumor necrosis (HE10X).
Two years after the operation, a relapse was diagnosed at the level of the epitrochlear lymph nodes. Epitrochlear lymphadenectomy was performed, and chemotherapy with paclitaxel and carboplatin was initiated postoperatively. At 7 months, the occurrence of metastases in the axillary nodes was highlighted. External radiotherapy in a total dose of 56 Gy and chemotherapy with cisplatin were administered. The response to radiotherapy and chemotherapy was satisfactory, with the reduction of the volume of the affected axillary nodes. We mention that it was not possible to evaluate tumor dissemination by PET- CT. At 6 months, a new recurrence appeared at the elbow (epitrochlear region). The anatomical relationships of the tumor made the amputation of the arm the only remaining option as far as surgery concerns, but this operation was not accepted by the patient. Treatment with cemiplimab was instituted, but the tumor at the level of the elbow and the lower third of the arm continued to grow. The growth and necrosis of the tumor led to the functional impotence of the right upper limb and to problems of social integration determined by the persistent smell (even if the dressing was done twice a day) (Figure 2: A). As a result, the patient accepted the amputation of the arm. Arm amputation through scapulo-humeral disarticulation was performed approximately 6 years after the onset of symptoms, the postoperative evolution being without complications (Figure 2: B, C, D). The patient has adapted to his current physical infirmity; he currently has a good general condition and an optimistic attitude. He is currently following treatment with cemiplimab, with good tolerance except for grade II pruritus and with good radiological response by the RECIST criteria.
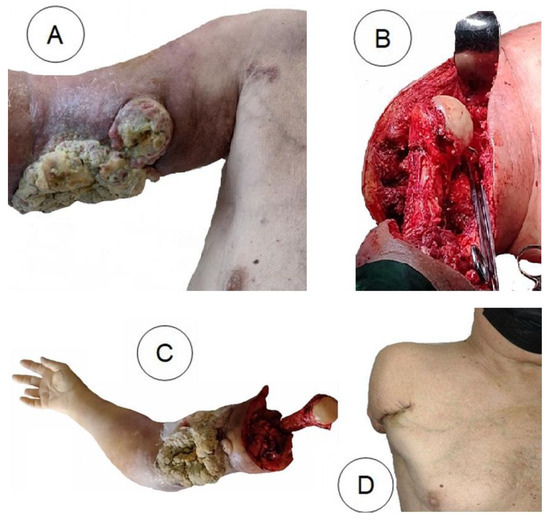
Figure 2.
A: Voluminous, ulcerated, partially necrotic tumour on the arm. B, C: Amputation of the arm. D: 6 months postoperatively
Discussions
Having a slow growth rate and non-specific symptomatology, squamous cell carcinoma of the nail bed is often diagnosed in advanced stages [8,9]. The first factor presumed to lead to the delay of the diagnosis is the late presentation of the patient, which is explained by the reduced intensity of the symptoms [2]. The similarity of the symptoms with other chronic diseases of the nail unit is the second major factor involved in the delay in the diagnosis [2]. Establishing the diagnosis years after the onset of symptoms is common [8,9]. Dijksterhuis mentions delays in diagnosis between 2 and 480 months, with an average of 55 months [2]. Unfortunately, this aspect affects the functional outcome of the patient, as the partial amputation of the finger is regarded in many cases as the only option. In this particular case, the amputation of two phalanges of the IV finger was imperative. But in cases where the disease is located at the level of the thumb, the amputation seriously affects the functionality of the hand. And in the case that is presented, due to the evolution of the disease, it reached a major mutilation through the amputation of the right arm. Consequently, the need to establish a diagnosis as early as possible is imperative.
The clinician must also consider the rare diseases of the nail unit. For a dermatologist, the diagnosis is not surprising, but usually the patient presents himself to the general practitioner, to the surgeon or to an emergency service. In any situation where a long-term disease at the level of the last phalanx does not respond to the usual treatment, the patient should be referred to a dermatologist to perform a biopsy.
When examining the nail and the periungual region clinically, two signs are observed more frequently in patients with carcinoma of the nail bed: distal onycholysis and localized hyperkeratosis [10]. But a simple examination with the naked eye does not provide categorical information for diagnosis. Dermoscopy provides a more accurate image of the nail and periungual lesions: distal onycholysis, localized hyperkeratosis, polycyclic/fuzzy lesion edges, nonparallel lesion edges, splinter hemorrhages, longitudinal parallel white lines, and nail thickening [10,11]. Localized hyperkeratosis is significantly associated with nail bed carcinoma [10], but there is no pathognomonic criteria that allows a well- defined diagnosis by dermoscopy [1,2,10]. Even if dermoscopy cannot establish a positive diagnosis, it is essential for the exclusion of some benign conditions, for establishing a suspicious diagnosis, for identifying which cases requiring biopsy and for choosing the most suitable biopsy technique [10].
Ultrasound of the nail unit requires a linear multiple frequency 15MHz transducer as the minimum standard, or if available very high-frequency (> 20MHz) and ultra- high-frequency transducer (30–70 MHz), which allows the exploration of very small (< 2 mm diameter) structures [12,13]. The ultrasonography can assess the nail plate (as a bilaminar hyperechoic structure), the nail bed (between nail plate and cortex of the distal phalanx), the distal phalanx ( as a hyperechoic lineal interphase under the nail bed), and the nail Matrix (as an ill-defined hypoechoic area that surrounds the proximal nail plate area [12,14]. Carcinoma of the nail bed appears as an hypoechoic area with irregular shape, ill-defined borders, peripheral hypoechoic projections, bone remodeling with cortical erosion, black shadowing artifact, multiple peripheral vascular poles, and sometimes with a central anechoic avascular area corresponding to central tumor necrosis [15,16].
Optical coherence tomography is a noninvasive method that uses the Michelson interferometry with infrared light. The detection of reflected light which passes through a "transparent mirror" type surface allows bi- and three- dimensional morphological reconstruction [17]. Optical coherence tomography allows the highlighting of structures of the nail unit [18], but there are no specific signs for nail bed carcinoma.
During the MRI evaluation, the examined finger is held in a special loop flex and phased-array multichannel wrist coil [19]. MRI could reveal an ill-defined infiltrative margin, hypointense signal on T1 weighted images, intermediate to hyperintense signal on T2-weighted images, with heterogenous enhancement after contrast material administration [16,19].
These methods guide the diagnosis, but none of them can replace the role of the biopsy. The indication for biopsy may result from imaging aspects that raise the suspicion of a subungual tumor or may be established based on clinical suspicion. In the discussed case, it was decided to perform the biopsy considering the long evolution and no tendency towards healing of a "chronic paronychia".
The nail biopsy technique takes into account the anatomy of the nail apparatus. It is important that the harvested tissue is adequate to avoid uninformative or falsely reassuring results. The anatomical elements of the nail unit are the nail plate, the nail bed, the nail matrix, the eponychium, the hyponychium and the nail fold (Figure 3) [20].
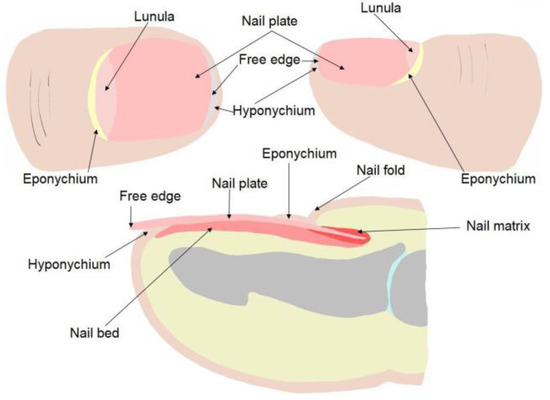
Figure 3.
The components of the nail unit
Depending on the clinical suspicion and the possible origin of the suspected pathology, one or another biopsy technique can be chosen: with or without excision (total / partial) of the nail plate, punch biopsy or incisional biopsy, transversal or longitudinal elliptical excision (Figure 4) [21,22,23,24,25,26]. The choice of the biopsy technique is made in order to obtain a relevant tissue for the histopathological examination. In the case of the presented patient, it was decided to excise the nail and collect a longitudinal elliptical fragment from the nail bed.
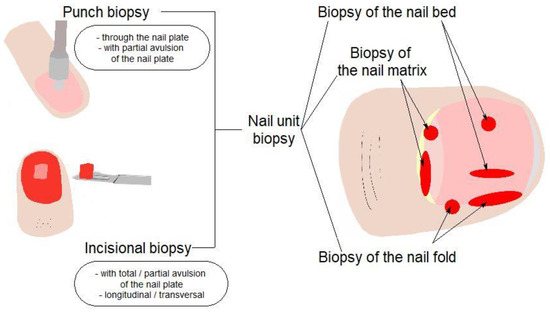
Figure 4.
Types of nail biopsy
After establishing the diagnosis, the choice of treatment must cover two objectives: the oncological and the functional result. Amputation is the last option, but R0 resection is the primary goal. The choice of technique depends on the tumor stage. For the early stages, Mohs surgery is an optimal solution [1,27]. However, bone invasion requires amputation of the distal phalanx of the finger. Evaluation by MRI or radiography is necessary, even if the differentiation between actual invasion and periosteal reaction is sometimes difficult [28]. In the discussed patient, the indication for partial amputation of the finger was established based on the radiography of the hand, whereas the histopathological examination confirmed a bone invasion (pT4).
The presented case shows that surgical treatment alone is often insufficient in some cases. The aggressiveness of the disease was not correctly assessed. The partial amputation of the affected finger was not followed by an adjuvant treatment, taking into account the reduced risk of metastasis of this tumor. Even if metastasis is rare, its consequences in terms of functional and vital prognosis are serious. It is therefore obvious the need to assess some clinical and histopathological criteria on the basis of which to begin the adjuvant treatment. For cutaneous squamous carcinomas, the high-risk criteria include location, size, definition of the tumor margin, immunosuppression, growth rate, neurological symptoms, degree of differentiation and histological subtype, tumor thickness (Clarck level), perineural, lymphatic or vascular invasion [29,30,31,32]. However, there are no consensus guidelines regarding the choice of adjuvant treatment in patients with R0 resection [33].
The discovery of lymph node metastases 2 years after finger amputation changed the patient's prognosis. In squamous cell carcinoma of the nail bed, the appearance of ganglion metastases is rare anyway, and metastases at the level of the epitrochlear lymph nodes is yet again a quite rare situation. Retrospectively, we wondered whether the use of the sentinel ganglion technique at the time of finger amputation would have been helpful. The value of this technique in the treatment of cutaneous squamous carcinomas is not clearly established [29].
There is no specific recommendation for systemic chemotherapy for metastatic cases of nail bed carcinoma; the indications remain the same as for other squamous carcinomas. Platinum-based drugs (cisplatin, carboplatin) alone or in combination with 5-Fluoro-uracil and epidermal growth factor inhibitors (cetuximab, panitumumab) are used [29,30,31,32,33]. Recently, the treatment with cemiplimab (a monoclonal antibody which acts as a checkpoint inhibitor targeting the cellular pathway PD-1) was introduced, and the results are promising [29,30,31,32,33,34]. In the discussed case, cemiplimab treatment was available when the disease advanced, the patient having a bulky tumor in the arm. Cemiplimab treatment alone could not ensure the regression of such a large tumor, so amputation of the arm was considered.
Accepting the operation was a difficult decision for the patient. The understanding and evaluation of a disability refers to the physical, attitudinal, institutional/ organizational and information/ communication barriers that the subject has to face further on. There are important differences between the medical model and the social model of disability [35]. The patient accepted the amputation, appreciating the importance of 2 arguments: the functionality of the arm was already compromised and the social rejection due to the smell produced by the necrotic tumor had become obvious. In accepting the amputation, the discussed patient had to choose between the obvious physical infirmity and an apparent physical normality, but accompanied by a huge social handicap. It is difficult for the doctor to present the patient with the option of a mutilating but palliative operation. In this case, amputation of the arm was the only option, even if it cannot be considered a radical operation. The disease is still considered systemic and is ought to be treated in consequence.
Conclusions
The possibility of carcinoma of the nail bed should be considered when seemingly trivial chronic diseases of the nail unit do not respond to usual treatment. Although there are valuable methods for guidance, biopsy remains the main method of diagnosis. The earlier the diagnosis is established, the less mutilating the following treatment would be. Metastasis is rare, but when it occurs it seriously affects the vital, functional and social prognosis of the patient. Modern treatments with immune checkpoint inhibitors have shown promising results, but early diagnosis of these rare tumors remains essential for a curative treatment.
Compliance with ethical standards
Any aspect of the work covered in this manuscript has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
Conflict of interest disclosure
There are no known conflicts of interest in the publication of this article. The manuscript was read and approved by all authors.
References
- Starace M, Alessandrini A, Dika E, Piraccini BM. Squamous cell carcinoma of the nail unit. Dermatol Pract Concept. 2018, 8, 238–244. [Google Scholar] [CrossRef]
- Dijksterhuis A, Friedeman E, van der Heijden B. Squamous Cell Carcinoma of the Nail Unit: Review of the Literature. J Hand Surg Am. 2018, 43, 374–379e2. [Google Scholar] [CrossRef] [PubMed]
- Xu W, Mao D, Wen G. Squamous Cell Carcinoma of the Nail Unit After Trauma: A Case Report. Clin Cosmet Investig Dermatol. 2022, 15, 1737–1741. [Google Scholar] [CrossRef] [PubMed]
- Hedberg ML, Berry CT, Moshiri AS, et al. Molecular Mechanisms of Cutaneous Squamous Cell Carcinoma. Int J Mol Sci. 2022, 23, 3478. [Google Scholar] [CrossRef]
- Dika E, de Biase D, Lambertini M, Alessandriniet AM, et al. Mutational landscape in squamous cell carcinoma of the nail unit. Exp Dermatol. 2022, 31, 854–861. [Google Scholar] [CrossRef]
- Tambe SA, Patil PD, Saple DG, Kulkarni UY. Squamous Cell Carcinoma of the Nail Bed: The Great Mimicker. J Cutan Aesthet Surg. 2017, 10, 59–60. [Google Scholar] [CrossRef]
- Vashisht D, Singh PY, Tewari R, Baveja S. Squamous cell carcinoma of nail bed: A great mimicker. Med J Armed Forces India. 2018, 74, 190–192. [Google Scholar] [CrossRef]
- Meesiri, S. Subungual squamous cell carcinoma masquerading as chronic common infection. J Med Assoc Thai. 2010, 93, 248–251. [Google Scholar]
- Acuña Pinzon CL, Nieves Condoy JF, Zúñiga Vázquez LA, Chavez Perez G, Chavarría Chavira JL. Squamous Cell Carcinoma of the Nail, an Underdiagnosed and Underestimated Entity: A Series of Two Cases. Cureus. 2021, 13, e14826. [Google Scholar] [CrossRef]
- Teysseire S, Dalle S, Duru G, et al. Dermoscopic Features of Subungual Squamous Cell Carcinoma: A Study of 44 Cases. Dermatology. 2017, 233, 184–191. [Google Scholar] [CrossRef]
- Srivastava R, Manfredini M, Rao BK. Noninvasive imaging tools in dermatology. Cutis. 2019, 104, 108–113. [Google Scholar]
- Alfageme F, Wortsman X, Catalano O, et al. European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) Position Statement on Dermatologic Ultrasound. Ultraschall Med. 2021, 42, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Crisan D, Wortsman X, Alfageme F, et al. Ultrasonography in dermatologic surgery: revealing the unseen for improved surgical planning. J Dtsch Dermatol Ges. 2022, 20, 913–926. [Google Scholar] [CrossRef] [PubMed]
- Wortsman X, Jemec GB. Ultrasound imaging of nails. Dermatol Clin. 2006, 24, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Sechi A, Alessandrini A, Patrizi A, et al. Ultrasound features of the subungual glomus tumor and squamous cell carcinomas. Skin Res Technol. 2020, 26, 867–875. [Google Scholar] [CrossRef]
- Baek HJ, Lee SJ, Cho KH, et al. Subungual tumors: clinicopathologic correlation with US and MR imaging findings. Radiographics. 2010, 30, 1621–1636. [Google Scholar] [CrossRef]
- Constantin VD, Socea B, Gaspar BS, Epistatu D, Paunica I, Dumitriu AS, Paunica S, Silaghi A. Limb amputations; etiopathogenesis, diagnosis and the multidisciplinary therapeutic approach. J Mind Med Sci. 2022, 9, 209–223. [Google Scholar] [CrossRef]
- Psomadakis CE, Marghoob N, Bleicher B, Markowitz O. Optical coherence tomography. Clin Dermatol. 2021, 39, 624–634. [Google Scholar] [CrossRef]
- Mundada P, Becker M, Lenoir V, Stefanelli S, et al. High resolution MRI of nail tumors and tumor-like conditions. Eur J Radiol. 2019, 112, 93–105. [Google Scholar] [CrossRef]
- Haneke, E. Anatomy of the nail unit and the nail biopsy. Semin Cutan Med Surg. 2015, 34, 95–100. [Google Scholar] [CrossRef]
- Rich, P. Nail biopsy. Indications and methods. J Dermatol Surg Oncol. 1992, 18, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Rich, P. Nail biopsy: indications and methods. Dermatol Surg. 2001, 27, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Jellinek, NJ. Nail surgery: practical tips and treatment options. Dermatol Ther. 2007, 20, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Grover C, Chaturvedi UK, Reddy BS. Role of nail biopsy as a diagnostic tool. Indian J Dermatol Venereol Leprol. 2012, 78, 290–298. [Google Scholar] [CrossRef]
- Tanasescu D, Moisin A, Fleaca R, et al. Modern therapeutic options in diabetic foot ulcer. J Mind Med Sci. 2022, 9, 285–293. [Google Scholar] [CrossRef]
- Grover C, Bansal S. Nail Biopsy: A User's Manual. Indian Dermatol Online J. 2018, 9, 3–15. [Google Scholar] [CrossRef]
- Dika E, Fanti PA, Patrizi A, et al. Mohs Surgery for Squamous Cell Carcinoma of the Nail Unit: 10 Years of Experience. Dermatol Surg. 2015, 41, 1015–1019. [Google Scholar] [CrossRef]
- Grover C, Bansal S, Varma A, Jakhar D. Radiological Imaging of Nail Disorders (PART II) - Radiological Features of Nail Disease. Indian Dermatol Online J. 2022, 13, 701–709. [Google Scholar] [CrossRef]
- Work Group; Invited Reviewers, Kim JYS, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018, 78, 560–578. [Google Scholar] [CrossRef]
- Stratigos AJ, Garbe C, Dessinioti C, et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: Part 1. epidemiology, diagnostics and prevention. Eur J Cancer. 2020, 128, 60–82. [Google Scholar] [CrossRef]
- Schmults CD, Blitzblau R, Aasi SZ, et al. NCCN Guidelines® Insights: Squamous Cell Skin Cancer, Version 1.2022. J Natl Compr Canc Netw. 2021, 19, 1382–1394. [Google Scholar] [CrossRef] [PubMed]
- Maubec, E. Update of the Management of Cutaneous Squamous-cell Carcinoma. Acta Derm Venereol. 2020, 100, adv00143. [Google Scholar] [CrossRef] [PubMed]
- Stratigos AJ, Garbe C, Dessinioti C, et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: Part 2. Treatment. Eur J Cancer. 2020, 128, 83–102. [Google Scholar] [CrossRef] [PubMed]
- de Jong E, Lammerts MUPA, Genders RE, Bouwes Bavinck JN. Update of advanced cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2022, 36, 6–10. [Google Scholar] [CrossRef]
- Haegele JA, Hodge S. Disability Discourse: Overview and Critiques of the Medical and Social Models. Quest. 2016, 68, 193–206. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |