Introduction
As a chronic infectious inflammatory condition, the periodontal disease gradually deteriorates the tissues that support the teeth. In addition to damaging the adjacent bone and periodontal ligament, it also affects the immune cells [
1,
2]. Besides the slow and persistent bacterial colonization, the dysregulation of the immuno- inflammatory responses of the host, through elevated levels of pro-inflammatory mediators produced in the inflamed periodontal tissues like interleukin 1, interleukin-6 (IL-6), C-reactive protein (CRP), tumor necrosis factor alpha (TNF-α), ultimately leads to important periodontal destructions [
3,
4]. Originally described as chemotactic mediators of leukocytes, the cytokines are now acknowledged to be secreted by a number of different cell types and can be expressed either constitutively or in response to inflammatory stimuli, such as bacterial infections [
5]. Gingival crevicular fluid (GCF) is an inflammatory exudate that accumulates within the gingival crevice. Its levels increase in patients with periodontal disease, reflecting the inflammatory state of the periodontium, but it also may be considered a true representation of systemic inflammation [
6].
In terms of cancer-related mortality worldwide, colorectal cancer (CRC) ranked third and is also the third most common cancer in the US, while in the European Union in 2020, it was placed on second place regarding both incidence and mortality [
7,
8]. Compared to males, women experience a 25% lower incidence and mortality rate [
9]. Strong associations between disease incidence and male sex or increased age have been regularly shown in epidemiological research. The occurrence of CRC can be attributed to inherited and environmental risk factors [
9,
10]. The proportion of individuals with CRC with a positive family history appears to range from 10% to 20%, with a variable risk that depends on the degree or number of the affected relatives and also the age of CRC diagnosis [
9,
10]. Despite the fact that several genome-wide association studies of colorectal cancer have successfully identified cancer susceptibility genes (common single- nucleotide polymorphisms) associated with CRC risk, the majority of heritability-causing factors are still elusive and need more research. A well-defined hereditary colorectal cancer syndrome affects a subpopulation of about 5-7% of patients with colorectal cancer [
9,
11]. Smoking, excessive alcohol consumption, increased body weight, eating red and processed meat, and other environmental lifestyle variables, that are generally controllable, raise the risk of CRC [
10].
Periodontitis susceptibility may increase the risk of CRC, suggesting that oral health may have a role in the development of CRC [
12]. A recent study reported no significant increase in malignancy in patients with periodontitis and stated that benign colorectal tumors are more likely to be developed in patients with periodontitis than malignant ones [
13], while other reports found a 21- 44% increased risk for the occurrence and development of CRC in patients with periodontitis [
8,
14].
The cytokines play a number of different roles, including cell survival, proliferation, angiogenesis, and migration, while in cancer, chemokines primarily control angiogenesis and stimulate tumor-specific immune responses [
5]. The colon has a variety of cell types that express inflammatory cytokine receptors and respond to cytokines, including TNF-α, IL-1β, IL-6, and others [
15]. TNF-α and IL-6 are important pro-inflammatory cytokines that play crucial roles in the development of CRC, as they are created by both the tumor cells themselves, as well as by the host cells in response to the tumor or other bacteria [
5,
16,
17,
18]. Activating the oncogenic transcription factors signal transducer and activator of transcription 3 (STAT3) and nuclear factor-kappa B (NF-kB) allows tumor- infiltrating leukocytes (TILs) to effectively promote the development of human CRC cell lines. Comparatively to those from lamina propria mononuclear cells (LPMCs), T cells from TILs exhibit a functional switch which leads them to release an abundance of cytokines associated to T helper type 17 (Th17), TNF-α, and IL-6. The mitogenic impact of supernatants on CRC cells is diminished by simultaneous neutralization of IL-17A and TNF-α, which disables NF-kB signaling, and IL-22 and IL-6, which disables STAT3 signaling [
16,
17].
This study was designed to evaluate the periodontal status, the levels of IL-6 and TNF-α in GCF and test the possible correlations between them in hospitalized patients with CRC.
Materials and Methods
Study Design, Setting
The current pilot study is a cross-sectional, transversal study, which was designed in accordance with the STROBE guidelines [
19].
The research was initiated after obtaining approval from the ethics committee. All procedures were approved by the Research Ethics Commission of the University of Medicine and Pharmacy of Craiova, no. 127/09.12.2019, and the Clinical County Hospital of Emergency of Craiova, no. 3273/21.01.2020, and were performed in accordance with the guidelines of the Declaration of Helsinki. All participants agreed to sign an informed consent form before participating.
The research was conducted from September 2021 to May 2022. The patients were selected from the 1st Department of Surgery of the Clinical County Hospital of Emergency of Craiova, Romania.
Participants
The study participants were patients with CRC addressing the 1st Department of Surgery of the Clinical County Hospital of Emergency of Craiova who received, after periodontal examination, the diagnosis of periodontitis, divided into two groups based on the stages of CRC depending on its spread (the lower the number, the less cancer has spread), according to the American Joint Committee on Cancer (AJCC) staging system [
20]: group A (stage III-IV) and group B (stage I-II).
The selection criteria for CRC patients consisted of adult patients (>18 years old) regardless of gender (i) with a confirmed diagnosis of CRC, through previous investigations; (ii) that could follow and understand basic instructions.
The selection criteria used for the inclusion of periodontitis patients followed the 2018 Classification of Periodontal Disease guidelines [
21,
22]. Patients with at least two non-adjacent teeth or a minimum of 5 teeth were diagnosed with periodontitis if they displayed interdental clinical attachment loss (CAL) at 2 non-adjacent teeth or more, vestibular/oral CAL higher than/equal to 3 mm, with probing pocket depth (PPD) greater than/equal to 3mm in minimum 2 teeth [
21,
22]. They presented stage I-IV periodontitis, but due to the small number of patients, the subdivision into subgroups based on both the stage of CRC and periodontitis was not performed.
General exclusion criteria were: (i) the refuse/ the inability to sign the informed consent form; (ii) the patient could not undergo an oral examination; (iii) anti- inflammatory or antibiotic medication in the last 30 days prior to initial sampling of gingival crevicular fluid; (iv) systemic diseases, such as diabetes mellitus, cardiovascular diseases, hepatic disorders and rheumatoid arthritis.
The sample of our study was represented by 74 patients with CRC, assigned to one of the two study groups, based on cancer’s stages, as follows: (i) stage III-IV: 51 patients (A-group); (ii) stage I-II: 23 patients (B-group).
Periodontal Examination
The patients received a clinical periodontal examination, by the same well-trained dentist (F.M.N.). With the exception of third molars and any remaining root tips, all teeth were examined using a UNC15 periodontal probe (Hu- Friedy, Chicago, IL, USA) at 6 sites for each tooth (mesio- vestibular, centro-vestibular, disto-vestibular, mesio-lingual, centro-lingual, and disto-lingual), regarding the immediate full millimeter. The following variables were recorded: PPD, CAL and bleeding on probing (BOP). CAL and PPD were measured in millimeters and for each patient, they were obtained by summing the measured values and dividing them by the number of examined sites. The percentage of probed pockets that bled was recorded in order to measure BOP.
Gingival Crevicular Fluid Sampling
Two GCF samples were collected from each participant for the immunological evaluation, with a 30-seconds interval between them. GCF was collected from the tooth with the deepest periodontal pocket, following the clinical periodontal evaluation. Each tooth was isolated using cotton rolls, and then dried by air, as a precaution against sample contamination. The supragingival plaque was eliminated using a different cotton roll. Using a sterile paper cone, size 60 (Dentsply-De-Trey®, Ballaigues, Switzerland), GCF samples were obtained using the intracrevicular method through the absorption technique. The paper cone was inserted in the gingival sulcus for 30 seconds and then placed into polyethylene microtubes with 50 µL saline buffer solution (PBS). The samples were conserved until they were used for immunological assessment by being kept at -80°C.
Immunological Assessment
The targeted pro-inflammatory mediators (IL-6 and TNF-α) from GCF samples were quantitatively assessed through an enzyme-linked immunosorbent assay (ELISA).
Invitrogen IL-6 Human ELISA Kit, ThermoFisher Scientific (Waltham, Massachusetts, USA; range 1.56-100 pg/mL), and Invitrogen TNF alpha Human ELISA Kit, ThermoFisher Scientific (Waltham, Massachusetts, USA; range 7.8-500 pg/mL) commercial kits were used in accordance with the manufacturer's instructions and recommended methodology. During the procedure, a common optical analyzer with a 450 nm wavelength was employed.
Statistical Analysis
All data analyses were performed using GraphPad Prism 9.3.1 (GraphPad Software, San Diego, CA, USA). Data expressed as mean, standard deviation and median were subjected to statistical analysis in order to detect differences between subgroups using the Mann–Whitney test, due to the small sample size. The existence of statistical correlations between the different datasets using Spearman’s coefficients was assessed (weak correlation rho = 0,2 – 0,4; moderate correlation rho = 0,4 – 0,6; strong correlation rho = 0,6 – 0,8; very strong correlation rho = 0,8 – 1). A p-value of less than 0.05 was considered to be statistically significant.
Results
Patients Data
The age of the 74 eligible patients ranged from 39 to 79 years, and the median age was 56.81 years (SD = 11.77). For patients with stage III-IV CRC (A-group), the median age was 61.33 (SD = 11.29), and for the patients with stage I-II CRC (B-group), the median age was 46.78 (SD = 4.22).
The study sample was distributed by gender: 43 were males (58.1%) and 31 were females (41.89%). In A-group, 29 (56.9%) of the participants were males, and in B-group, 14 (60.9%) of the participants were males.
Periodontal Parameters
In A-group, 82.35% (n=42) presented stage III-IV periodontitis and 17.64% (n=9) presented stage I-II periodontitis.
In B-group, 69.56% (n=16) presented stage I-II periodontitis and 30.43% (n=7) presented stage III-IV periodontitis. Periodontal parameters are described in
Table 1.
Statistical Differences between the Assessed Parameters
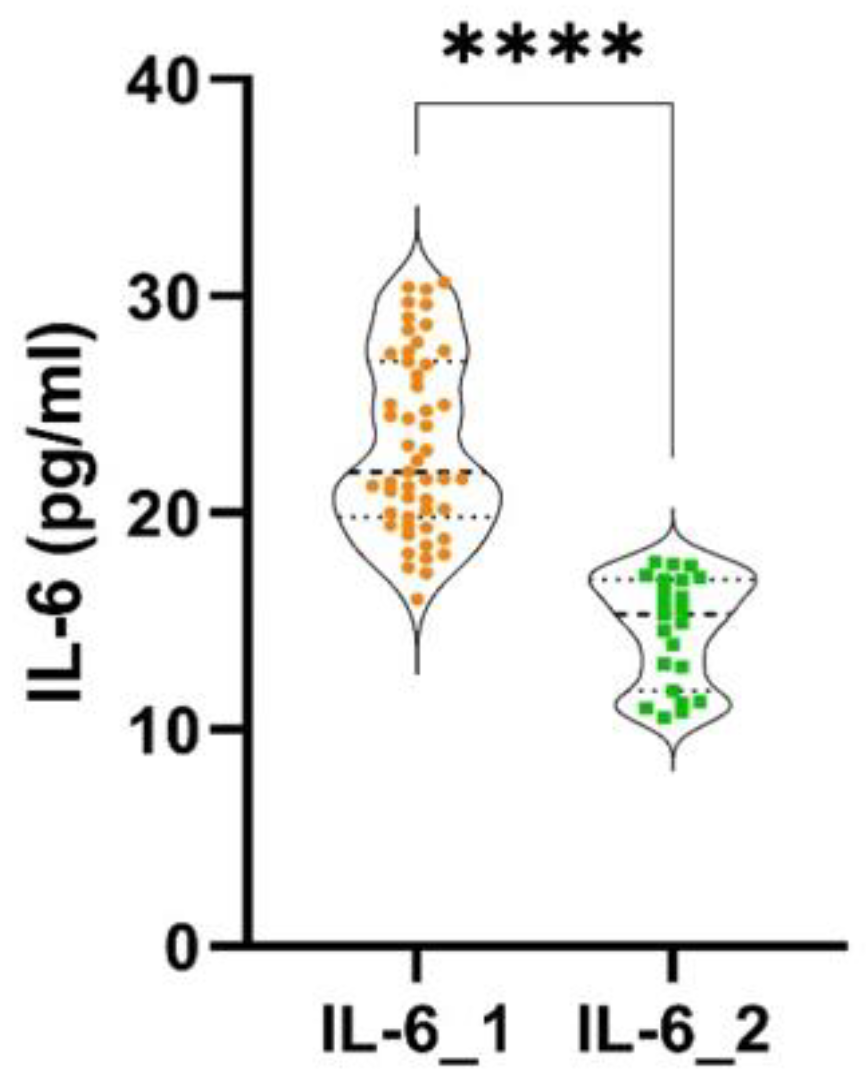
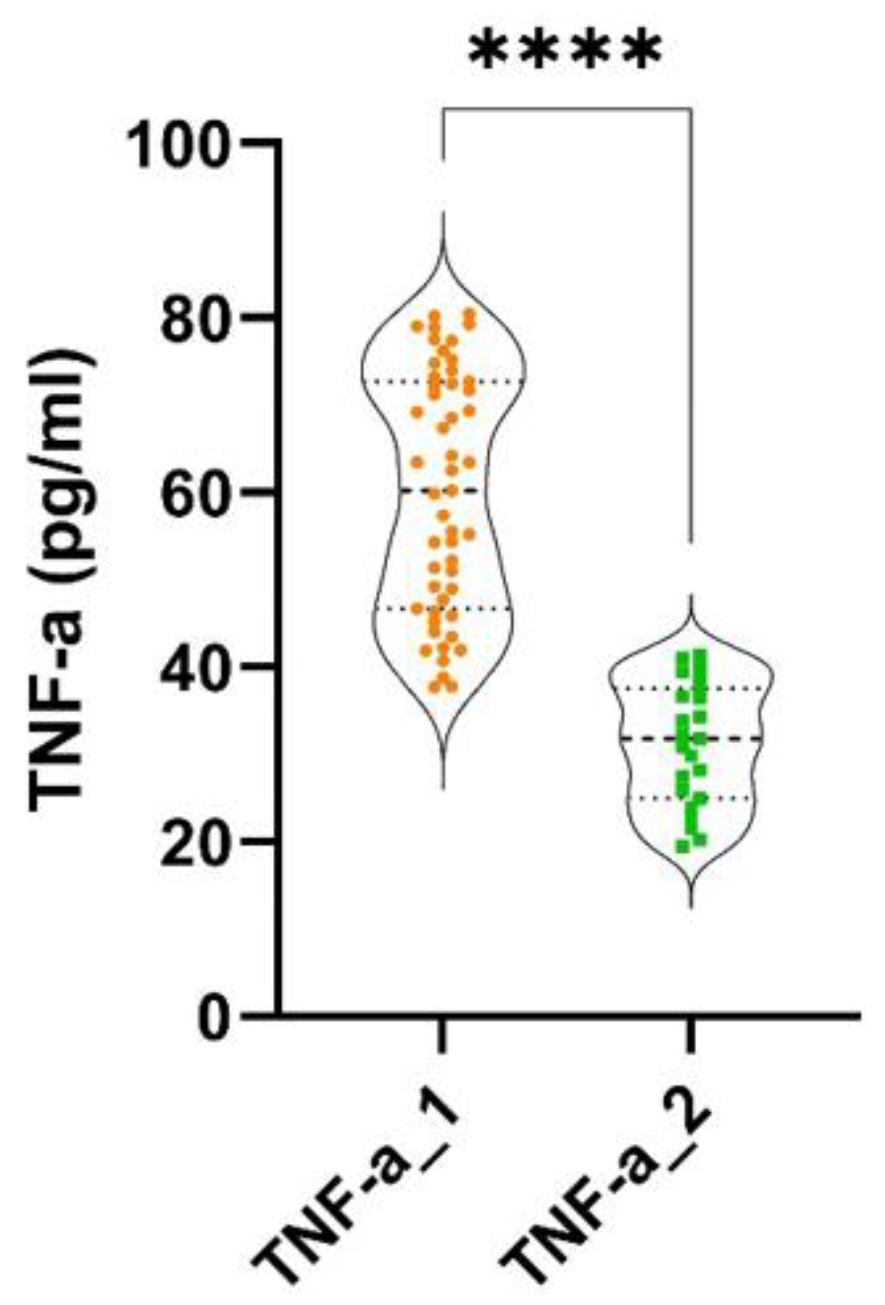
Statistically significant differences were found between the two groups, regarding probing pocket depth (PPD) (p < 0.0001), clinical attachment loss (CAL) (p < 0.0001) and bleeding on probing (BOP) (p < 0.0001). Statistically significant differences were discovered between the two groups, regarding the levels of IL-6 (p < 0.0001) and TNF-α (p < 0.0001), as shown in
Figure 1 and
Figure 2.
Correlations between Inflammatory Markers in Gingival Crevicular Fluid and Periodontal Indices in A-group
Very strong and statistically significant correlations were discovered between IL-6 and PPD (rho = 0.96, p < 0.0001) and between the levels of IL-6 and BOP (rho = 0.93, p < 0.0001). A moderate and negative statistically significant correlation was found between IL-6 and CAL (rho = -0.56, p < 0.0001) (
Table 2).
Very strong and statistically significant correlations were found between TNF-α and PPD (rho = 0.95, p < 0.0001) and between TNF-α and BOP (rho = 0.93, p < 0.0001). A moderate and negative statically significant correlation was discovered between TNF-α and CAL (rho = -0.6, p < 0.0001) (
Table 2).
Correlations between Inflammatory Markers in Gingival Crevicular Fluid and Periodontal Indices in B-group
A moderate and statistically significant correlation was found between the levels of IL-6 and PPD (rho = 0.47, p = 0.031). A strong and statistically significant correlation was found between the levels of IL-6 and BOP (rho = 0.76, p = 0.028). A weak and negative statistically not significant correlation was discovered between IL-6 and CAL (rho = - 0.33, p > 0.05) (
Table 3).
Moderate and statistically not significant correlations were found between the levels of TNF-α and PPD (rho = 0.48, p > 0.05) and between TNF-α and BOP (rho = 0.51, p > 0.05). A weak and negative statistically not significant correlation was found between TNF-α and CAL (rho = - 0.36, p > 0.05) (
Table 3).
Discussions
Our research revealed statistically significant differences regarding the periodontal parameters investigated between groups. A-group showed increased values for PPD, CAL and BOP. In our study, 82.35% of the patients from A-group, with stage III-IV CRC, had extensive forms of periodontitis, stages III or IV periodontitis, while 69.56% of the patients from B-group with stage I-II CRC, presented with stages I or II periodontitis. These findings suggest a possible association between the severities of both diseases, CRC and periodontitis.
Due to the small number of patients, their division into sub-groups based on both the stage of CRC and the stage of periodontitis was not achieved, but the increased prevalence of certain stages of periodontitis, depending on the cancer stage, might point towards a positive association between the severity of both diseases, CRC and periodontitis. These associations and correlations motivate future research on a higher sample of patients for a better understanding of the bi-directional relationship. There is not much epidemiological research examining a possible link between periodontal parameters and CRC. However, various mechanisms, including inflammation, nutrition, and perhaps bacterial infection, support this connection [
23], but to our knowledge, more accurate studies regarding the clinical parameters of periodontal disease in CRC patients have not been developed. It was hypothesized that periodontitis makes it easier for germs to spread to other regions of the body, where dysbiosis, persistent inflammation and epithelial barrier breakdown mix together to induce carcinogenesis [
24]. Moreover, CRC mortality was elevated in the presence of periodontal disease [
25], and in individuals who have had gastrointestinal surgery, periodontal disease is an independent risk factor for post- operative infectious complications [
26].
Neoplastic tissue formation has been connected to low- grade systemic inflammation [
27]. According to research undertaken over the past few decades, chronic systemic inflammation, which is also present in periodontitis, has a variety of consequences on the development of CRC, including the induction of gene mutations, promoting primary tumor invasion, suppression of apoptosis, proliferation of tumorous tissues, promotion of cell growth, angiogenesis, metastasis and inhibiting anti-tumor immunity [
28,
29,
30]. It has been shown that IL-6 increases chemotherapeutic resistance in CRC by activating autophagy via the IL-6/JAK2/BECN1 pathway. The association between JAK2 and BECN1, in which JAK2 phosphorylates BECN1 at Y333, is initiated mechanistically by IL-6. Through controlling PI3KC3 complex development, BECN1 Y333 phosphorylation is essential for BECN1 activation and IL-6-induced autophagy. Additionally, BECN1 Y333 phosphorylation has been proposed to be an indicator for chemotherapy resistance and poor CRC prognosis [
30].
The correlations between the pro-inflammatory markers analyzed (IL-6 and TNF-α) and the periodontal parameters (PPD, CAL, BOP) were very strong and statistically significant in patients with stage III-IV CRC, whereas the same correlations in B-group were weaker and statistically not significant. The fact that the correlations were stronger in patients with more advanced CRC stages might point to a complex relationship between the severity of periodontitis, pro-inflammatory makers and the evolution of CRC. Recent research has demonstrated that the systemic inflammatory response influences the interactions between cancerous and healthy cells, promoting tumor growth even before metastatic dissemination [
29]. The role of cytokines in the initiation, promotion, invasion, and metastasis of cancer is supported by a wealth of data. During a chronic inflammatory process, like periodontitis, cytokines like TNF-α and IL-6 stimulate the production of free radicals that can damage DNA and perhaps result in mutations that will develop tumors. Pro-inflammatory cytokines that increase cell proliferation and decrease apoptosis are also favorable for tumor growth, whereas anti-inflammatory cytokines aid in tumor immune evasion. TNF-α and IL-6 are pro- inflammatory cytokines that increase the invasiveness of malignancies and are crucial for metastasis and angiogenesis [
31,
32,
33,
34]. In a mouse model of sporadic CRC, excessive amounts of IL-17A, IL-21, IL-22, TNF-α and IL-6 are also generated, and this has been associated to enhanced STAT3/NF-kB activation. BP-1-102, an oral bioavailable drug that inhibits STAT3/NF-kB activation and cross-talk, reduces colon tumorigenesis in mice and decreases the production of STAT3/NF-kB-activating cytokines in the tumor-bearing regions [
16].
Our patients with stage III-IV CRC showed increased GCF levels of IL-6, in comparison with stage I-II patients, highlighting a possible involvement of the cytokine in more advanced CRC stages. Patients who had greater amounts of IL-6 in their CRC tissues had frequent metastasis and shorter overall survival times than those who had lower levels of IL-6 expression [
17,
29,
31,
35]. IL- 6 is significantly elevated in CRC patients compared to healthy controls. In mouse models, the tumors with overexpression of IL-6 tended to grow more quickly than those with IL-6 deletion, as IL-6 promotes the inhibition of apoptosis, thus promoting proliferation. It also stimulates tumor growth by facilitating the transformation of non- cancerous cells into tumor stem cells. In CRC, the IL- 6/JAK2/BECN1 pathway of IL-6 triggers autophagy and enhances chemotherapy resistance [
30]. Additionally, because IL-6 draws a variety of immune cells into the tumor microenvironment, it promotes the release of other pro-inflammatory cytokines [
30].
Significant differences regarding the levels of TNF-α were revealed between the two groups, with more advanced stages of CRC patients presenting higher values of this pro-inflammatory marker. This could suggest a possible implication of the pro-inflammatory maker in the evolution and development of CRC. Early phases of carcinogenesis, such as angiogenesis and invasion, appear to be more affected by TNF-α than later stages of carcinogenesis, as it was suggested that it induces DNA damage, thereby promoting tumorigenesis [
31]. It was demonstrated that the anti-TNF treatment, which targets inflammation, inhibited the development of CRC [
36].
In certain, but not all studies, plasma levels of specific pro-inflammatory cytokines or inflammatory biomarkers were linked to an elevated risk of CRC [
12,
15]. Even though we did not investigate the serum levels of IL-6 or TNF-α, our results showed that their GCF levels were significantly higher in patients diagnosed with stage III-IV CRC. When compared to healthy controls, individuals with CRC had statistically significant higher blood levels of IL- 1β, IL-6, IL-8 and TNF-α. The concentrations of IL-1β were strongly associated with the clinical characteristics of the tumors, while the levels of TNF-α were frequently connected with more advanced CRC stages and bone metastasis [
17,
29,
31,
34]. TNF-α starts an inflammatory cascade that activates and promotes CRC development in vivo, and anti- TNF-α antibodies were proven to reduce metastasis [
17,
31]. Another study reported that the mean scores for the IL-6 serums of the CRC group and the healthy group did not differ significantly, while the TNF-α serum level of the CRC group was statistically significantly lower than that of the control group [
33].
Additionally, it has been observed that individuals with recurring malignancies had higher circulating IL-6 levels, which are also reportedly enhanced by chemotherapy and radiation. Circulating IL-6 levels have been demonstrated to be predictive markers of survival and predictors of response to therapy in numerous forms of cancer, and they often correlate with tumor size, stage, and metastasis in patients with CRC [
30].
Increased blood levels of IL-6 and TNF-α are directly related to the clinical stage of the illness and have a strong association with CRC. This can be used to find patients with a poor prognosis and diagnose cancer in earlier stages [
33,
37]. Combining treatment with autophagy inhibitors or medications that block the IL-6/JAK2/BECN1 signaling pathway may be a promising way for managing CRC cancer [
30].
Recent findings imply that greater focus should be placed on preventive dental health interventions and that patients with periodontitis may benefit from improved cancer surveillance [
38]. In addition, as part of the CRC treatment plan, periodontal therapy and good periodontal health would indirectly reduce systemic inflammation and, therefore, the environment that promotes CRC development [
4].
A 21-day period of experimental gingivitis induction was shown to significantly elevate levels of CRP and IL-6 in research on participants who were otherwise healthy [
39]. Moreover, the considerable decrease in dental plaque and gingival inflammation seen in individuals with generalized gingivitis is linked to systemic effects since there was a decrease in overall inflammation, determined by serum CRP and IL-6 levels [
39].
According to some research, inhibiting TNF signaling may be able to stop tumor growth even in cases where CRC is already present, potentially leading to the development of efficient anti-cancer treatments [
29]. Preclinical and translational data indicate that IL-6 plays significant roles in tumor malignancies and that when IL-6 is overexpressed, IL-6 signaling inhibition may be therapeutic for cancer as its expression is correlated with cancer medication resistance [
35].
Therefore, the major, long-term effects of periodontal disease on systemic health can be lessened and controlled by preventing or identifying it early and also the advantages of periodontal therapy for restoring disturbed homeostasis arise [
24,
40,
41]. Oral healthcare professionals should concentrate on these specific individuals through early detection prevention programs, campaigns to promote oral health, accurate diagnosis techniques, and appropriate therapies. Additionally, all medical workers must contribute to a holistic concept of health that does not separate the mouth from the body. The connection between periodontal disease and other systemic chronic inflammatory illnesses will become more important since there are several therapeutic methods, such as cytokine- based therapy approaches, that have the potential to enhance both periodontal disease and systemic health [
24,
41].
A wide range of exclusion criteria, like anti- inflammatory or antibiotic medication in the last 30 days prior to sampling and systemic diseases, such as diabetes mellitus, cardiovascular diseases, hepatic disorders and rheumatoid arthritis, were used in order to produce the most accurate results possible; thus, a reduced number of patients were included in the study. Because our research was implemented during the COVID-19 pandemic, the number of patients decreased even more, which could be considered a limitation of the study. Due to the small number of patients, it was not possible to divide the patients into subgroups based on both the stage of CRC and the stage of periodontitis. Further research that includes a larger number of patients would be needed, in order to split the participants in several study groups, according to the pathologies they have.
Contributions
Conceptualization, F.M.N., S.R, A.S., D.N.M., P.S.; methodology, F.M.N., M.V.B., P.S.; validation, A.S., D.N.M., D.M.P.; formal analysis, M.V.B., A.T.S.; investigation, F.M.N., D.N.G., B.S.U., D.N.M., S.R.; data curation, F.M.N., D.N.G., D.M.P.; writing—original draft preparation, F.M.N., A.S., D.N.G., D.M.P., B.S.U., M.V.B., P.S.; writing—review and editing, F.M.N., S.R., D.N.M., A.T.S., V.M.S., D.I.G., P.S.; visualization, F.M.N., A.S., P.S.; supervision, V.M.S., D.I.G., P.S.