Imaging of Pathologies of the Temporal Bone and Middle Ear: Inflammatory Diseases, Their Mimics and Potential Complications—Pictorial Review
Abstract
1. Introduction, Anatomy and Clinical Features
2. Imaging
3. Clinical Features and Differential Diagnoses
3.1. External Auditory Canal
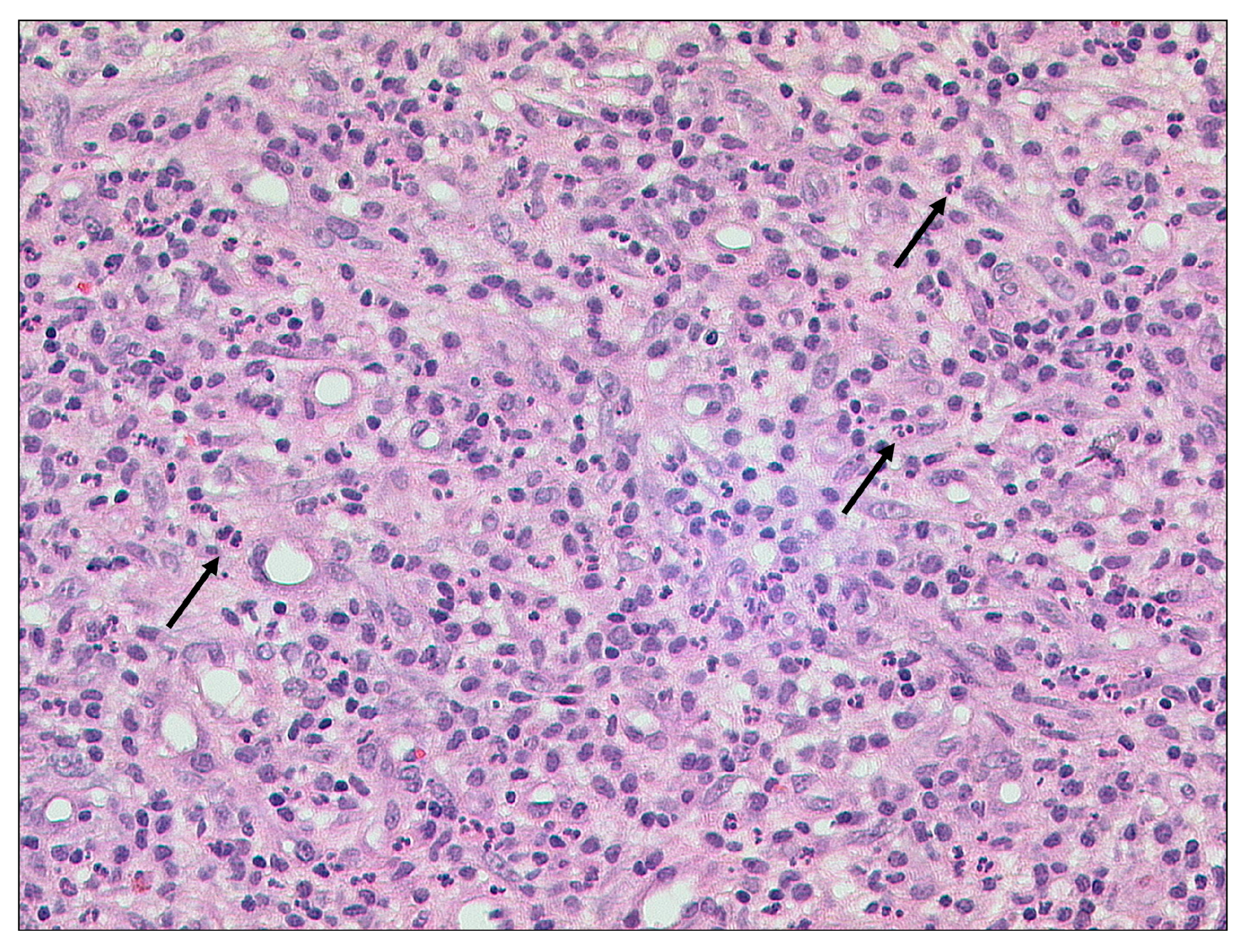
3.1.1. Otitis Externa Maligna
3.1.2. Cholesteatoma of the External Ear Canal
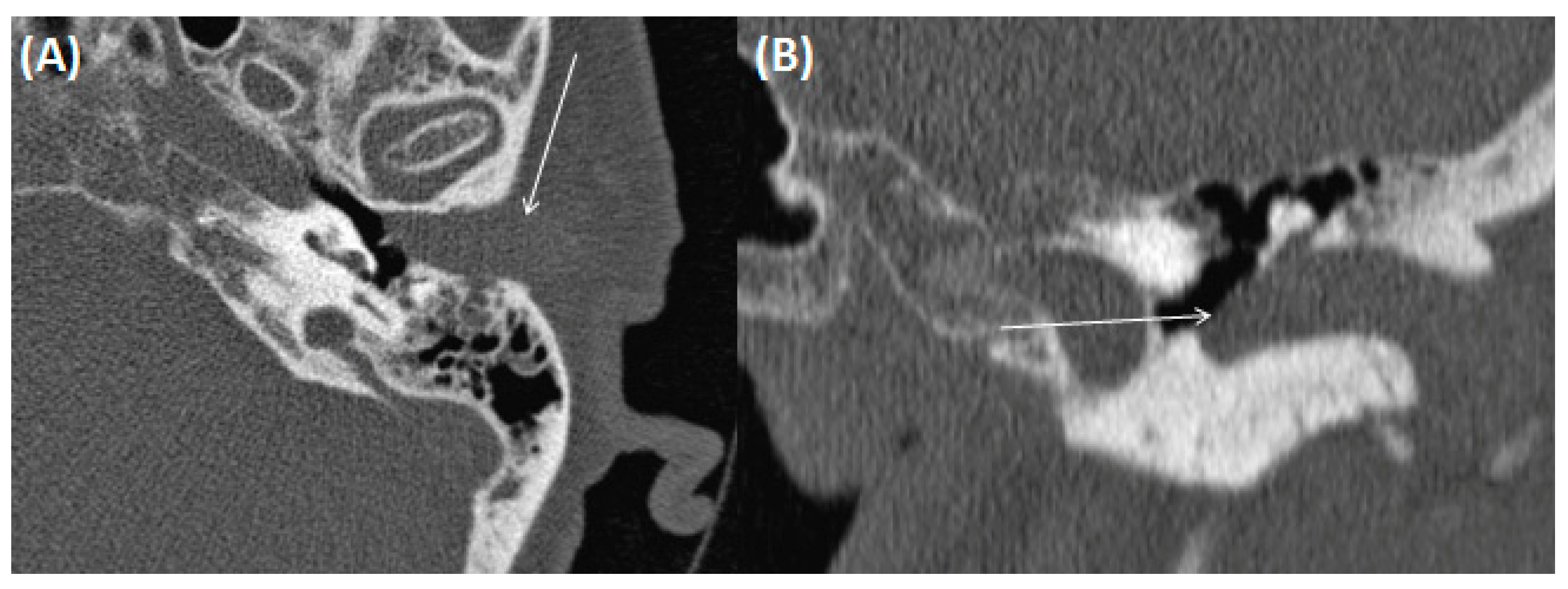
3.1.3. Auditory Canal Exostosis
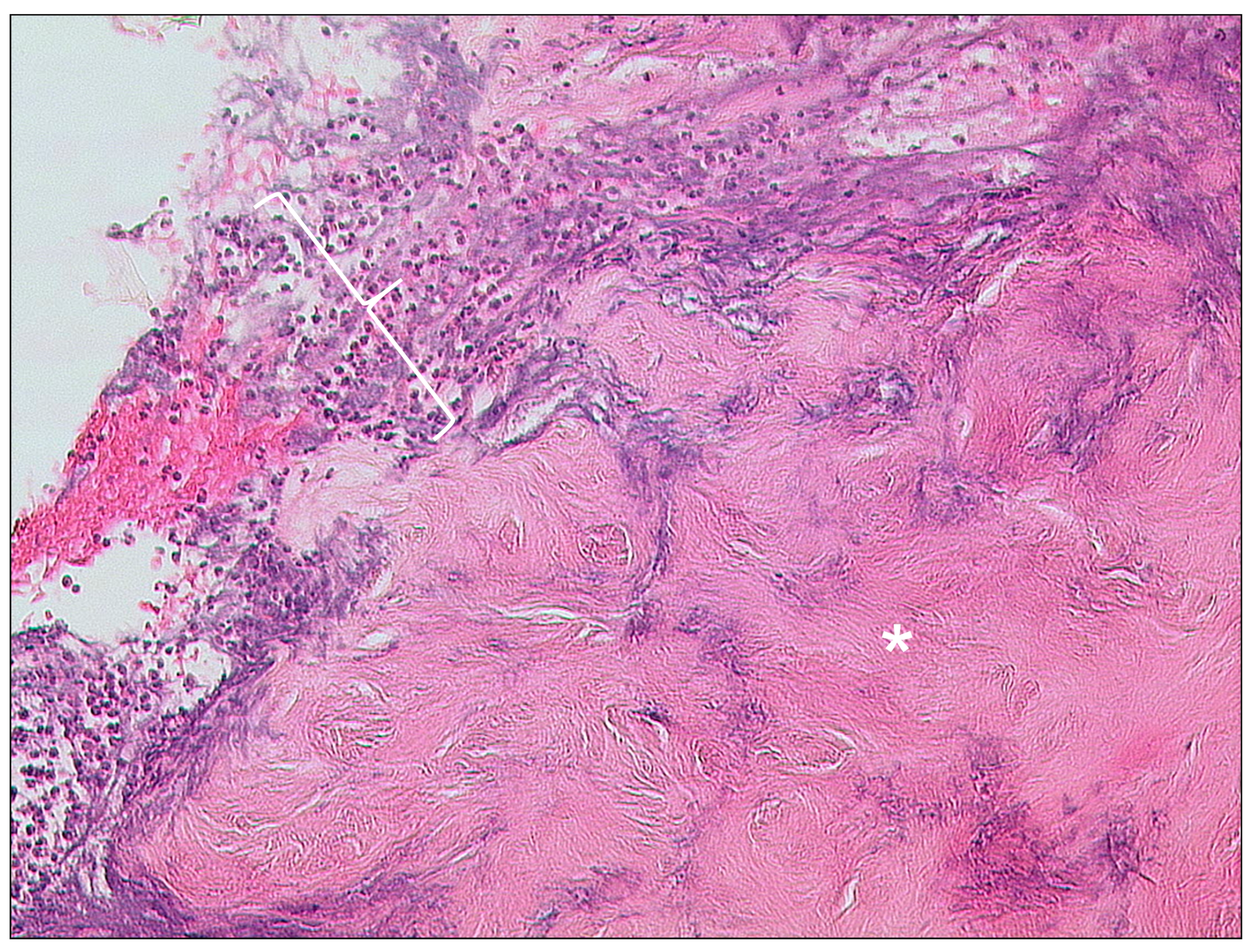
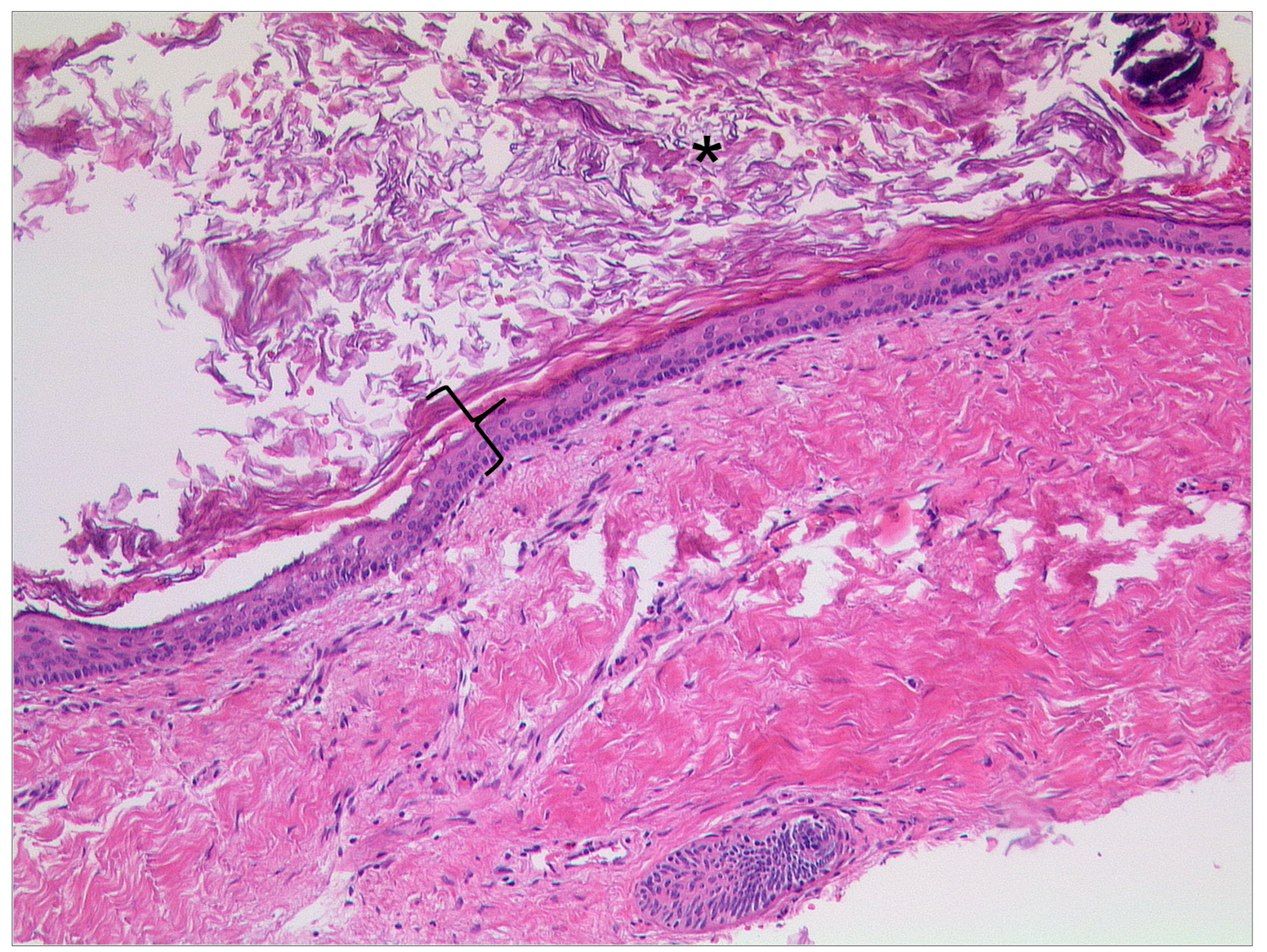
3.1.4. Keratosis Obturans
3.2. Middle Ear and Mastoid
3.2.1. Acute/Chronic Otitis Media
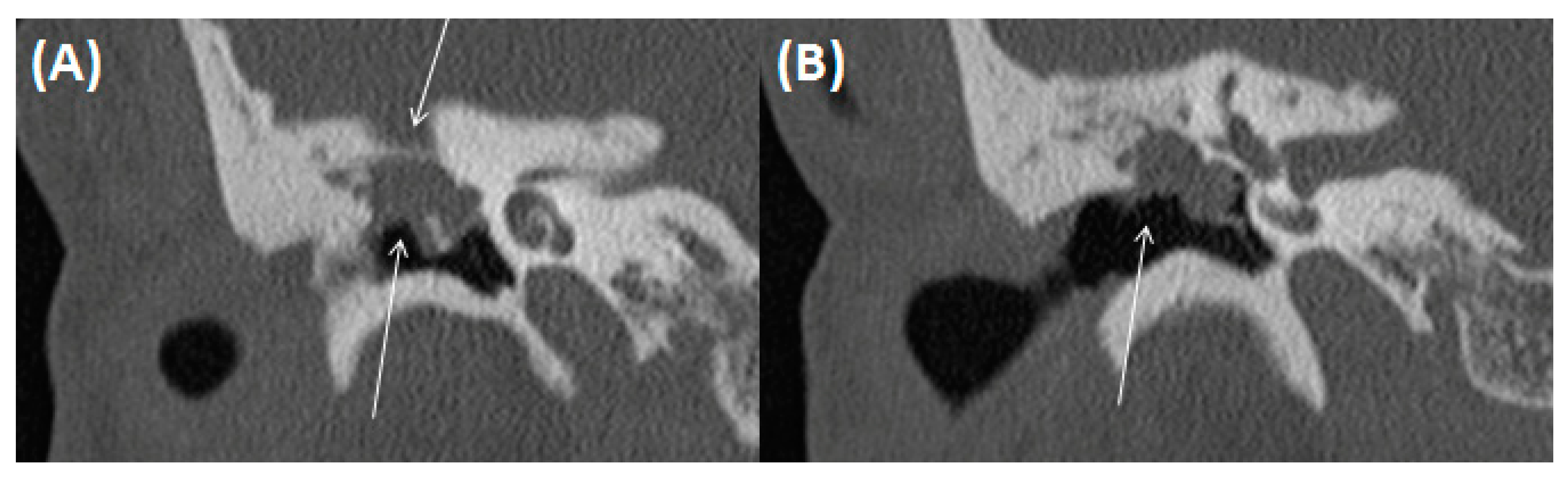
3.2.2. (Oto-)Mastoiditis
3.2.3. Tympanosclerosis
3.2.4. Cholesteatoma of the Middle Ear
3.2.5. Cholesterol Granuloma
3.3. Inner Ear
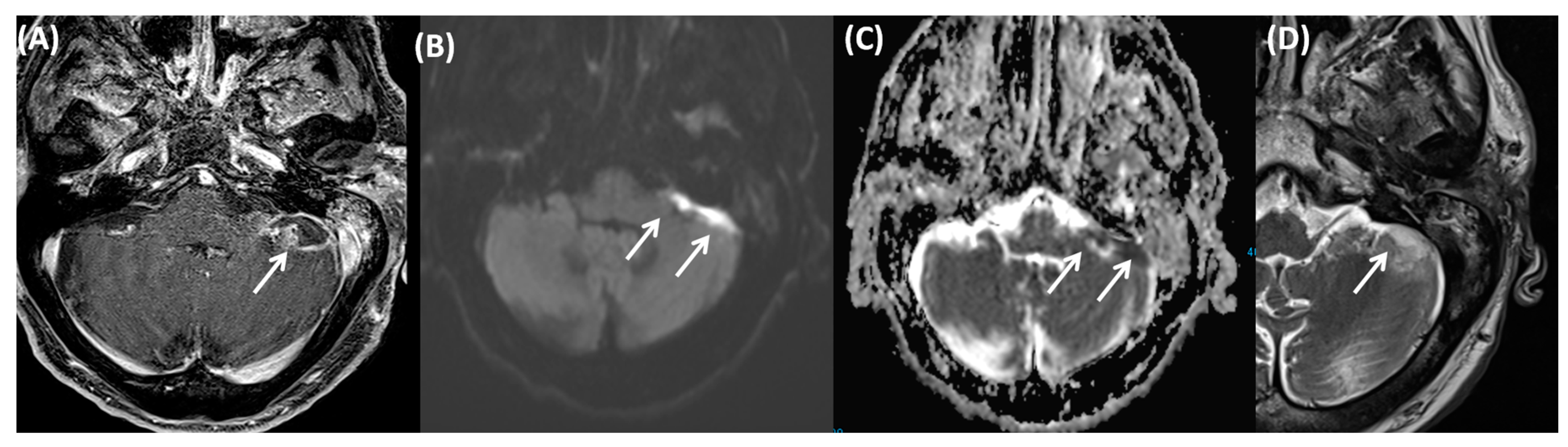
3.3.1. Tympanogenic Labyrinthitis
3.3.2. Otogene Purulent Meningitis
4. Complications
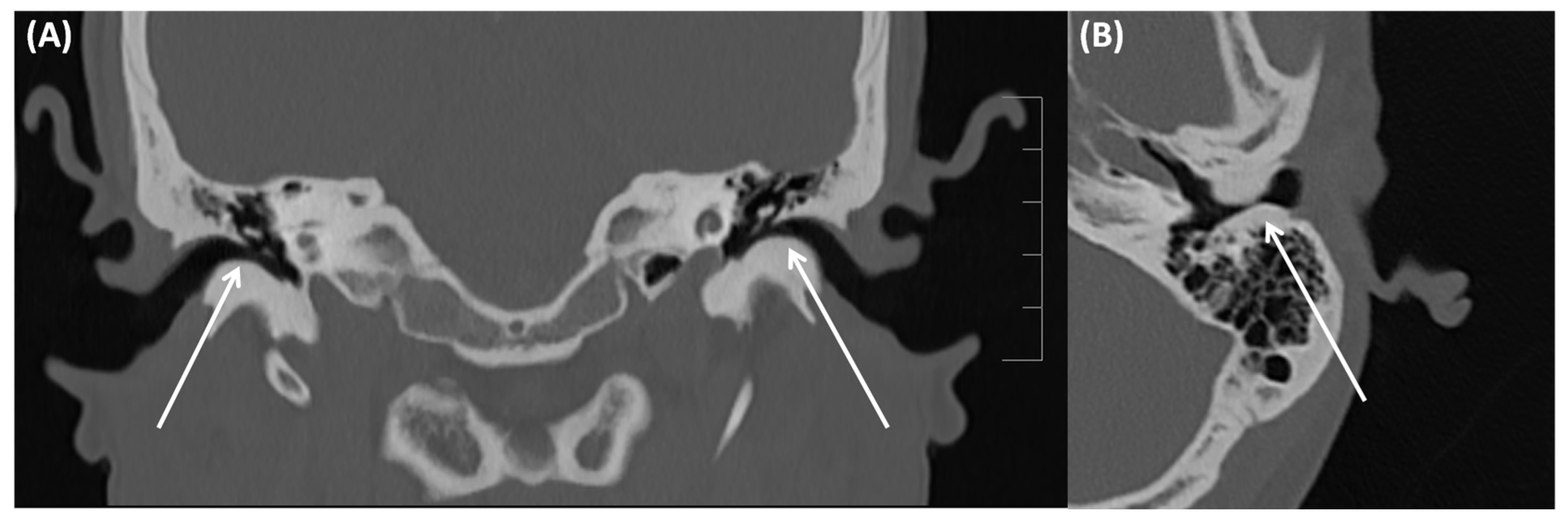
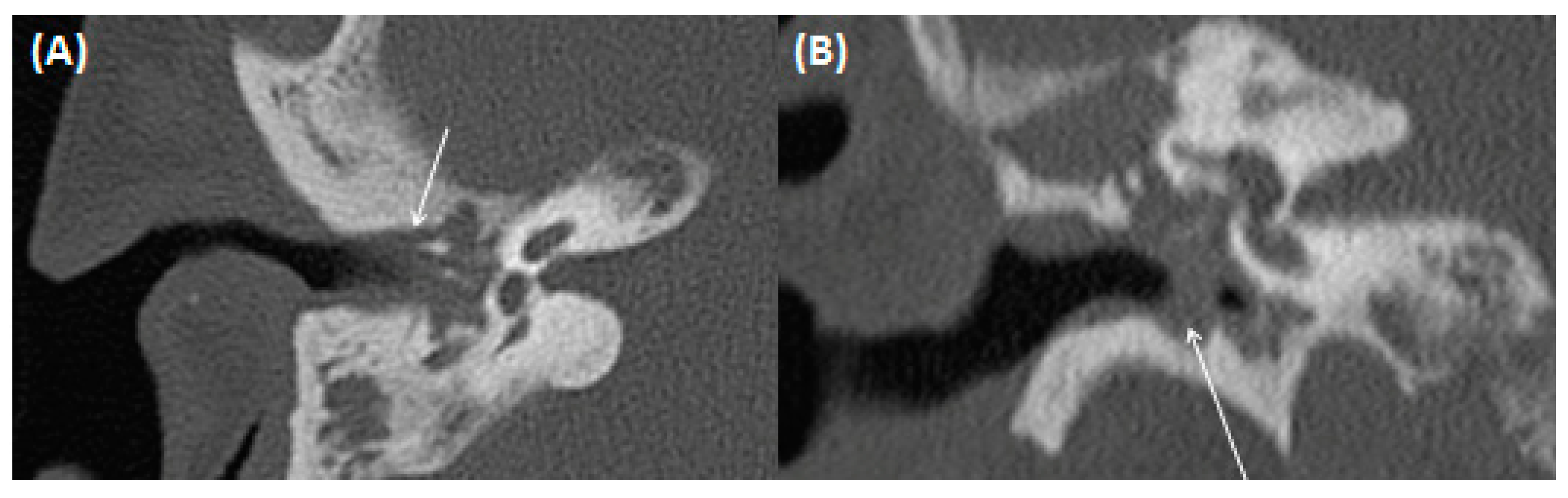
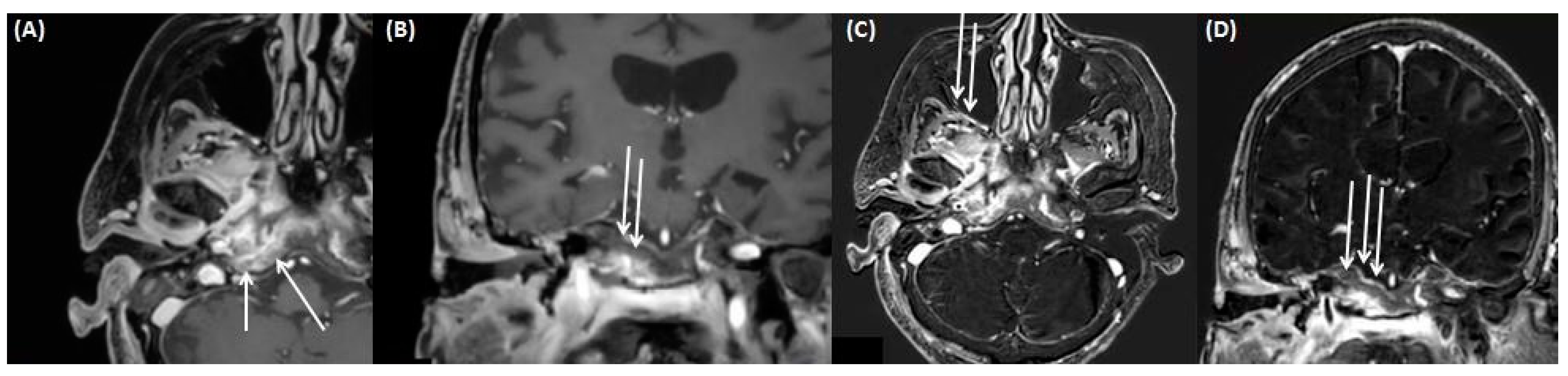
4.1. Gradenigo’s Syndrome
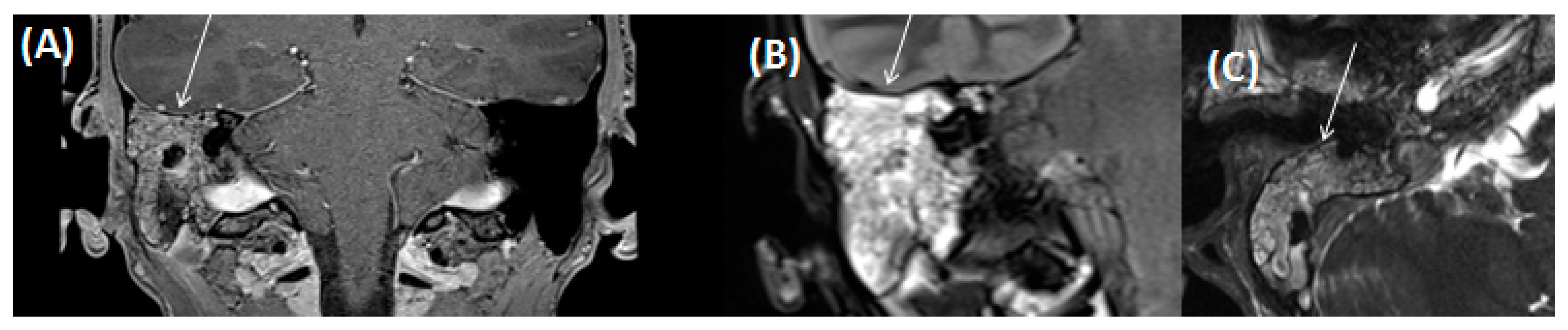
4.2. Intracerebral Abscess/Emphysema/Meningitis
4.3. Cerebral Venous Sinus Thrombosis
4.4. Otitic Hydrocephalus
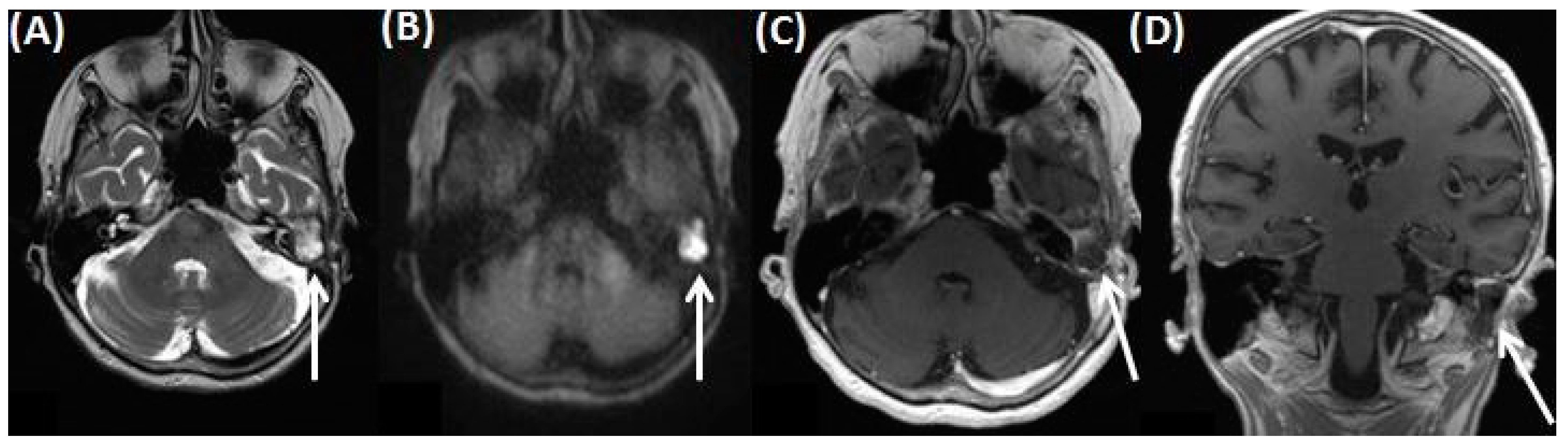
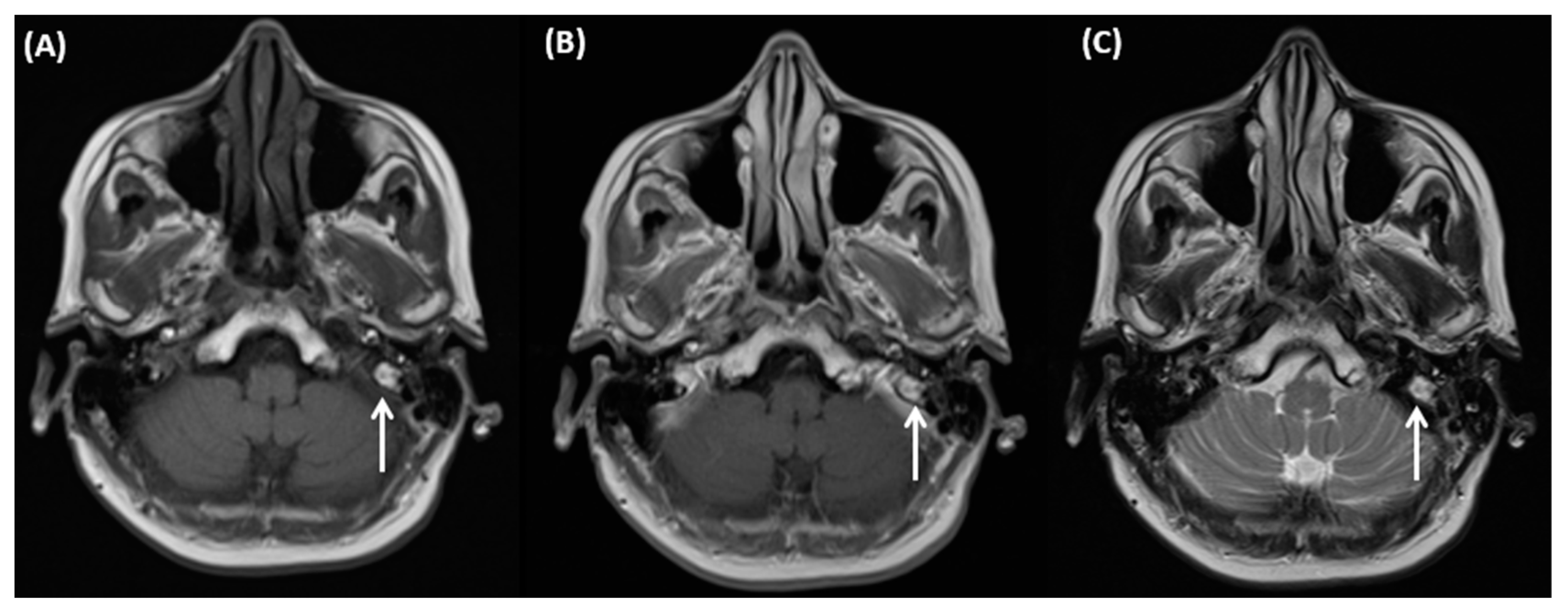
4.5. Ramsay Hunt Syndrome
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CSF | cerebrospinal fluid space |
| CT | computed tomography |
| DWI | diffusion weighted imaging |
| EAC | external auditory canal |
| EACC | external ear canal cholesteatoma |
| EPI | echo planar imaging |
| FLAIR | Fluid-attenuated inversion recovery |
| HE | heamatoxylin-eosin |
| HU | Hounsfield units |
| MRI | Magnetic resonance imaging |
| RHS | Ramsay Hunt syndrome |
References
- Juliano, A.F.; Ginat, D.T.; Moonis, G. Imaging Review of the Temporal Bone: Part I. Anatomy and Inflammatory and Neoplastic Processes. Radiology 2013, 269, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Fatterpekar, G.M.; Mukherji, S.K.; Lin, Y.; Alley, J.G.; Stone, J.A.; Castillo, M. Normal Canals at the Fundus of the Internal Auditory Canal: CT Evaluation. J. Comput. Assist. Tomogr. 1999, 23, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, M. Anatomy, Head and Neck, Ear Internal Auditory Canal (Internal Auditory Meatus, Internal Acoustic Canal). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Marchioni, D.; Rubini, A.; Soloperto, D. Endoscopic Ear Surgery: Redefining Middle Ear Anatomy and Physiology. Otolaryngol. Clin. N. Am. 2021, 54, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, M.; Di Lullo, A.M.; Cantone, E.; Scala, G.; Elefante, A.; Russo, C.; Brunetti, L.; Motta, G.; Iengo, M. Cholesteatoma vs. granulation tissue: A differential diagnosis by DWI-MRI apparent diffusion coefficient. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 2237–2243. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, D.C.; Epure, V.; Oprea, D.; Zamfir-Chiru-Anton, A. Persistent Stapedial Artery, Oval Window Atresia and Congenital Stapes Agenesis—Case Report. Medicina 2023, 59, 461. [Google Scholar] [CrossRef] [PubMed]
- Iima, M.; Sakamoto, R.; Kakigi, T.; Yamamoto, A.; Otsuki, B.; Nakamoto, Y.; Toguchida, J.; Matsuda, S. The Efficacy of CT Temporal Subtraction Images for Fibrodysplasia Ossificans Progressiva. Tomography 2023, 9, 768–775. [Google Scholar] [CrossRef]
- Waldeck, S.; Overhoff, D.; Alizadeh, L.; Becker, B.V.; Port, M.; Froelich, M.F.; Brockmann, M.A.; Schumann, S.; Vogl, T.J.; Schoenberg, S.O.; et al. Photon-Counting Detector CT Virtual Monoengergetic Images for Cochlear Implant Visualization—A Head to Head Comparison to Energy-Integrating Detector CT. Tomography 2022, 8, 1642–1648. [Google Scholar] [CrossRef] [PubMed]
- Sartoretti-Schefer, S.; Scherler, M.; Wichmann, W.; Valavanis, A. Contrast-enhanced MR of the facial nerve in patients with posttraumatic peripheral facial nerve palsy. AJNR Am. J. Neuroradiol. 1997, 18, 1115–1125. [Google Scholar]
- De Raeve, L.; Cumpăt, M.-C.; van Loo, A.; Costa, I.M.; Matos, M.A.; Dias, J.C.; Mârțu, C.; Cavaleriu, B.; Gherguț, A.; Maftei, A.; et al. Quality Standard for Rehabilitation of Young Deaf Children Receiving Cochlear Implants. Medicina 2023, 59, 1354. [Google Scholar] [CrossRef]
- Tsuno, N.S.G.; Tsuno, M.Y.; Neto, C.A.F.C.; Noujaim, S.E.; Decnop, M.; Pacheco, F.T.; Souza, S.A.; Fonseca, A.P.A.; Garcia, M.R.T. Imaging the External Ear: Practical Approach to Normal and Pathologic Conditions. RadioGraphics 2022, 42, 522–540. [Google Scholar] [CrossRef]
- Nakanishi, H.; Tono, T.; Kawano, H. Incidence of External Auditory Canal Exostoses in Competitive Surfers in Japan. Otolaryngol. Head Neck Surg. 2011, 145, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Jung, Y.H.; Oh, J. Clinical Characteristics of Keratosis Obturans and External Auditory Canal Cholesteatoma. Otolaryngol. Head Neck Surg. 2015, 152, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Stefan, I.; Stefanescu, C.D.; Vlad, A.M.; Zainea, V.; Hainarosie, R. Postoperative Outcomes of Endoscopic versus Microscopic Myringoplasty in Patients with Chronic Otitis Media—A Systematic Review. Medicina 2023, 59, 1074. [Google Scholar] [CrossRef] [PubMed]
- Campion, T.; Taranath, A.; Pinelli, L.; Ugga, L.; Nash, R.; Talenti, G.; Dahmoush, H.; D’Arco, F. Imaging of temporal bone inflammations in children: A pictorial review. Neuroradiology 2019, 61, 959–970. [Google Scholar] [CrossRef]
- Stefanescu, E.H.; Balica, N.C.; Motoi, S.B.; Grigorita, L.; Georgescu, M.; Iovanescu, G. High-Resolution Computed Tomography in Middle Ear Cholesteatoma: How Much Do We Need It? Medicina 2023, 59, 1712. [Google Scholar] [CrossRef]
- Imamura, K.; Hosoya, M.; Kasuya, K.; Shimanuki, M.N.; Shinden, S.; Ogawa, K.; Oishi, N. Labyrinthine destruction caused by inflammatory pseudotumor of the temporal bone: A report of three cases and review of the literature. Laryngoscope Investig. Otolaryngol. 2021, 6, 857–865. [Google Scholar] [CrossRef]
- Da Costa, C.F.; Polanski, J.F. Wegener Granulomatosis: Otologic Manifestation as First Symptom. Int. Arch. Otorhinolaryngol. 2015, 19, 266–268. [Google Scholar] [CrossRef][Green Version]
- Talmor, G.; Vakil, M.; Tseng, C.; Svider, P.; Ying, M.; Eloy, J.A. Petrous Apicitis: A Systematic Review and Case Presentation. Otol. Neurotol. 2022, 43, 753–765. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Fan, Y.-K.; Wu, K.-C.; Shu, M.-T.; Yang, C.-C. The incidence of tympanogenic labyrinthitis ossificans. J. Laryngol. Otol. 2014, 128, 618–620. [Google Scholar] [CrossRef]
- Bruschini, L.; Fortunato, S.; Tascini, C.; Ciabotti, A.; Leonildi, A.; Bini, B.; Giuliano, S.; Abbruzzese, A.; Berrettini, S.; Menichetti, F.; et al. Otogenic Meningitis: A Comparison of Diagnostic Performance of Surgery and Radiology. Open Forum Infect. Dis. 2017, 4, ofx069. [Google Scholar] [CrossRef]
- Grandis, J.R.; Curtin, H.D.; Yu, V.L. Necrotizing (malignant) external otitis: Prospective comparison of CT and MR imaging in diagnosis and follow-up. Radiology 1995, 196, 499–504. [Google Scholar] [CrossRef]
- Lamry, N.A. Synchronous Occurrence of Bilateral Malignant Otitis Externa: Report of a Rare Case. Korean J. Fam. Med. 2021, 42, 483–486. [Google Scholar] [CrossRef]
- Leahy, T.W.; Sader, C. A rare case of bilateral malignant otitis externa and osteomyelitis with lower cranial nerve sequelae. BMJ Case Rep. 2011, 2011, bcr0320113957. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.J.; Han, M.H.; Oh, S.H.; Song, J.J.; Chang, K.H. MRI findings and spreading patterns of necrotizing external otitis: Is a poor outcome predictable? Clin. Radiol. 2006, 61, 495–504. [Google Scholar] [CrossRef]
- Bernheim, J.; Sade, J. Histopathology of the soft parts in 50 patients with malignant external otitis. J. Laryngol. Otol. 1989, 103, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Qian, M.; Li, J.; Xu, J.; Chen, H. Clinical Analysis of 85 Cases of External Auditory Canal Cholesteatoma Surgery under Specialized Endoscopy. Biomed Res. Int. 2022, 2022, 9190241. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, M.F.; Williamson, I.G.; Sudhoff, H.H. Cholesteatom. Praxis 2011, 100, 1247–1250. [Google Scholar] [CrossRef] [PubMed]
- Hertz, J.; Siim, C. External auditory canal cholesteatoma and benign necrotising otitis externa: Clinical study of 95 cases in the Capital Region of Denmark. J. Laryngol. Otol. 2018, 132, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Widmann, G.; Henninger, B.; Kremser, C.; Jaschke, W. MRI Sequences in Head & Neck Radiology—State of the Art. Fortschr. Röntgenstr. 2017, 189, 413–422. [Google Scholar]
- Ismaeel, A.M.; El-Tantawy, A.M.; Eissawy, M.G.; Gomaa, M.A.; Rahman, A.A.; Elkholy, T.; Hamead, K. The Clinical Role of Diffusion-Weighted MRI for Detecting Residual Cholesteatoma in Canal Wall up Mastoidectomy. Indian J. Otolaryngol. Head Neck Surg. 2022, 74 (Suppl. 3), 3911–3918. [Google Scholar] [CrossRef]
- Fischer, N.; Plaikner, M.; Schartinger, V.H.; Kremser, C.; Riechelmann, H.; Schmutzhard, J.; Gottfried, T.; Dejaco, D.; Tauber, H.; Josip, E.; et al. MRI of middle ear cholesteatoma: The importance of observer reliance from diffusion sequences. J. Neuroimaging 2022, 32, 120–126. [Google Scholar] [CrossRef]
- Henninger, B.; Kremser, C. Diffusion weighted imaging for the detection and evaluation of cholesteatoma. World J. Radiol. 2017, 9, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Benson, J.; Carlson, M.; Lane, J. Non-EPI versus Multishot EPI DWI in Cholesteatoma Detection: Correlation with Operative Findings. AJNR Am. J. Neuroradiol. 2021, 42, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Karandikar, A.; Goh, J.; Loke, S.C.; Yeo, S.B.; Tan, T.Y. Mucous retention cyst of temporal bone: A mimic of cholesteatoma on DW-MRI. Am. J. Otolaryngol. 2013, 34, 753–754. [Google Scholar] [CrossRef] [PubMed]
- Kroon, D.F.; Lawson, M.L.; Derkay, C.S.; Hoffmann, K.; McCook, J. Surfer’s Ear: External Auditory Exostoses are More Prevalent in Cold Water Surfers. J. Otolaryngol. Head Neck Surg. 2002, 126, 499–504. [Google Scholar] [CrossRef]
- Reddy, V.M.; Abdelrahman, T.; Lau, A.; Flanagan, P.M. Surfers’ awareness of the preventability of ‘surfer’s ear’ and use of water precautions. J. Laryngol. Otol. 2011, 125, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Barbon, D.A.; Hegde, R.; Li, S.; Abdelbaki, A.; Bajaj, D. Bilateral External Auditory Exostoses Causing Conductive Hearing Loss: A Case Report and Literature Review of the Surfer’s Ear. Cureus 2017, 9, e1810. [Google Scholar] [CrossRef] [PubMed]
- Turetsky, D.B.; Vines, F.S.; Clayman, D.A. Surfer’s ear: Exostoses of the external auditory canal. AJNR Am. J. Neuroradiol. 1990, 11, 1217–1218. [Google Scholar] [PubMed]
- Wong, B.J.F.; Cervantes, W.; Doyle, K.J.; Karamzadeh, A.M.; Boys, P.; Brauel, G.; Mushtaq, E. Prevalence of External Auditory Canal Exostoses in Surfers. Arch. Otolaryngol. Head Neck Surg. 1999, 125, 969–972. [Google Scholar] [CrossRef]
- Pace-Baizan, A.; Hawke, M. Exostosis of the external auditory canal: An interesting histopathological finding. J. Laryngol. Otol. 1991, 105, 844–846. [Google Scholar] [CrossRef]
- Åberg, B.; Westin, T.; Tjellström, A.; Edström, S. Clinical characteristics of cholesteatoma. Am. J. Otolaryngol. 1991, 12, 254–258. [Google Scholar] [CrossRef]
- Persaud, R.; Hajioff, D.; Thevasagayam, M.; Wareing, M.; Wright, A. Keratosis obturans and external ear canal cholesteatoma: How and why we should distinguish between these conditions. Clin. Otolaryngol. Allied Sci. 2004, 29, 577–581. [Google Scholar] [CrossRef]
- Naiberg, J.; Berger, G.; Hawke, M. The Pathologic Features of Keratosis Obturans and Cholesteatoma of the External Auditory Canal. Arch. Otolaryngol. 1984, 110, 690–693. [Google Scholar] [CrossRef]
- Kuczkowski, J.; Mikaszewski, B.; Narożny, W.J. Immunohistochemical and histopathological features of keratosis obturans and cholesteatoma of the external auditory canal. Atypical keratosis obturans. J. Laryngol. Otol. 2004, 118, 249–251, author reply 250–251. [Google Scholar] [CrossRef][Green Version]
- Poudyal, P.; Nepal, G.; Yadav, S.K.; Neupane, Y.; Dutta, H.; Pokhrel, S.; Gaire, P. Keratosis obturans: A rare cause of facial nerve palsy. Clin. Case Rep. 2022, 10, e05410. [Google Scholar] [CrossRef]
- Rosenfeld, R.M.; Shin, J.J.; Schwartz, S.R.; Coggins, R.; Gagnon, L.; Hackell, J.M.; Hoelting, D.; Hunter, L.L.; Kummer, A.W.; Payne, S.C.; et al. Clinical Practice Guideline: Otitis Media with Effusion (Update). Otolaryngol. Head Neck Surg. 2016, 154 (Suppl. 1), S1–S41. [Google Scholar] [CrossRef]
- Danishyar, A.; Ashurst, J.V. Acute Otitis Media. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kusak, A.; Rosiak, O.; Durko, M.; Grzelak, P.; Pietruszewska, W. Diagnostic imaging in chronic otitis media: Does CT and MRI fusion aid therapeutic decision making?—A pilot study. Otolaryngol. Pol. 2018, 72, 1–5. [Google Scholar] [CrossRef]
- Lee, B.; Bae, Y.J.; Choi, B.Y.; Kim, Y.S.; Han, J.H.; Kim, H.; Kim, J.H. Construction of an MRI-based decision tree to differentiate autoimmune and autoinflammatory inner ear disease from chronic otitis media with sensorineural hearing loss. Sci. Rep. 2021, 11, 19171. [Google Scholar] [CrossRef]
- Wong, K.; Arrighi-Allisan, A.E.; Fan, C.J.; Wanna, G.B.; Cosetti, M.K.; Perez, E.R. A Review of Noninfectious Diseases Masquerading as Acute Mastoiditis. Otolaryngol. Head Neck Surg. 2022, 167, 901–911. [Google Scholar] [CrossRef]
- Orhan, K.; Nishiyama, H.; Tadashi, S.; Shumei, M.; Furukawa, S. MR of 2270 TMJs: Prevalence of radiographic presence of otomastoiditis in temporomandibular joint disorders. Eur. J. Radiol. 2005, 55, 102–107. [Google Scholar] [CrossRef]
- Moya, P.A.; Malinvaud, D.; Mimoun, M.; Huart, J.; Bonfils, P. Tuberculous otomastoiditis: Advantage of MRI in the treatment survey. Rev. Laryngol. Otol. Rhinol. 2008, 129, 301–304. [Google Scholar]
- Stähelin-Massik, J.; Podvinec, M.; Jakscha, J.; Rüst, O.N.; Greisser, J.; Moschopulos, M.; Gnehm, H.E. Mastoiditis in children: A prospective, observational study comparing clinical presentation, microbiology, computed tomography, surgical findings and histology. Eur. J. Pediatr. 2008, 167, 541–548. [Google Scholar] [CrossRef]
- Barry, J.Y.; Reghunathan, S.; Jacob, A. Tympanosclerosis Presenting as Mass: Workup and Differential. Case Rep. Otolaryngol. 2016, 2016, 9821493. [Google Scholar] [CrossRef]
- Yildiz, S.; Balık, A.Ö.; Toros, S.Z. Is ossicular chain fixation predictable for tympanosclerosis on preoperative temporal bone computed tomography? Eur. Arch. Otorhinolaryngol. 2021, 278, 2789–2794. [Google Scholar] [CrossRef]
- Bhaya, M.H.; Schachern, P.A.; Morizono, T.; Paparella, M.M. Pathogenesis of tympanosclerosis. Otolaryngol. Head Neck Surg. 1993, 109, 413–420. [Google Scholar] [CrossRef]
- Larem, A.; Altamimi, Z.A.R.; Aljariri, A.A.; Haidar, H.; Elsotouhy, A.; Alsaadi, A.; Alqahtani, A. Reliability of high-resolution CT scan in diagnosis of ossicular tympanosclerosis. Laryngoscope Investig. Otolaryngol. 2021, 6, 540–548. [Google Scholar] [CrossRef]
- Kuo, C.-L.; Shiao, A.-S.; Yung, M.; Sakagami, M.; Sudhoff, H.; Wang, C.-H.; Hsu, C.-H.; Lien, C.-F. Updates and Knowledge Gaps in Cholesteatoma Research. BioMed Res. Int. 2015, 2015, 854024. [Google Scholar] [CrossRef]
- Vercruysse, J.-P.; De Foer, B.; Pouillon, M.; Somers, T.; Casselman, J.; Offeciers, E. The value of diffusion-weighted MR imaging in the diagnosis of primary acquired and residual cholesteatoma: A surgical verified study of 100 patients. Eur. Radiol. 2006, 16, 1461–1467. [Google Scholar] [CrossRef]
- Fukuda, A.; Morita, S.; Harada, T.; Fujiwara, K.; Hoshino, K.; Nakamaru, Y.; Homma, A. Value of T1-weighted magnetic resonance imaging in cholesteatoma detection. Otol. Neurotol. 2017, 38, 1440–1444. [Google Scholar] [CrossRef]
- Lincot, J.; Veillon, F.; Riehm, S.; Babay, N.; Matern, J.-F.; Rock, B.; Dallaudière, B.; Meyer, N. Middle ear cholesteatoma: Compared diagnostic performances of two incremental MRI protocols including non-echo planar diffusion-weighted imaging acquired on 3T and 1.5T scanners. J. Neuroradiol. 2015, 42, 193–201. [Google Scholar] [CrossRef]
- Razek, A.A.; Huang, B.Y. Lesions of the Petrous Apex: Classification and Findings at CT and MR Imaging. RadioGraphics 2012, 32, 151–173. [Google Scholar] [CrossRef]
- Pace, A.; Iannella, G.; Riminucci, M.; Corsi, A.; Magliulo, G. Tympano-Mastoid Cholesterol Granuloma: Case Report and Review of the Literature. Clin. Med. Insights Case Rep. 2020, 13, 1179547620958728. [Google Scholar] [CrossRef]
- Hoa, M.; House, J.W.; Linthicum, F.H.; Go, J.L. Petrous apex cholesterol granuloma: Pictorial review of radiological considerations in diagnosis and surgical histopathology. J. Laryngol. Otol. 2013, 127, 339–348. [Google Scholar] [CrossRef]
- Jang, C.H.; Park, S.Y.; Wang, P. A Case of Tympanogenic Labyrinthitis Complicated by Acute Otitis Media. Yonsei Med. J. 2005, 46, 161–165. [Google Scholar] [CrossRef]
- Casselman, J.W.; Kuhweide, R.; Ampe, W.; Meeus, L.; Steyaert, L. Pathology of the membranous labyrinth: Comparison of T1- and T2-weighted and gadolinium-enhanced spin-echo and 3DFT-CISS imaging. AJNR Am. J. Neuroradiol. 1993, 14, 59–69. [Google Scholar]
- Dobben, G.D.; Raofi, B.; Mafee, M.F.; Kamel, A.; Mercurio, S. Otogenic Intracranial Inflammations: Role of Magnetic Resonance Imaging. Top. Magn. Reson. Imaging 2000, 11, 76–86. [Google Scholar] [CrossRef]
- Vazquez, E.; Castellote, A.; Piqueras, J.; Mauleon, S.; Creixell, S.; Pumarola, F.; Figueras, C.; Carreño, J.-C.; Lucaya, J. Imaging of Complications of Acute Mastoiditis in Children. RadioGraphics 2003, 23, 359–372. [Google Scholar] [CrossRef]
- Kolenda, J.; Carr, M.M.; Lemckert, R.J.; Ummat, S.K. Intracranial sinus thrombosis secondary to ear disease in an adolescent. J. Otolaryngol. 1997, 26, 203–206. [Google Scholar]
- Bozan, N.; Düzenli, U.; Yalinkilic, A.; Ayral, A.; Parlak, M.; Turan, M.; Kiroglu, A.F. Gradenigo Syndrome Induced by Suppurative Otitis Media. J. Craniofacial Surg. 2018, 29, e645–e646. [Google Scholar] [CrossRef]
- Vitale, M.; Amrit, M.; Arora, R.; Lata, J. Gradenigo’s syndrome: A common infection with uncommon consequences. Am. J. Emerg. Med. 2017, 35, 1388.e1–1388.e2. [Google Scholar] [CrossRef]
- Taklalsingh, N.; Falcone, F.; Velayudhan, V. Gradenigo’s syndrome in a patient with chronic suppurative otitis media, petrous apicitis, and meningitis. Am. J. Case Rep. 2017, 18, 1039–1043. [Google Scholar] [CrossRef]
- Motamed, M.; Kalan, A. Gradenigo’s syndrome. Postgrad. Med. J. 2000, 76, 559–560. [Google Scholar] [CrossRef]
- Beran, A.; Aladamat, N.; Alchalabi, M.; Mhanna, M.; Srour, O.; Khader, Y.; Kayyali, A. Atypical Gradenigo Syndrome in an Elderly Man Resolved with Mastoidectomy and Petrous Apicectomy. Eur. J. Case Rep. Intern. Med. 2022, 9, 003344. [Google Scholar] [CrossRef]
- Quesada, J.; Kong, A.; Tweddle, E. An unusual case of acute otitis media resulting in Gradenigo syndrome: CT and MRI findings. Radiol. Case Rep. 2021, 16, 3903–3907. [Google Scholar] [CrossRef]
- Muzumdar, D.; Biyani, N.; Deopujari, C. Subdural empyema in children. Child Nerv. Syst. 2018, 34, 1881–1887. [Google Scholar] [CrossRef]
- Kempf, H.-G.; Wiel, J.; Issing, P.R.; Lenarz, T. Otogenic brain abscess. Laryngorhinootologie 1998, 77, 462–466. [Google Scholar] [CrossRef]
- Derkaoui, A.; Khatouf, M. Acute otitis media complicated with pneumocephalus and pneumococcal meningitis. Pan Afr. Med. J. 2015, 22, 389. [Google Scholar] [CrossRef]
- Penido, N.D.O.; Borin, A.; Iha, L.C.; Suguri, V.M.; Onishi, E.; Fukuda, Y.; Cruz, O.L.M. Intracranial complications of otitis media: 15 years of experience in 33 patients. Otolaryngol. Head Neck Surg. 2008, 132, 37–42. [Google Scholar] [CrossRef]
- Raja, K.; Parida, P.K.; Alexander, A.; Surianarayanan, G. Otogenic Lateral Sinus Thrombosis: A Review of Fifteen Patients and Changing Trends in the Management. Int. Arch. Otorhinolaryngol. 2018, 22, 208–213. [Google Scholar] [CrossRef]
- Leichtle, A.; Hoffmann, T.; Wigand, M. Otitis media: Definition, pathogenesis, clinical presentation, diagnosis and therapy. Laryngorhinootologie 2018, 97, 497–508. [Google Scholar] [CrossRef]
- Kelly, K.E.; Jackler, R.K.; Dillon, W.P. Diagnosis of Septic Sigmoid Sinus Thrombosis with Magnetic Resonance Imaging. Otolaryngol. Neck Surg. 1991, 105, 617–624. [Google Scholar] [CrossRef]
- Viswanatha, B. Otitic hydrocephalus: A report of 2 cases. Ear Nose Throat J. 2010, 89, E34–E37. [Google Scholar] [CrossRef]
- Wahid, F.I.; Khan, A.; Khan, I.A. Complications of chronic suppurative otitis media: Challenge for a developing country. Kulak Burun Bogaz Ihtis Derg 2014, 24, 265–270. [Google Scholar] [CrossRef]
- Symonds, C.D. Otitic Hydrocephalus. Brain 1931, 54, 55–57. [Google Scholar] [CrossRef]
- Mehta, A.K.; Singh, V.K. Otitic hydrocephalus, a rare complication of CSOM. Med. J. Armed Forces India 1991, 55, 63–64. [Google Scholar] [CrossRef]
- Zainine, R.; Sellami, M.; Charfeddine, A.; Beltaief, N.; Sahtout, S.; Besbes, G. Ramsay Hunt syndrome. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2012, 129, 22–25. [Google Scholar] [CrossRef]
- Robillard, Z.R.B.; Hilsinger, R.L., Jr.; Adour, K.K. Ramsay Hunt facial paralysis: Clinical analysis of 185 patients. Otolaryngol. Head Neck Surg. 1986, 95, 292–297. [Google Scholar] [CrossRef]
- Hunt, J.R. On herpetic inflammations of the geniculate ganglion, a new syndrome and its complication. J. Nerv. Ment. Dis. 1907, 34, 73–96. [Google Scholar] [CrossRef]
- Wagner, G.; Klinge, H.; Sachse, M.M. Ramsay Hunt syndrome. J. Dtsch. Dermatol. Ges. 2012, 10, 238–244. [Google Scholar] [CrossRef]
- Choi, J.W.; Lee, J.; Lee, D.-H.; Shin, J.E.; Kim, C.-H. Mastoid effusion on temporal bone MRI in patients with Bell’s palsy and Ramsay Hunt syndrome. Sci. Rep. 2021, 11, 3127. [Google Scholar] [CrossRef]


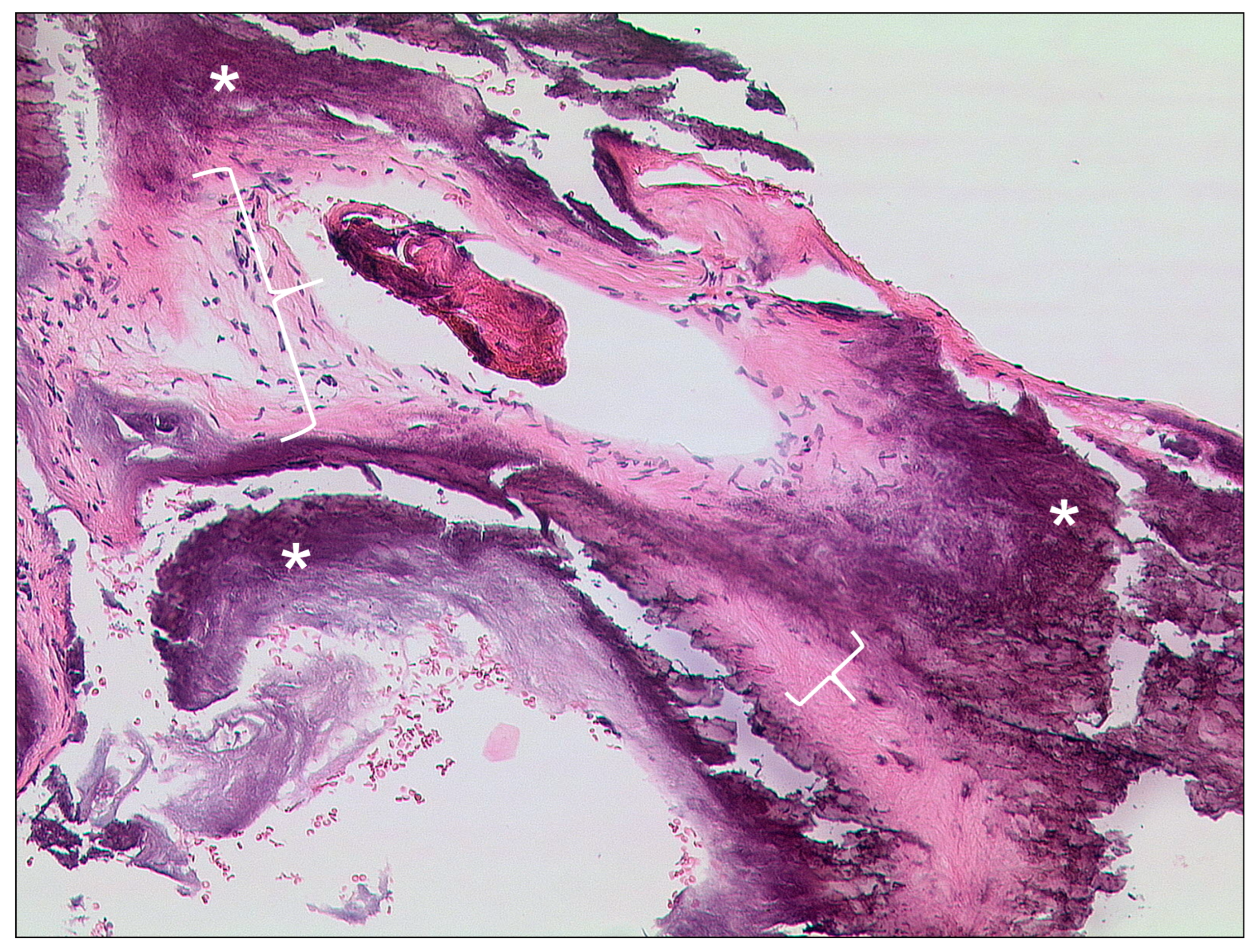
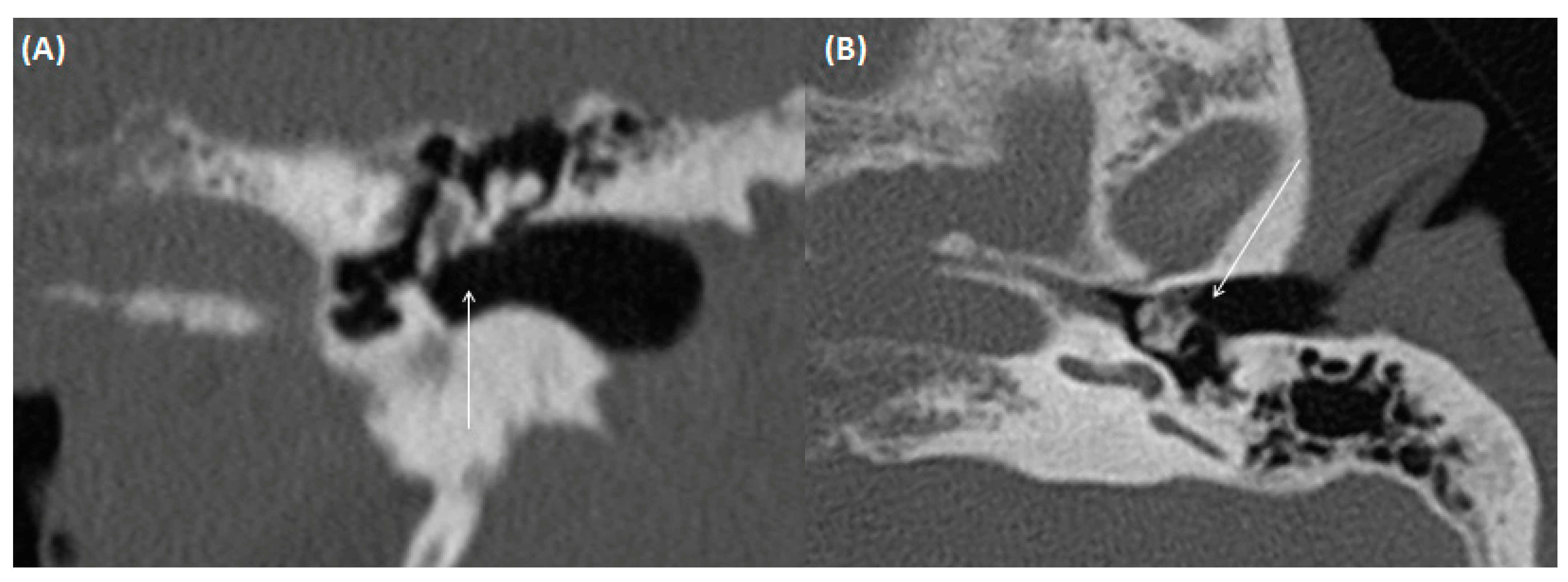
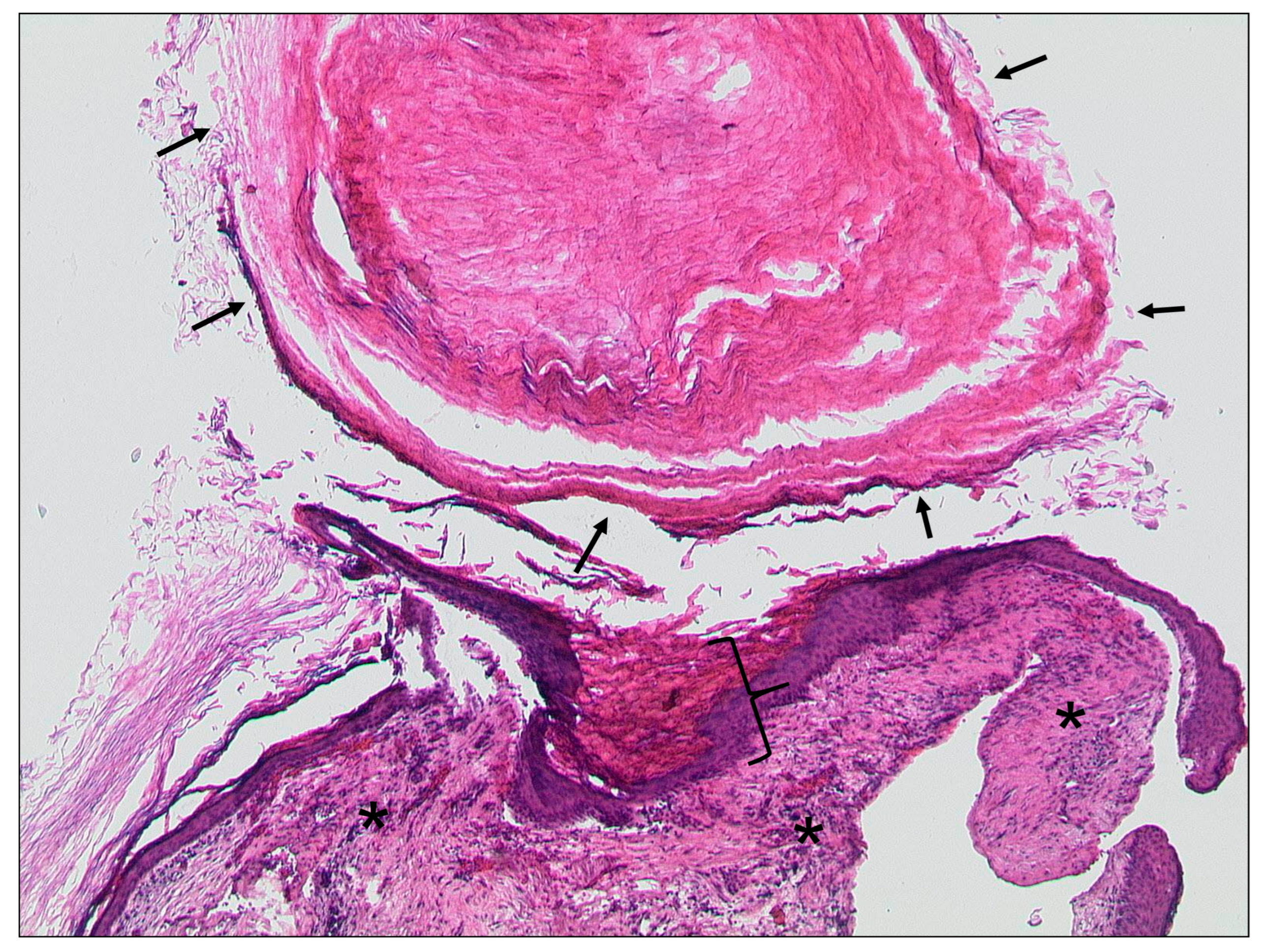
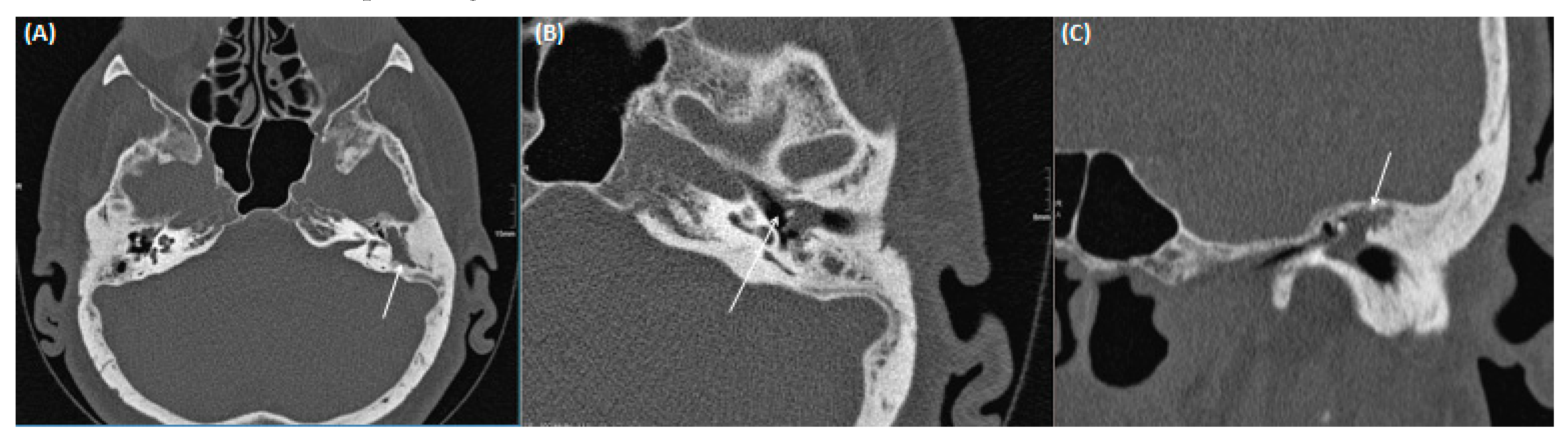

| External auditory canal | |
| Middle ear and mastoid | |
| Inner ear |
| Localization | Ossicular Displacement | Extension to Mastoid Antrum | |
|---|---|---|---|
| Pars flaccida cholesteatoma | Prussak’s space | Medial | Lateral to incus |
| Pars tensa cholesteatoma | Posterosuperior retraction | Lateral | Medial to incus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kloth, C.; Beck, A.; Sollmann, N.; Beer, M.; Horger, M.; Thaiss, W.M. Imaging of Pathologies of the Temporal Bone and Middle Ear: Inflammatory Diseases, Their Mimics and Potential Complications—Pictorial Review. Tomography 2023, 9, 2190-2210. https://doi.org/10.3390/tomography9060170
Kloth C, Beck A, Sollmann N, Beer M, Horger M, Thaiss WM. Imaging of Pathologies of the Temporal Bone and Middle Ear: Inflammatory Diseases, Their Mimics and Potential Complications—Pictorial Review. Tomography. 2023; 9(6):2190-2210. https://doi.org/10.3390/tomography9060170
Chicago/Turabian StyleKloth, Christopher, Annika Beck, Nico Sollmann, Meinrad Beer, Marius Horger, and Wolfgang Maximilian Thaiss. 2023. "Imaging of Pathologies of the Temporal Bone and Middle Ear: Inflammatory Diseases, Their Mimics and Potential Complications—Pictorial Review" Tomography 9, no. 6: 2190-2210. https://doi.org/10.3390/tomography9060170
APA StyleKloth, C., Beck, A., Sollmann, N., Beer, M., Horger, M., & Thaiss, W. M. (2023). Imaging of Pathologies of the Temporal Bone and Middle Ear: Inflammatory Diseases, Their Mimics and Potential Complications—Pictorial Review. Tomography, 9(6), 2190-2210. https://doi.org/10.3390/tomography9060170









