Best Therapeutic Practices in the Management of Obstetric Sepsis
Abstract
:Introduction
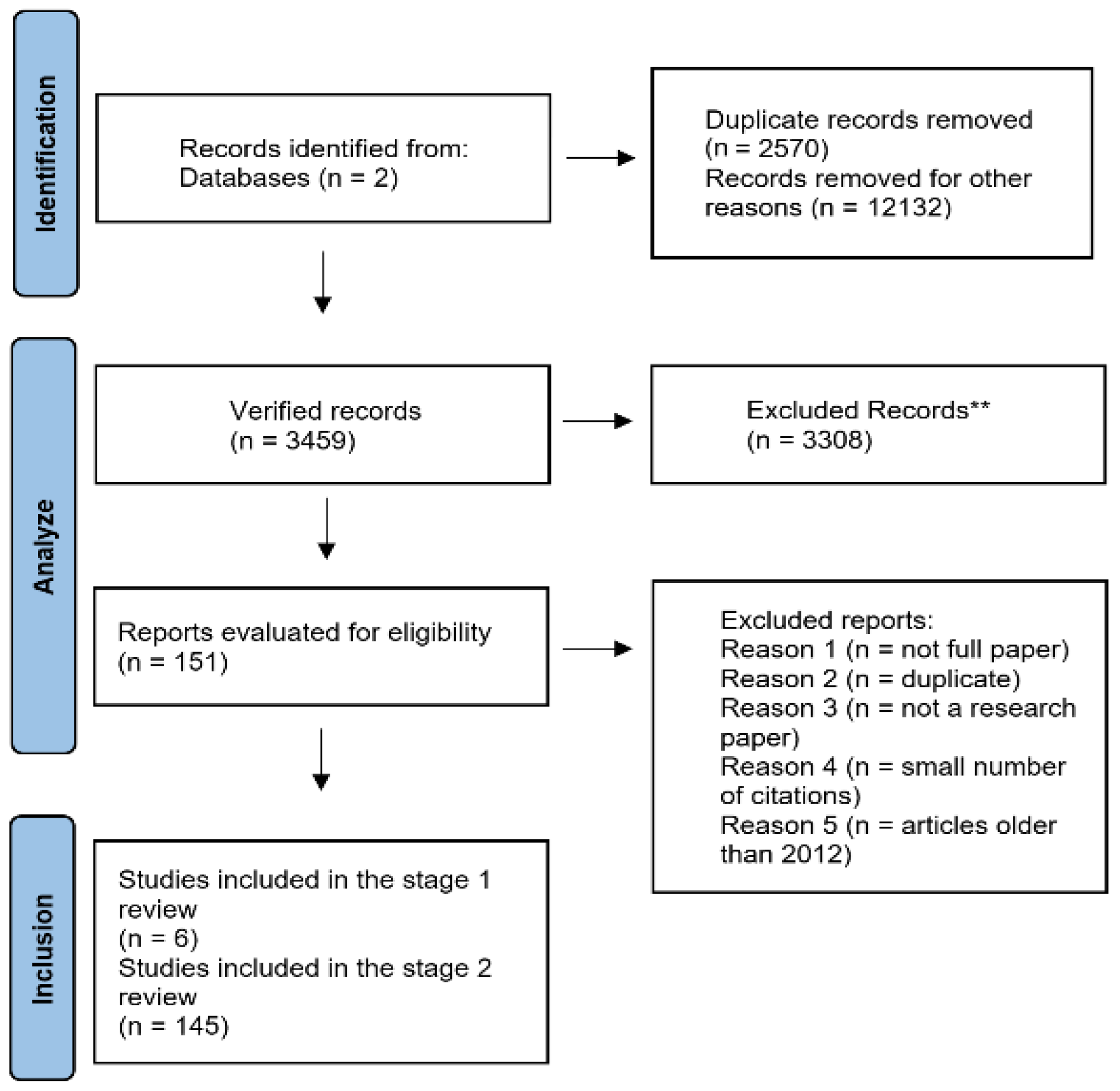
Materials and Methods
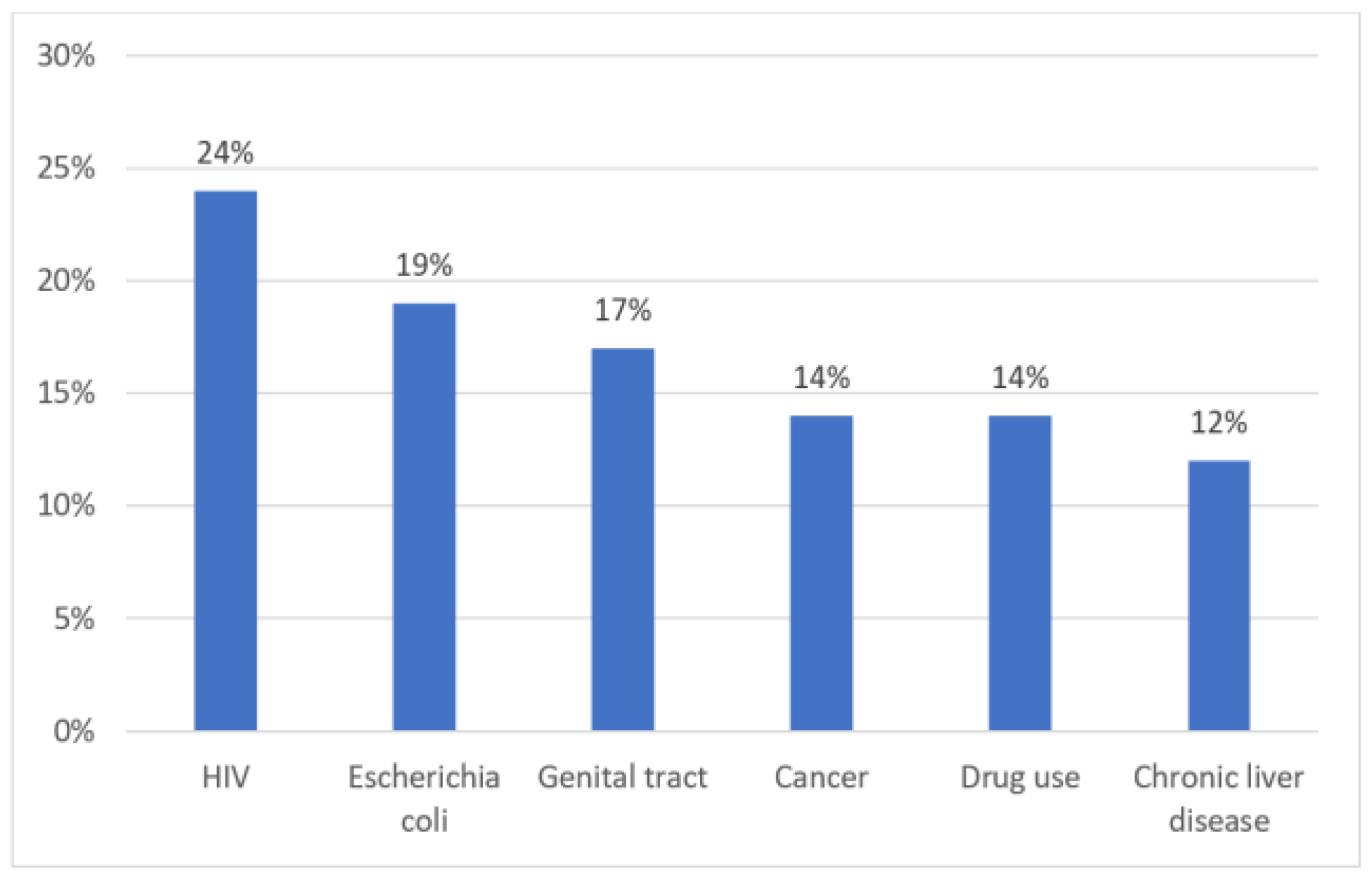
Results
Discussions
Conclusions
Compliance with ethical standards
Conflict of interest disclosure
References
- Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Giouleka S, Boureka E, Tsakiridis I, et al. Sepsis in Pregnancy and the Puerperium: A Comparative Review of Major Guidelines. Obstet Gynecol Surv. 2023, 78, 237–248. [Google Scholar] [CrossRef]
- Jury I, Thompson K, Hirst JE. A scoping review of maternal antibiotic prophylaxis in low- and middle- income countries: Comparison to WHO recommendations for prevention and treatment of maternal peripartum infection. Int J Gynaecol Obstet. 2021, 155, 319–330. [Google Scholar] [CrossRef]
- Escobar MF, Echavarría MP, Zambrano MA, et al. Maternal sepsis. Am J Obstet Gynecol MFM. 2020, 2, 100149. [Google Scholar] [CrossRef]
- Lucas DN, Robinson PN, Nel MR. Sepsis in obstetrics and the role of the anaesthetist. Int J Obstet Anesth. 2012, 21, 56–67. [Google Scholar] [CrossRef]
- Abutheraa N, Grant J, Mullen AB. An Observational Cohort Study Evaluating Antimicrobial Use in Peripartum Sepsis: A Tendency towards Overdiagnosis? Pharmacy (Basel). 2020, 8, 211. [Google Scholar] [CrossRef]
- Lapinsky, SE. Obstetric infections. Crit Care Clin. 2013, 29, 509–520. [Google Scholar] [CrossRef]
- Brizuela V, Cuesta C, Bartolelli G, et al. Availability of facility resources and services and infection-related maternal outcomes in the WHO Global Maternal Sepsis Study: a cross-sectional study. Lancet Glob Health. 2021, 9, e1252–e1261. [Google Scholar] [CrossRef]
- Bonet M, Nogueira Pileggi V, Rijken MJ, et al. Towards a consensus definition of maternal sepsis: results of a systematic review and expert consultation. Reprod Health. 2017, 14, 67. [Google Scholar] [CrossRef]
- Filetici N, Van de Velde M, Roofthooft E, Devroe S. Maternal sepsis. Best Pract Res Clin Anaesthesiol. 2022, 36, 165–177. [Google Scholar] [CrossRef]
- Stanescu AD, Balalau DO, Ples L, Paunica S, Balalau C. Postpartum depression: Prevention and multimodal therapy. J Mind Med Sci. 2018, 5, 163–168. [Google Scholar] [CrossRef]
- Brown KN, Arafeh JM. Obstetric Sepsis: Focus on the 3-Hour Bundle. J Perinat Neonatal Nurs. 2015, 29, 213–221. [Google Scholar] [CrossRef]
- Islam MM, Nasrin T, Walther BA, et al. Prediction of sepsis patients using machine learning approach: A meta-analysis. Comput Methods Programs Biomed. 2019, 170, 1–9. [Google Scholar] [CrossRef]
- Albright CM, Ali TN, Lopes V, et al. The Sepsis in Obstetrics Score: a model to identify risk of morbidity from sepsis in pregnancy. Am J Obstet Gynecol. 2014, 211, 39.e1–39.e398. [Google Scholar] [CrossRef]
- Aarvold AB, Ryan HM, Magee LA, von Dadelszen P, Fjell C, Walley KR. Multiple Organ Dysfunction Score Is Superior to the Obstetric-Specific Sepsis in Obstetrics Score in Predicting Mortality in Septic Obstetric Patients. Crit Care Med. 2017, 45, e49–e57. [Google Scholar] [CrossRef]
- Leisman DE, Doerfler ME, Ward MF, et al. Survival Benefit and Cost Savings From Compliance With a Simplified 3-Hour Sepsis Bundle in a Series of Prospective, Multisite, Observational Cohorts. Crit Care Med. 2017, 45, 395–406. [Google Scholar] [CrossRef]
- Ferrer R, Martin-Loeches I, Phillips G, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014, 42, 1749–1755. [Google Scholar] [CrossRef]
- Holanda AMC, de Amorim MMR, Bezerra SMB, Aschoff LMS, Katz L. Risk factors for death in patients with sepsis admitted to an obstetric intensive care unit: A cohort study. Medicine (Baltimore). 2020, 99, e23566. [Google Scholar] [CrossRef]
- Al-Ostad G, Kezouh A, Spence AR, Abenhaim HA. Incidence and risk factors of sepsis mortality in labor, delivery and after birth: population-based study in the USA. J Obstet Gynaecol Res. 2015, 41, 1201–1206. [Google Scholar] [CrossRef]
- Shields A, de Assis V, Halscott T. Top 10 Pearls for the Recognition, Evaluation, and Management of Maternal Sepsis. Obstet Gynecol. 2021, 138, 289–304. [Google Scholar] [CrossRef]
- Loghin MG, Gorescki PG, Sima RM, Pleș L, Balan DG, et al. The obstetrical management of HIV-positive pregnancy. J Mind Med Sci. 2022, 9, 111–117. [Google Scholar] [CrossRef]
- Bowyer L, Robinson HL, Barrett H, et al. SOMANZ guidelines for the investigation and management sepsis in pregnancy. Aust N Z J Obstet Gynaecol. 2017, 57, 540–551. [Google Scholar] [CrossRef]
- Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med. 2018, 44, 925–928. [Google Scholar] [CrossRef]
- Woodd SL, Montoya A, Barreix M, et al. Incidence of maternal peripartum infection: A systematic review and meta-analysis. PLoS Med. 2019, 16, e1002984. [Google Scholar] [CrossRef]
- Iancu G, Serban D, Badiu CD, et al. Tyrosine kinase inhibitors in breast cancer (Review). Exp Ther Med. 2022, 23, 114. [Google Scholar] [CrossRef]
- Bebell LM, Ngonzi J, Siedner MJ, et al. HIV Infection and risk of postpartum infection, complications and mortality in rural Uganda. AIDS Care. 2018, 30, 943–953. [Google Scholar] [CrossRef]
- Knowles SJ, O’Sullivan NP, Meenan AM, Hanniffy R, Robson M. Maternal sepsis incidence, aetiology and outcome for mother and fetus: a prospective study. BJOG. 2015, 122, 663–671. [Google Scholar] [CrossRef]
- Serban D, Badiu DC, Davitoiu D, et al. Systematic review of the role of indocyanine green near-infrared fluorescence in safe laparoscopic cholecystectomy (Review). Exp Ther Med. 2022, 23, 187. [Google Scholar] [CrossRef]
- Wilkie GL, Prabhu M, Ona S, et al. Microbiology and Antibiotic Resistance in Peripartum Bacteremia. Obstet Gynecol. 2019, 133, 269–275. [Google Scholar] [CrossRef]
- Acosta CD, Kurinczuk JJ, Lucas DN, et al. Severe maternal sepsis in the UK, 2011-2012: a national case- control study. PLoS Med. 2014, 11, e1001672. [Google Scholar] [CrossRef]
- Polprasarn P, Thongwon T. Modified Early Obstetric Warning Criteria Predict Maternal Morbidity in the Immediate Postpartum Period: A Case-Control Study. Nurs Womens Health 2023. [Google Scholar] [CrossRef]
- Attia Hussein Mahmoud H, Parekh R, Dhandibhotla S, et al. Insight Into Neonatal Sepsis: An Overview. Cureus. 2023, 15, e45530. [Google Scholar] [CrossRef]
- Balki I, Baghirzada L, Walker A, Lapinsky S, Balki M. Incidence, morbidity, and associated factors for sepsis in women hospitalized for delivery: a nationwide retrospective observational population-based study in Canada. Can J Anaesth. 2022, 69, 298–310. [Google Scholar] [CrossRef]
- Bălălău OD, Bacalbașa N, Stănescu DA. Cesarean scar defects and placental abnormalities: a three-year survey study. J Mind Med Sci. 2017, 4, 156–162. [Google Scholar] [CrossRef]
- Pohl N, Bauer ME, Pancaro C. Characteristics and Outcomes of Obstetric Sepsis by Three Sets of Clinical Diagnostic Criteria - a Retrospective Study. Am J Perinatol 2023. [Google Scholar] [CrossRef]
- Olaru OG, Stanescu AD, Raduta C, et al. Caesarean section versus vaginal birth in the perception of woman who gave birth by both methods. J Mind Med Sci. 2021, 8, 127–132. [Google Scholar] [CrossRef]
- Guo L, Han W, Su Y, et al. Perinatal risk factors for neonatal early-onset sepsis: a meta-analysis of observational studies. J Matern Fetal Neonatal Med. 2023, 36, 2259049. [Google Scholar] [CrossRef]
- Wieser MN, Gourounti K, Sarantaki A. Modes of birth and their impact on the psychological and physical health of women. J Mind Med Sci. 2021, 8, 1–4. [Google Scholar] [CrossRef]
- Society for Maternal-Fetal Medicine (SMFM), Shields AD, Plante LA, Pacheco LD, Louis JM; SMFM Publications Committee. Electronic address: pubs@smfm.org. Society for Maternal-Fetal Medicine Consult Series #67: Maternal sepsis. Am J Obstet Gynecol. 2023, 229, B2–B19. [Google Scholar] [CrossRef]
- He D, Zhang L, Hu H, et al. Effect of early vasopressin combined with norepinephrine on short-term mortality in septic shock: A retrospective study based on the MIMIC-IV database. Am J Emerg Med. 2023, 69, 188–194. [Google Scholar] [CrossRef]
| Purpose | A retrospective cohort study of pregnant and postpartum patients with suspected SIRS or sepsis was performed. |
| Number of subjects included | 850 women were included. |
| Inclusion criteria | Women at high risk of sepsis were included. Only those who had blood cultures or a flu swab sent to the clinical laboratory were included. Blood cultures or an influenza swab were used as surrogate markers for a patient presenting with signs or symptoms of sepsis. |
| Results | Of the 850 hospitalized patients. 9 were admitted to the intensive care unit (1.1%), 32 of the women were in the telemetry unit (3.8%), and none died. The most common diagnosis at presentation was influenza-like illness (ILI) (60.4%), followed by viral non-respiratory syndrome (11.1%), pyelonephritis (5.3%), endometritis (4.5%), pneumonia (2.4%), mastitis (1.2%), chorioamnionitis (0.7%) and septic abortion (0.6%). |
| Conclusions | A sepsis scoring system of the S.O.S. type (Obstetric Sepsis Score) was used for all pregnancy-specific physiological changes. This system was able to identify pregnant and postpartum patients at risk of admission to the intensive care unit for sepsis within 48 hours of presentation to the emergency department. |
| Limitations | The study is retrospective and was conducted at only one institution. Patients included were only those with signs of sepsis in the emergency department and not those who became septic after admission. |
| Purpose | The study aimed to analyze five mortality prediction scores (one obstetric-based and four general) in the septic obstetric population and compare them with a nonobstetric septic control group. |
| Number of subjects included | 797 women were included. |
| Inclusion criteria | The women were in the 16-50 age group with a diagnosis or suspicion of sepsis. All pregnant and postpartum patients up to 6 weeks postpartum were included. An age- and sex-matched non-obstetric control population was drawn from a single-center critical care population. |
| Results | The Obstetrics Sepsis Score, designed specifically for sepsis in obstetric populations, was no better than overall severity of illness scoring systems. Additionally, the Sepsis in Obstetric Performance Score was not different in an obstetric sepsis population compared to a nonobstetric sepsis population. |
| Conclusions | The obstetric-specific S.O.S. (Septic Obstetric Patients) score has been shown to have poor predictive value for mortality in both septic obstetric and nonobstetric populations. Also, disease severity scores based on organ failure, such as the MODS (Multiple Organ Dysfunction Score), are superior to the obstetric-specific SOS score in an obstetric population. Indeed, the MODS score performs equally well in obstetric and nonobstetric (age- and sex- equivalent) populations. |
| Limitations | Even though there was a large number of patients in the databases, the final figures of the septic cohort are small and therefore there is a significant geographical variation in the mortality figures. |
| Purpose | Determining mortality and costs associated with adherence to an aggressive 3-hour sepsis bundle versus nonadherence to a greater or equal bundle element for patients with severe sepsis and septic shock. | |
| Number of subjects included | Cohort 1: five tertiary and six community hospitals. Cohort 2: single tertiary, academic medical center. Cohort 3: five tertiary and four community hospitals. | |
| Inclusion criteria | Consecutive sample of all patients with severe sepsis and septic shock (defined as: infection, ≥ 2 systemic inflammatory response syndrome and hypoperfusion organ dysfunction) identified through a quality initiative. The exposure was full 3-hour bundle compliance. Bundle elements are as follows: 1) blood cultures before antibiotics; 2) parenteral antibiotics administered less than or equal to 180 minutes from greater than or equal to two systemic inflammatory response syndrome `and` lactate ordered, or less than or equal to 60 minutes from `time- zero`, whichever occurs earlier; 3) dairy result available less than or equal to 90 minutes postorder; and 4) 30 mL/kg IV crystalloid bolus initiated less than or equal to 30 minutes from `time zero`. | |
| Results | Cohort 1: 5,819 total patients; 1,050 (18.0%) bundle compliant. Mortality: 604 (22.6%) versus 834 (26.5%); CI, 0.9– 7.1%; adjusted odds ratio, 0.72; CI, 0.61– 0.86; p value is less than 0.001. Cohort 2: 1,697 total patients; 739 (43.5%) bundle compliant. Mortality: 99 (13.4%) versus 171 (17.8%), CI, 1.0– 7.9%; adjusted odds ratio, 0.60; CI, 0.44– 0.80; p value is equal to 0.001. Mean costs: $14,845 versus $20,056; CI, – $4,798 to –5,624; adjusted β, –$2,851; CI, –$4,880 to –822; p value is equal to 0.006. Cohort 3: 7,239 total patients; 2,115 (29.2%) bundle compliant. Mortality: 383 (18.1%) versus 1,078 (21.0%); CI, 0.9– 4.9%; adjusted odds ratio, 0.84; CI, 0.73– 0.96; p value is equal to 0.013. Mean costs: $17,885 versus $22,108; CI, – $2,783 to –5,663; adjusted β, –$1,423; CI, –$2,574 to –272; p value is equal to 0.015. | |
| Conclusions | In three independent cohorts, 3-hour bundle compliance was associated with improved survival and cost savings. | |
| Limitations | Compliant groups had lower frequency of some comorbidities and organ dysfunction criteria. Nonexperimental findings cannot show causality. | |
| Purpose | The aim of the study was to analyze the relationship between the timing of antibiotic administration and mortality. |
| Number of subjects included | One hundred sixty-five ICUs in Europe, the United States, and South America. |
| Inclusion criteria | 28,150 patients with severe sepsis and septic shock were entered. |
| Results | After diagnosis of sepsis, a total of 17,990 people were given antibiotics and then included in the analysis. The cohort as a whole had an in-hospital death rate of 29.7%. There was a statically significant increase in the probability of death associated with the number of hours of delay in first antibiotic administration. In-hospital mortality adjusted for severity (sepsis severity score), source of ICU admission (emergency department, ward, versus ICU), and geographic region increased steadily after 1 hour of time to antibiotic administration. Results were similar in patients with severe sepsis and septic shock, regardless of the number of organ failure. |
| Conclusions | Delay in first antibiotic administration was associated with increased in-hospital mortality. |
| Limitations | The appropriateness of antibiotic therapy in this patient population has not been analyzed. The study did not look at the reasons for the delay or the cause of the delay in antibiotic administration. |
| Purpose | The study aimed to analyze the risk factors for death in patients with sepsis admitted to the obstetric intensive care unit of a hospital. |
| Number of subjects included | 155 patients |
| Inclusion criteria | 155 patients with sepsis |
| Results | 14.2% (n= 22) died. Risk factors for death were septic shock at the time of hospitalization (relative risk [RR]= 3.45; 95% confidence interval [CI]: 1.64–7.25), need for vasopressors during hospitalization (RR= 17.32; 95% CI: 4.20 –71.36), lactate levels >2 mmol/L at diagnosis (RR=4.60; 95% CI: 1.05– 20.07) and Sequential Organ Failure Assessment score >2 at diagnosis (RR= 5.97; 95% CI: 1.82–19.94). |
| Following multiple logistic regression analysis, only the need for vasopressors during hospitalization remained as a risk factor associated with death (odds ratio [OR]= 26.38; 95% CI: 5.87–118.51). | |
| Conclusions | The need for vasopressors during hospitalization is associated with death in obstetric patients with sepsis. |
| Limitations | The analysis was performed only on one center. The study was based on the review of medical records, the fact that some data were missing may raise doubts about the chronology of certain events. |
| Purpose | Estimation of the incidence rate and mortality rate of sepsis, as well as the associated risk factors for their development during pregnancy, labor, delivery and the postpartum period. |
| Number of subjects included | 5 million births |
| Inclusion criteria | The 1998–2008 database from the Healthcare Utilization and Cost Project, death from sepsis during admission for delivery, was used. |
| Results | The overall incidence of maternal sepsis was 29.4 per 100,000 births (95% CI: 28.0–30.9) with a sepsis case fatality rate of 4.4 per 100 births (95% CI: 3.5-5.6). Both the incidence of maternal sepsis and the sepsis-related mortality rate have increased over the past decade. |
| Conclusions | Mortality from maternal sepsis during labor and delivery is a growing and important problem in Westernized countries. |
| Limitations | The study was limited to a single geographic area. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Gorecki, G.P.; Balalau, O.D.; Comandasu, D.E.; Stanescu, A.D.; Tomescu, D.R. Best Therapeutic Practices in the Management of Obstetric Sepsis. J. Mind Med. Sci. 2023, 10, 304-311. https://doi.org/10.22543/2392-7674.1436
Gorecki GP, Balalau OD, Comandasu DE, Stanescu AD, Tomescu DR. Best Therapeutic Practices in the Management of Obstetric Sepsis. Journal of Mind and Medical Sciences. 2023; 10(2):304-311. https://doi.org/10.22543/2392-7674.1436
Chicago/Turabian StyleGorecki, Gabriel Petre, Oana Denisa Balalau, Diana Elena Comandasu, Anca Daniela Stanescu, and Dana Rodica Tomescu. 2023. "Best Therapeutic Practices in the Management of Obstetric Sepsis" Journal of Mind and Medical Sciences 10, no. 2: 304-311. https://doi.org/10.22543/2392-7674.1436
APA StyleGorecki, G. P., Balalau, O. D., Comandasu, D. E., Stanescu, A. D., & Tomescu, D. R. (2023). Best Therapeutic Practices in the Management of Obstetric Sepsis. Journal of Mind and Medical Sciences, 10(2), 304-311. https://doi.org/10.22543/2392-7674.1436



