Abstract
Sarcopenic obesity involves the co-occurrence of sarcopenia and obesity, yields more health risks than either condition alone, thus requiring prevention and treatment for healthy aging. In this review, the literature on the diagnosis of sarcopenic obesity, the mechanisms of its pathogenesis and treatment with emphasis on exercise and dietary protein were revised. Changes in body composition and sex hormones with age, myocellular mechanisms, inflammation, oxidative stress, physical inactivity, and insufficient protein intake are the main factors associated with the pathogenesis of sarcopenic obesity. Treatment targets weight loss while preserving muscle mass and function. Promising approaches involve high-protein and low-calorie diets combined with aerobic and resistance exercises. In addition to the total amount of protein taken daily, the amount consumed per meal, the type, source, and essential amino acid content of the protein are also important factors.
Keywords:
sarcopenic obesity; sarcopenia; obesity; pathogenesis; therapy; exercise; diet; protein intake Introduction
The proportion of the elderly population in the world has been increasing over the years. While the population aged 60 and over was 1 billion, aged 65 and over was 700 million in 2019, these numbers are estimated to reach 2.1 billion and 1.5 billion, respectively, in 2050 [1,2]. The increase in the elderly population in society brings about a rapid rise in the importance of health problems in this age group. Sarcopenia is one of the most prominent health problems in the elderly. A study of community-dwelling older adults (mean age 67 years) in the UK found the prevalence of sarcopenia to be 4.6% in men and 7.9% in women [3]. A study conducted in the United States reported that the prevalence of sarcopenia was 36.5% in older adults with a mean age of 70.1 years [4].
Obesity is a significant health problem that has become an epidemic, its prevalence increasing almost threefold since 1975 [5]. Both sarcopenia [6] and obesity [7] are associated with many metabolic and cardiovascular diseases. Sarcopenic obesity, which means the coexistence of these two conditions, shows more negative effects on morbidity and mortality through a synergistic effect [8,9,10]. Sarcopenic obesity is associated with a higher risk of diabetes [11], dyslipidemia [11], metabolic syndrome [2], restrictive lung disease [12], osteoporosis [13]. Therefore, it is necessary to prevent or treat sarcopenic obesity for healthy aging.
Although sarcopenic obesity is associated with aging, it is a preventable and treatable condition [14]. However, there is no approved drug therapy for the treatment of sarcopenic obesity yet. Therefore, lifestyle interventions consisting of nutrition and exercise form the cornerstone of treatment [9,10,15]. In the treatment of sarcopenic obesity, ESPEN stated that the primary goal is to maintain muscle strength and function, and the secondary goal is to provide body weight loss, but also emphasized that this loss should be from fat, not muscle [16]. However, for an effective treatment, the diagnosis of sarcopenic obesity and its pathophysiology should be well-known. Therefore, this review will examine the sarcopenic obesity diagnostic criteria, pathophysiology, and treatment strategies focusing on exercise and dietary protein intake and will address the issue of sarcopenic obesity holistically.
Discussions
Sarcopenic obesity is generally expressed as the coexistence of sarcopenia and obesity. Although there is no consensus on its definition, the criteria used in sarcopenia and obesity diagnosis also constitute the criteria for sarcopenic obesity [9]. However, there are different definitions in the literature regarding sarcopenia and obesity that confuses the diagnosis of sarcopenic obesity.
Sarcopenia
The diagnosis of sarcopenia is generally based on two or three parameters of low muscle mass, low muscle strength, and low physical performance [9]. While European Working Group on Sarcopenia in Older People (EWGSOP, 2010) [17] and Asian Working Group for Sarcopenia (AWGS, 2014) [18] recommended evaluating low muscle mass and low muscle strength or low physical performance parameters in the diagnosis of sarcopenia, International Working Group on Sarcopenia (IWGS, 2011) [19] and Society of Sarcopenia, Cachexia and Wasting Disorder (SSCWD, 2011) [20] suggested evaluating low muscle mass and low physical performance parameters without considering low muscle strength. On the other hand, the Foundation for the National Institutes of Health (FINH, 2014) [21] supported that all three parameters should be evaluated together in the definition of sarcopenia. The EWGSOP updated the sarcopenia diagnostic criteria in 2019 [22] and defined the cut-off points for these criteria (Table 1). Accordingly, it was emphasized that low muscle strength was the primary parameter in the definition of sarcopenia, and low physical performance was used to evaluate the degree of sarcopenia. While pre-sarcopenia is characterized by low muscle mass without impact on muscle strength or physical performance, sarcopenia is characterized by low muscle mass plus low muscle strength or low physical performance. In addition, meeting all three criteria indicates severe sarcopenia [17].

Table 1.
Criteria for the diagnosis of sarcopenia according to EWGSOP.
Obesity
Obesity is defined by the World Health Organization (WHO) as “abnormal or excessive fat accumulation that poses a health risk”. A BMI ≥30 kg/m2 based on body mass index (BMI) in the evaluation of obesity indicates the presence of obesity [5]. However, the fact that Chinese, Ethiopians, and Indonesians with similar BMI have higher fat percentages compared to Blacks and Polynesians [23] indicates that BMI alone is not a confident parameter for evaluating body fat percentages. Evaluation of waist circumference in addition to BMI for obesity diagnosis increases the reliability. But it should be underlined that the cut-off points for the diagnosis of obesity in waist circumference, as in BMI [24], differ according to ethnic groups [24,25,26]. In addition, changes in body composition with aging, such as shortening in stature, increase in adipose tissue, and muscle loss, make it inconvenient to use BMI in obesity diagnosis in the elderly [27]. Therefore, direct evaluation of body fat percentage in the diagnosis of sarcopenic obesity is seen as a more reliable method compared to BMI and waist circumference measurements [8,9]. Methods such as Dual Energy X-Ray Absorptiometry (DEXA), Bioelectric Impedance Analysis (BIA), Magnetic Resonance Imaging (MRI), and Computed Tomography (CT) can be used to evaluate body fat and muscle mass. It has been reported that DEXA can be used as a reference method in the determination of muscle and fat mass for the sarcopenia diagnosis [28].
Sarcopenic obesity
Recently, the European Society for Clinical Nutrition and Metabolism (ESPEN) and the European Association for the Study of Obesity (EASO) have published a consensus on a two-step algorithm for screening and identifying sarcopenic obesity [29]. Accordingly, two factors were identified in screening for sarcopenic obesity: (1) low skeletal muscle mass with high BMI or waist circumference and (2) low muscle function. In sarcopenic obesity screening, the diagnostic evaluation should be performed in individuals who meet both criteria. In addition, primarily decreased skeletal muscle function in the diagnosis of sarcopenic obesity; subsequent assessment of body composition (high-fat mass and low muscle mass) has been suggested. Cut-off points can be used in different ethnic groups for these parameters were also determined.
The differences in the parameters used in the diagnosis of sarcopenic obesity, the methods used in the evaluation of these parameters, and the cut-off points cause the rates in prevalence studies to differ. For example, in a study evaluating sarcopenic obesity with eight different definitions, the prevalence was found to vary between 4.4% and 84.0% in men and between 3.6% and 94% in women [30]. In addition, these differences cause variations in the evaluation, comparison, and monitoring of the effectiveness of the methods used in sarcopenic obesity treatment. For this reason, establishing agreed diagnostic criteria for sarcopenic obesity is critical in taking more appropriate steps on the subject.
Pathophysiology of Sarcopenic Obesity
The pathophysiology of sarcopenic obesity is multifactorial. Various risk factors consisting of changes in body composition and sex hormones due to aging, sedentary lifestyle, unhealthy eating habits, systemic inflammation, and oxidative stress are the primary mechanisms thought to be involved in the pathogenesis of sarcopenic obesity (Figure 1).
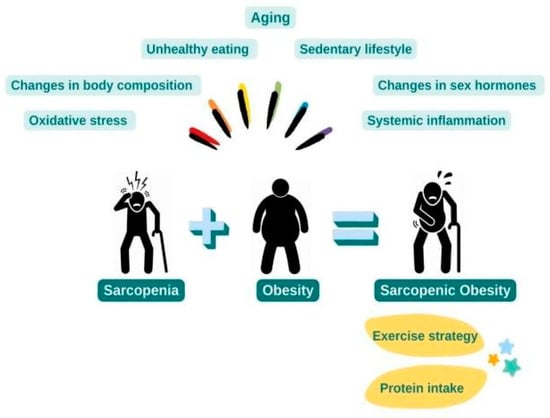
Figure 1.
Pathophysiology and Treatment of Sarcopenic Obesity.
Changes in body composition
It has been reported that lean body mass increases until the age of 45-55 in women and 60 years in men and then begins to decrease [31]. Skeletal muscle atrophy is inevitable with aging. Loss of muscle fibers starts around the age of the fifties and differs according to the usual physical activity level of the individual. It has also been suggested that these losses are parallel to the losses in the motor units in terms of timing and magnitude, so the mechanism responsible for both damages is the same [32].
The decrease in lean body mass, physical activity level, mitochondrial volume, and oxidative capacity with age causes the resting metabolic rate to slow down [10]. Decreased Resting energy expenditure and physical activity can lead to an increase in body fat tissue. However, Chumlea et al.'s study stated that total body fat increased for both genders until the ages of 55-65 years old and started to decrease in advanced old age [31]. This decrease in adipose tissue may be explained by reduced appetite and energy intake [33]. As a result, a decrease in lean tissue mass and an increase or decrease in fat mass may be observed with age. In addition, vertebral compression in old age can cause a shortening of stature and an increase in BMI even if the same body weight is maintained [34].
In addition to changes in adipose tissue, there are differences in the distribution of fat in the body with age. Intramuscular and hepatic lipid accumulations are higher in older adults than younger adults [35]. With advancing age, fats generally have an ectopic distribution in the liver, muscles, and pancreas [15,35,36,37].
Changes in sex hormones
Sex hormones have a strong effect on both body fat distribution and skeletal muscle mass. There are estrogen receptors in brown and white adipocytes, and estrogen shows its effects by binding to estrogen receptor-α in these cells. The binding of estrogen to the receptor in brown adipocytes activates AMP-protein kinase, which in turn activates the peroxisome proliferative activation receptor- gamma coactivator 1α, resulting in an increase in uncoupled protein 1 expression. Estrogen increases lipolysis and decreases adipogenesis by decreasing lipoprotein lipase activity and increasing beta-adrenergic receptor activity in white adipocytes [38].
The self-repairing and regenerating properties of skeletal muscles occur through satellite cells. Estrogen and testosterone have a regulatory effect on the proliferation of satellite cells. By activating satellite cells, these hormones can suppress inflammation in skeletal muscle and regenerate muscle tissue [39,40,41]. In addition, testosterone, an anabolic hormone, increases protein synthesis in muscle cells and reduces protein degradation, thus positively affecting muscle hypertrophy and myogenesis [42].
The decrease in sex hormones with age in women and men leads to the loss of the protective effects of these hormones on fat and muscle tissue, and as a result, the risk of sarcopenic obesity increases.
Inflammatory mechanisms
Physical inactivity, low protein intake, and increased adiposity increase the infiltration of immune cells into adipose tissue and the release of inflammatory cytokines from adipocytes [43]. Adipokines and cytokines released from adipocytes and immune cells cause low-grade chronic inflammation, increase insulin resistance, and promote ectopic fat distribution. Low-grade chronic inflammation and intramuscular lipid deposition lead to mitochondrial dysfunction. Therefore, mitochondrial β-oxidation of lipids is impaired, lipid peroxidation increases, and lipid intermediates and reactive oxygen metabolites accumulate in myocytes. All these processes cause further increases in insulin resistance, inflammation, oxidative stress, and lipotoxicity [9,10,43]. It was determined that the highest hs-CRP levels were observed in individuals with sarcopenic obesity in a large-scale study, and hs-CRP was 1.17, 2.23, and 3.23 times higher in groups with obesity, sarcopenia, and sarcopenic obesity, respectively, compared to healthy individuals [44].
Myoselüler mechanisms
With aging, both the amount and size of muscle fibers decrease [32,45]. However, age-related loss of skeletal muscle mass is primarily due to reductions in the size of muscle fibers, particularly type II muscle fibers [45]. Imbalances between muscle protein synthesis and breakdown cause muscle fiber atrophy, and it is thought that “anabolic resistance” underlies this deterioration [46]. Anabolic resistance is the reduction/impairment of muscle protein synthesis response to dietary protein intake. An increase in splanchnic amino acid sequestration and reductions in digestive and absorptive capacity, the presence of postprandial amino acids, muscle perfusion, uptake of dietary amino acids into muscles, and anabolic signal proteins are some of the causes of anabolic resistance in the elderly [47].
Intramyocellular lipid accumulation leads to mitochondrial dysfunction, the generation of reactive oxygen species, and increased inflammation. All these factors associated with obesity and aging cause demyelination, motor loss, and axonal atrophy. As a result, neuromuscular connections are weakened, and physical functions such as muscle contraction are reduced [43,48,49]. In addition, decreases in the signaling and functionality of muscle satellite cells during aging also cause decreases in muscle functions [50,51].
Treatment of Sarcopenic Obesity: Exercise and protein intake
The most promising option for sarcopenic obesity treatment is lifestyle changes consisting of diet and exercise. In this review, the subject of diet will be examined through the protein component of the diet (Figure 1).
Exercise
Physical inactivity is a risk factor for sarcopenic obesity [52]. Meta-analysis [53,54] and systematic reviews [55,56] of exercise intervention in individuals with sarcopenic obesity showed that exercises, especially resistance exercises, were effective in increasing muscle mass and reducing fat mass. Both effects of exercise are very important in the treatment. When the exercise types were examined, it was determined that the programs combining resistance and aerobic exercises were more effective in increasing muscle strength [55]. According to the meta- analysis study of Hsu et al. (2019), resistance exercises and a combination of aerobic and resistance exercises reduced body fat percentage more compared to aerobic exercises, grip strength increased only in resistance exercises, and walking speed increased in a combination of aerobic and resistance exercises [53]. However, studies show that most adults, especially the elderly, exercise much below the recommended level of exercise [57,58]. In a systematic review examining why osteopenia and osteoporosis patients do not follow exercise recommendations, many different factors were identified, such as lack of time, fear of falling or injury, problems in transportation, and the thought that physical activity is not necessary [59]. For this reason, the elderly should be informed about the necessity and importance of physical activity, and the conditions for safe exercise should be provided. Whole-body electromyostimulation, a new exercise concept activating up to 16 regions simultaneously, is one of the topics of interest in sarcopenic obesity treatment. According to Yang et al. (2022)'s meta-analysis [60], whole-body electromyostimulation significantly decreased the sarcopenia Z score and increased the skeletal muscle mass index and appendicular muscle mass in individuals with sarcopenic obesity.
Protein Intake
Studies on the use of dietary protein or protein supplements in the treatment of sarcopenic obesity are not as consistent as studies examining the effects of exercise in the treatment of sarcopenic obesity. Variations between studies are due to methodological differences such as dose of protein concentration, essential amino acid content, initial protein intake, intake days (training days only or daily), the timing of protein intake, length of intervention, and dietary protein source.
Dietary protein is essential for muscle protein synthesis as it provides the amino acids needed for muscle protein anabolism [61]. Therefore, inadequate protein intake is a risk factor for sarcopenic obesity in general [8]. Older men aged 65 years with dietary protein intakes below the recommended amount were found to be 5.82 times more at risk of osteosarcopenic obesity compared to those with protein intakes at or above the recommended protein intake [62]. A national study also showed that elderly with sarcopenia and osteosarcopenia had lower dietary protein intake than healthy ones [63].
As the muscle protein synthesis response to anabolic stimuli is blunted in the elderly, more protein is needed to stimulate muscle protein synthesis [64]. In addition, chronic diseases such as congestive heart failure, chronic kidney disease, and diabetes in the elderly increase the requirement for protein since they increase inflammation and metabolism [65]. In a study conducted on women with sarcopenic obesity, it was observed that normal (0.8 g/kg desirable body weight)- and high (1.2 g/kg desirable body weight)-protein hypocaloric diets (20-25 kcal/kg desirable body weight) were effective in reducing obesity-related parameters. But the increase in sarcopenia-related muscle mass index was only seen in the high-protein hypocaloric diet and reduced muscle mass index over time in the normal-protein hypocaloric diet [66]. In this context, evidence-based recommendations support 1.0-1.2 g/kg body weight protein intake to maintain and increase muscle mass in the elderly aged 65 and over. This amount increases to 1.2-1.5 g/kg for the elderly with chronic or acute illness and up to 2.0 g/kg for malnourished patients with severe disease or injury. However, patients with severe kidney disease who did not undergo dialysis were excluded from these recommendations, and it was recommended to limit their protein intake [16,67].
In addition to the daily protein intake, the amount of protein consumed per meal is also prominent. Loenneke et al. (2016) found that more frequent consumption of meals containing 30-45 g of protein 1-2 times/day in adults aged 50-85 was associated with more lean leg mass and strength, and accordingly, recommended that consumption of meals containing 30-45 g of protein was a considerable strategy for increasing or maintaining body mass and muscle strength in later ages [68]. According to evidence-based recommendations, the amount of dietary protein/amino acid to be taken per meal to stimulate postprandial muscle protein synthesis in the elderly is 25-30 g protein containing 2.5-2.8 g leucine [67]. In addition, it is recommended that the amount of protein should be distributed throughout the day to stimulate muscle protein synthesis after each meal [16,64].
Protein source
The source of protein in the diet and its essential amino acid content can regulate muscle protein synthesis. The anabolic effects of proteins from animal foods on muscle protein synthesis are higher than proteins from plant sources. This is due to the difference in digestibility, amount of essential amino acids (especially leucine), and type of proteins [69].
A meta-analysis conducted on middle and older adults with and without sarcopenic obesity concluded that milk and dairy products significantly increased appendicular muscle mass, did not affect muscle strength parameters, and their effects on muscle function were contradictory [70]. Among milk proteins, whey proteins are known to support muscle protein anabolism better than caseins [71] because whey proteins have higher digestion and absorption rates and leucine contents than caseins [72]. In the treatment of sarcopenia, it has been stated that rapidly digestible protein sources have an effect beyond the amount of protein in the diet, and fast-digestible dietary proteins such as whey protein, which are rich in essential amino acids, branched-chain amino acids, and leucine, are more effective in stimulating muscle protein synthesis [71].
Leucine is a branched-chain amino acid and role in initiating mRNA translation, which is required to stimulate protein synthesis by directly activating the mammalian target 1 (mTORC1) signaling pathway of the rapamycin protein complex in skeletal muscle [71,73]. A meta- analysis study evaluating the effects of leucine supplementation on body composition reported that leucine promotes an increase in lean body mass and this effect of leucine is particularly pronounced in the elderly with sarcopenia [74]. According to evidence-based recommendations, the dose of leucine required to achieve maximum stimulation of postprandial muscle protein synthesis in the elderly is approximately 3-4 g/meal, which requires consuming 25-30 g protein/meal [73]. Cereda et al. (2022) reported that oral supplements containing whey protein, leucine, and vitamin D are an effective treatment strategy for the elderly with sarcopenia, and these can be used not only to improve clinical outcomes but also to reduce resource consumption in healthcare [75]. Camajani et al. (2022) also supported this view. In this study, a low- calorie diet applied for 45 days in postmenopausal women with sarcopenic obesity and insulin resistance was supplemented with a mixed drink containing 18 g whey protein (4.1 g leucine) and vitamin D (5.01 mcg), resulting in a daily protein intake has been raised to approximately 1.4 g/kg. It has been determined that this diet prevents muscle mass loss and significantly improves muscle strength measured by grip strength and muscle function measured by short physical performance battery, besides improving parameters related to obesity and insulin resistance [76].
Exercise and Protein Intake
A systematic review [55] of randomized controlled studies examining the effects of exercise alone or combined with nutritional supplements on sarcopenic obesity parameters in individuals with sarcopenic obesity included three studies examining the intervention of protein supplementation. In these studies, 40 g of protein powder containing 21 g of whey and 2.8 g of leucine [77]; 3 g of the leucine [78] and whey protein supplement was used to complete the protein intake to 1.7-1.8 g/kg/day [79]. At the end of the review, it was reported that though it was difficult to reach a clear conclusion due to the differences in the methodologies of the studies, protein supplementation did not significantly affect muscle strength. These results were supported in later meta- analyses. Accordingly, protein supplementation [53,60] or low-calorie high-protein diets (20-25 kcal/kg/day, 1.2 g/kg/day) [53] without exercise intervention did not significantly affect muscle mass in individuals with sarcopenic obesity but provided significant improvements in obesity parameters. This systematic review [55] and meta-analyses [53,60] determined that protein supplementation or low-calorie high-protein diets combined with exercise did not benefit muscle mass and strength compared to exercise interventions alone. The ineffectiveness of these diets and supplements in increasing muscle mass may be due to the inadequacy of whey and leucine intake. Studies in healthy elderly have shown that whey protein taken at low doses may not stimulate postprandial muscle protein synthesis, 30-40 g of whey protein is required to stimulate muscle protein synthesis [80,81], and that the primary determinant of anabolic response of muscle is the amount of leucine rather than total protein intake [82]. For example, Nabuco et al. (2019) [83] also found that the use of 35 g of hydrolyzed whey protein (containing 3.8 g of leucine) after resistance exercise performed three times/week for 12 weeks in women with sarcopenic obesity resulted in a more increase in appendicular smooth muscle tissue compared to placebo.
Despite all these contradictions regarding dietary protein intake or protein supplements, low-calorie, high- protein diets supplemented with whey and leucine for sarcopenic obesity treatment are promising.
Conclusions
Sarcopenic obesity is a grave health problem with its increasing prevalence and negative effects on health. However, there is no consensus on its definition and diagnostic criteria; This significantly limits the evaluation of the efficacy of the treatments under investigation and the comparability of different studies and treatments. Therefore, establishing standard diagnostic criteria for diagnosing sarcopenic obesity is essential for scientific studies and clinical practices to be carried out healthily. Existing literature shows that aerobic and resistance exercises supplemented with high protein hypocaloric diets rich in rapidly digestible and essential amino acids, especially leucine, may be helpful for sarcopenic obesity treatment. However, the optimal protein amount and type have not been determined, and more well-planned randomized controlled studies are needed for this condition.
Highlights
- ✓
- Although there is no consensus on its definition and diagnostic criteria, sarcopenic obesity is generally expressed as the coexistence of sarcopenia and obesity.
- ✓
- The etiology of sarcopenic obesity entails a complex interplay of factors, encompassing aging, alterations in body composition and sex hormones, systemic inflammation, and myocellular mechanisms.
- ✓
- Aerobic and resistance exercises supplemented with high protein hypocaloric diets rich in rapidly digestible and essential amino acids may be helpful for sarcopenic obesity treatment.
Acknowledgments
The author thanks Mehmet Fisünoğlu, associate professor at Hacettepe University, who provided advice for the subject of this review.
Compliance with ethical standards
Any aspect of the work covered in this manuscript has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
Conflict of interest disclosure
There are no known conflicts of interest in the publication of this article.
References
- World Health Organization. Aging. Available online: https://www.who.int/health-topics/ageing#tab=tab_1 (accessed on 9 May 2023).
- United Nations, Department of economic and social affairs, population division (2017). World population prospects: The 2017 Revision, key findings, and advance tables. Working Paper No. ESA/P/WP/248. Available online: https://population.un.org/wpp/publications/files/wpp2 017_keyfindings.pdf (accessed on 9 May 2023).
- Patel HP, Syddall HE, Jameson K, et al. Prevalence of sarcopenia in community-dwelling older people in the UK using the European Working Group on Sarcopenia in Older People (EWGSOP) definition: findings from the Hertfordshire Cohort Study (HCS). Age Ageing 2013, 42, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Brown JC, Harhay MO, Harhay MN. Sarcopenia and mortality among a population-based sample of community-dwelling older adults. J Cachexia Sarcopenia Muscle 2016, 7, 290–298. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and overweight. Available online: https://www.who.int/news-room/fact- sheets/detail/obesity-and-overweight (accessed on 14 June 2022).
- Nezameddin R, Itani L, Kreidieh D, El Masri D, Tannir H, El Ghoch M. Understanding Sarcopenic Obesity in Terms of Definition and Health Consequences: A Clinical Review. Curr Diabetes Rev. 2020, 16, 957–961. [Google Scholar] [CrossRef]
- Safaei M, Sundararajan EA, Driss M, Boulila W, Shapi'i A. A systematic literature review on obesity: Understanding the causes & consequences of obesity and reviewing various machine learning approaches used to predict obesity. Comput Biol Med. 2021, 136, 104754. [Google Scholar] [CrossRef]
- Kim YJ, Moon S, Yu JM, Chung HS. Implication of diet and exercise on the management of age-related sarcopenic obesity in Asians. Geriatr Gerontol Int. 2022, 22, 695–704. [Google Scholar] [CrossRef]
- Polyzos SA, Margioris AN. Sarcopenic obesity. Hormones 2018, 17, 321–331. [Google Scholar] [CrossRef]
- Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef]
- Lim HS, Park YH, Suh K, et al. Association between Sarcopenia, Sarcopenic Obesity, and Chronic Disease in Korean Elderly. J Bone Metab. 2018, 25, 187–193. [Google Scholar] [CrossRef]
- Lee SE, Park JH, Kim KA, Kang YS, Choi HS. Association Between Sarcopenic Obesity and Pulmonary Function in Korean Elderly: Results from the Korean National Health and Nutrition Examination Survey. Calcif Tissue Int. 2020, 106, 124–130. [Google Scholar] [CrossRef]
- Chung JH, Hwang HJ, Shin HY, Han CH. Association between Sarcopenic Obesity and Bone Mineral Density in Middle-Aged and Elderly Korean. Ann Nutr Metab. 2016, 68, 77–84. [Google Scholar] [CrossRef]
- Lee DC, Shook RP, Drenowatz C, Blair SN. Physical activity and sarcopenic obesity: definition, assessment, prevalence and mechanism. Future Sci OA. 2016, 2, FSO127. [Google Scholar] [CrossRef] [PubMed]
- Petroni ML, Caletti MT, Dalle Grave R, Bazzocchi A, Aparisi Gómez MP, Marchesini G. Prevention and Treatment of Sarcopenic Obesity in Women. Nutrients. 2019, 11, 1302. [Google Scholar] [CrossRef]
- Deutz NE, Bauer JM, Barazzoni R, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef]
- Morley JE, Abbatecola AM, Argiles JM, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011, 12, 403–409. [Google Scholar] [CrossRef]
- Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef]
- Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord. 1998, 22, 1164–1171. [Google Scholar] [CrossRef]
- World Health Organization. Regional Office for the Western Pacific. (2000). The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia. Available online: https://apps.who.int/iris/handle/10665/206936.
- WHO Consultation on Obesity (1999: Geneva, Switzerland) & World Health Organization. (2000). Obesity: preventing and managing the global epidemic: report of a WHO consultation. World Health Organization. Available online: https://apps.who.int/iris/handle/10665/42330.
- Lee SY, Park HS, Kim DJ, et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract. 2007, 75, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo L, Itani L, Gualtieri P, Pellegrini M, El Ghoch M, De Lorenzo A. New BMI Cut-Off Points for Obesity in Middle-Aged and Older Adults in Clinical Nutrition Settings in Italy: A Cross-Sectional Study. Nutrients. 2022, 14, 4848. [Google Scholar] [CrossRef]
- Buckinx F, Landi F, Cesari M, et al. Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle. 2018, 9, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Donini LM, Busetto L, Bischoff SC, et al. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Obes Facts. 2022, 15, 321–335. [Google Scholar] [CrossRef]
- Batsis JA, Barre LK, Mackenzie TA, Pratt SI, Lopez- Jimenez F, Bartels SJ. Variation in the prevalence of sarcopenia and sarcopenic obesity in older adults associated with different research definitions: dual-energy X-ray absorptiometry data from the National Health and Nutrition Examination Survey 1999-2004. J Am Geriatr Soc. 2013, 61, 974–980. [Google Scholar] [CrossRef]
- Chumlea WC, Guo SS, Kuczmarski RJ, et al. Body composition estimates from NHANES III bioelectrical impedance data. Int J Obes Relat Metab Disord. 2002, 26, 1596–1609. [Google Scholar] [CrossRef]
- Faulkner JA, Larkin LM, Claflin DR, Brooks SV. Age- related changes in the structure and function of skeletal muscles. Clin Exp Pharmacol Physiol. 2007, 34, 1091–1096. [Google Scholar] [CrossRef]
- Clegg ME, Godfrey A. The relationship between physical activity, appetite and energy intake in older adults: A systematic review. Appetite 2018, 128, 145–151. [Google Scholar] [CrossRef]
- Xu W, Perera S, Medich D, et al. Height loss, vertebral fractures, and the misclassification of osteoporosis. Bone. 2011, 48, 307–311. [Google Scholar] [CrossRef]
- St-Onge, MP. Relationship between body composition changes and changes in physical function and metabolic risk factors in aging. Curr Opin Clin Nutr Metab Care 2005, 8, 523–528. [Google Scholar]
- Delmonico MJ, Harris TB, Visser M, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009, 90, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Khoury T, Asombang AW, Berzin TM, Cohen J, Pleskow DK, Mizrahi M. The Clinical Implications of Fatty Pancreas: A Concise Review. Dig Dis Sci. 2017, 62, 2658–2667. [Google Scholar] [CrossRef] [PubMed]
- Lizcano F, Guzmán G. Estrogen Deficiency and the Origin of Obesity during Menopause. Biomed Res Int. 2014, 2014, 757461. [Google Scholar] [CrossRef]
- Geraci A, Calvani R, Ferri E, Marzetti E, Arosio B, Cesari M. Sarcopenia and Menopause: The Role of Estradiol. Front Endocrinol 2021, 12, 682012. [Google Scholar] [CrossRef]
- La Colla A, Pronsato L, Milanesi L, Vasconsuelo A. 17β-Estradiol and testosterone in sarcopenia: Role of satellite cells. Ageing Res Rev. 2015, 24, 166–177. [Google Scholar] [CrossRef]
- Velders M, Diel P. How sex hormones promote skeletal muscle regeneration. Sports Med. 2013, 43, 1089–1100. [Google Scholar] [CrossRef]
- Shigehara K, Kato Y, Izumi K, Mizokami A. Relationship between Testosterone and Sarcopenia in Older-Adult Men: A Narrative Review. J Clin Med. 2022, 11, 6202. [Google Scholar] [CrossRef]
- Lynch GM, Murphy CH, Castro EM. Inflammation and metabolism: the role of adiposity in sarcopenic obesity [published online ahead of print, 2020 Jul 16]. Proc Nutr Soc 2020, 1–13. [Google Scholar] [CrossRef]
- Park CH, Do JG, Lee YT, Yoon KJ. Sarcopenic obesity associated with high-sensitivity C-reactive protein in age and sex comparison: a two-center study in South Korea. BMJ Open. 2018, 8, e021232. [Google Scholar] [CrossRef]
- Nilwik R, Snijders T, Leenders M, et al. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol. 2013, 48, 492–498. [Google Scholar] [CrossRef]
- Wilkinson DJ, Piasecki M, Atherton PJ. The age- related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res Rev. 2018, 47, 123–132. [Google Scholar] [CrossRef]
- Burd NA, Gorissen SH, van Loon LJ. Anabolic resistance of muscle protein synthesis with aging. Exerc Sport Sci Rev. 2013, 41, 169–173. [Google Scholar] [CrossRef]
- Manini TM, Hong SL, Clark BC. Aging and muscle: a neuron's perspective. Curr Opin Clin Nutr Metab Care. 2013, 16, 21–26. [Google Scholar] [CrossRef]
- Iyer SR, Shah SB, Lovering RM. The Neuromuscular Junction: Roles in Aging and Neuromuscular Disease. Int J Mol Sci. 2021, 22, 8058. [Google Scholar] [CrossRef]
- Yamakawa H, Kusumoto D, Hashimoto H, Yuasa S. Stem Cell Aging in Skeletal Muscle Regeneration and Disease. Int J Mol Sci. 2020, 21, 1830. [Google Scholar] [CrossRef]
- Sousa-Victor P, García-Prat L, Muñoz-Cánoves P. Control of satellite cell function in muscle regeneration and its disruption in ageing. Nat Rev Mol Cell Biol. 2022, 23, 204–226. [Google Scholar] [CrossRef]
- Mendham AE, Goedecke JH, Micklesfield LK, et al. Understanding factors associated with sarcopenic obesity in older African women from a low-income setting: a cross-sectional analysis. BMC Geriatr. 2021, 21, 247. [Google Scholar] [CrossRef]
- Hsu KJ, Liao CD, Tsai MW, Chen CN. Effects of Exercise and Nutritional Intervention on Body Composition, Metabolic Health, and Physical Performance in Adults with Sarcopenic Obesity: A Meta-Analysis. Nutrients 2019, 11, 2163. [Google Scholar] [CrossRef]
- Hita-Contreras F, Bueno-Notivol J, Martínez-Amat A, Cruz- Díaz D, Hernandez AV, Pérez-López FR. Effect of exercise alone or combined with dietary supplements on anthropometric and physical performance measures in community-dwelling elderly people with sarcopenic obesity: A meta-analysis of randomized controlled trials. Maturitas. 2018, 116, 24–35. [Google Scholar] [CrossRef]
- Martínez-Amat A, Aibar-Almazán A, Fábrega-Cuadros R, et al. Exercise alone or combined with dietary supplements for sarcopenic obesity in community- dwelling older people: A systematic review of randomized controlled trials. Maturitas 2018, 110, 92–103. [Google Scholar] [CrossRef]
- Theodorakopoulos C, Jones J, Bannerman E, Greig CA. Effectiveness of nutritional and exercise interventions to improve body composition and muscle strength or function in sarcopenic obese older adults: A systematic review. Nutr Res. 2017, 43, 3–15. [Google Scholar] [CrossRef]
- American College of Sports Medicine, Chodzko-Zajko WJ, Proctor DN, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef]
- Kay MC, Carroll DD, Carlson SA, Fulton JE. Awareness and knowledge of the 2008 Physical Activity Guidelines for Americans. J Phys Act Health. 2014, 11, 693–698. [Google Scholar] [CrossRef]
- Rodrigues IB, Armstrong JJ, Adachi JD, MacDermid JC. Facilitators and barriers to exercise adherence in patients with osteopenia and osteoporosis: a systematic review. Osteoporos Int. 2017, 28, 735–745. [Google Scholar] [CrossRef]
- Yang JM, Luo Y, Zhang JH, et al. Effects of WB-EMS and protein supplementation on body composition, physical function, metabolism and inflammatory biomarkers in middle-aged and elderly patients with sarcopenic obesity: A meta-analysis of randomized controlled trials. Exp Gerontol. 2022, 166, 111886. [Google Scholar] [CrossRef]
- Yaegashi A, Kimura T, Hirata T, Tamakoshi A. Association of dietary protein intake with skeletal muscle mass in older adults: A systematic review. Geriatr Gerontol Int. 2021, 21, 1077–1083. [Google Scholar] [CrossRef]
- Choi MK, Bae YJ. Protein intake and osteosarcopenic adiposity in Korean adults aged 50 years and older. Osteoporos Int. 2020, 31, 2363–2372. [Google Scholar] [CrossRef]
- Yoo JI, Lee KH, Choi Y, Lee J, Park YG. Poor Dietary Protein Intake in Elderly Population with Sarcopenia and Osteosarcopenia: A Nationwide Population-Based Study. J Bone Metab. 2020, 27, 301–310. [Google Scholar] [CrossRef]
- Breen L, Phillips SM. Skeletal muscle protein metabolism in the elderly: Interventions to counteract the 'anabolic resistance' of ageing. Nutr Metab 2011, 8, 68 Published 2011 Oct 5. [Google Scholar] [CrossRef]
- Walrand S, Boirie Y. Optimizing protein intake in aging. Curr Opin Clin Nutr Metab Care. 2005, 8, 89–94. [Google Scholar] [CrossRef]
- Muscariello E, Nasti G, Siervo M, et al. Dietary protein intake in sarcopenic obese older women. Clin Interv Aging. 2016, 11, 133–140. [Google Scholar] [CrossRef]
- Bauer J, Biolo G, Cederholm T, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Loenneke JP, Loprinzi PD, Murphy CH, Phillips SM. Per meal dose and frequency of protein consumption is associated with lean mass and muscle performance. Clin Nutr. 2016, 35, 1506–1511. [Google Scholar] [CrossRef]
- van Vliet S, Burd NA, van Loon LJ. The Skeletal Muscle Anabolic Response to Plant- versus Animal- Based Protein Consumption. J Nutr. 2015, 145, 1981–1991. [Google Scholar] [CrossRef]
- Hanach NI, McCullough F, Avery A. The Impact of Dairy Protein Intake on Muscle Mass, Muscle Strength, and Physical Performance in Middle-Aged to Older Adults with or without Existing Sarcopenia: A Systematic Review and Meta-Analysis. Adv Nutr. 2019, 10, 59–69. [Google Scholar] [CrossRef]
- Boirie Y, Guillet C. Fast digestive proteins and sarcopenia of aging. Curr Opin Clin Nutr Metab Care 2018, 21, 37–41. [Google Scholar] [CrossRef]
- Auestad N, Layman DK. Dairy bioactive proteins and peptides: a narrative review. Nutr Rev. 2021, 79, 36–47. [Google Scholar] [CrossRef]
- Volpi E, Campbell WW, Dwyer JT, et al. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J Gerontol A Biol Sci Med Sci. 2013, 68, 677–681. [Google Scholar] [CrossRef]
- Komar B, Schwingshackl L, Hoffmann G. Effects of leucine-rich protein supplements on anthropometric parameter and muscle strength in the elderly: a systematic review and meta-analysis. J Nutr Health Aging. 2015, 19, 437–446. [Google Scholar] [CrossRef]
- Cereda E, Pisati R, Rondanelli M, Caccialanza R. Whey Protein, Leucine- and Vitamin-D-Enriched Oral Nutritional Supplementation for the Treatment of Sarcopenia. Nutrients 2022, 14, 1524. [Google Scholar] [CrossRef]
- Camajani E, Persichetti A, Watanabe M, et al. Whey Protein, L-Leucine and Vitamin D Supplementation for Preserving Lean Mass during a Low-Calorie Diet in Sarcopenic Obese Women. Nutrients 2022, 14, 1884. [Google Scholar] [CrossRef] [PubMed]
- Kemmler W, Teschler M, Weissenfels A, et al. Whole- body electromyostimulation to fight sarcopenic obesity in community-dwelling older women at risk. Resultsof the randomized controlled FORMOsA-sarcopenic obesity study. Osteoporos Int. 2016, 27, 3261–3270. [Google Scholar] [CrossRef]
- Kim H, Kim M, Kojima N, et al. Exercise and Nutritional Supplementation on Community-Dwelling Elderly Japanese Women With Sarcopenic Obesity: A Randomized Controlled Trial. J Am Med Dir Assoc. 2016, 17, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Kemmler W, Weissenfels A, Teschler M, et al. Whole-body electromyostimulation and protein supplementation favorably affect sarcopenic obesity in community- dwelling older men at risk: the randomized controlled FranSO study. Clin Interv Aging. 2017, 12, 1503–1513. [Google Scholar] [CrossRef]
- D'Souza RF, Marworth JF, Figueiredo VC, et al. Dose- dependent increases in p70S6K phosphorylation and intramuscular branched-chain amino acids in older men following resistance exercise and protein intake. Physiol Rep. 2014, 2, e12112. [Google Scholar] [CrossRef]
- Pennings B, Groen B, de Lange A, et al. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am J Physiol Endocrinol Metab. 2012, 302, E992–E999. [Google Scholar] [CrossRef]
- Devries MC, McGlory C, Bolster DR, et al. Leucine, Not Total Protein, Content of a Supplement Is the Primary Determinant of Muscle Protein Anabolic Responses in Healthy Older Women. J Nutr. 2018, 148, 1088–1095. [Google Scholar] [CrossRef]
- Nabuco HCG, Tomeleri CM, Fernandes RR, et al. Effect of whey protein supplementation combined with resistance training on body composition, muscular strength, functional capacity, and plasma-metabolism biomarkers in older women with sarcopenic obesity: A randomized, double-blind, placebo-controlled trial. Clin Nutr ESPEN. 2019, 32, 88–95. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |