The Tomosynthesis Broken Halo Sign: Diagnostic Utility for the Classification of Newly Diagnosed Breast Tumors
Abstract
1. Introduction
2. Materials and Methods
2.1. Approval by the Ethical Review Board (ERB)
2.2. Patient Inclusion and Case Classification
2.3. Technical Information
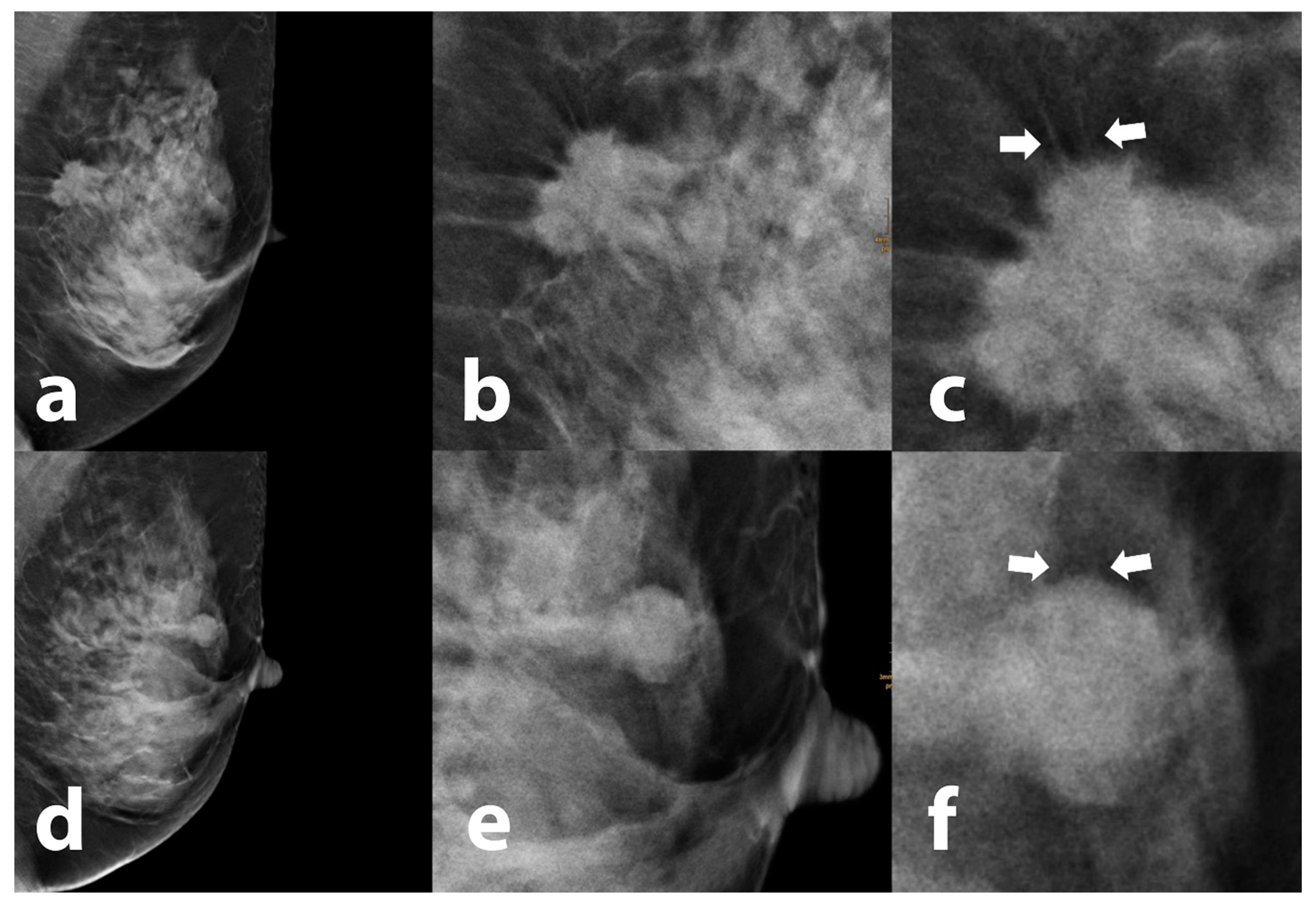
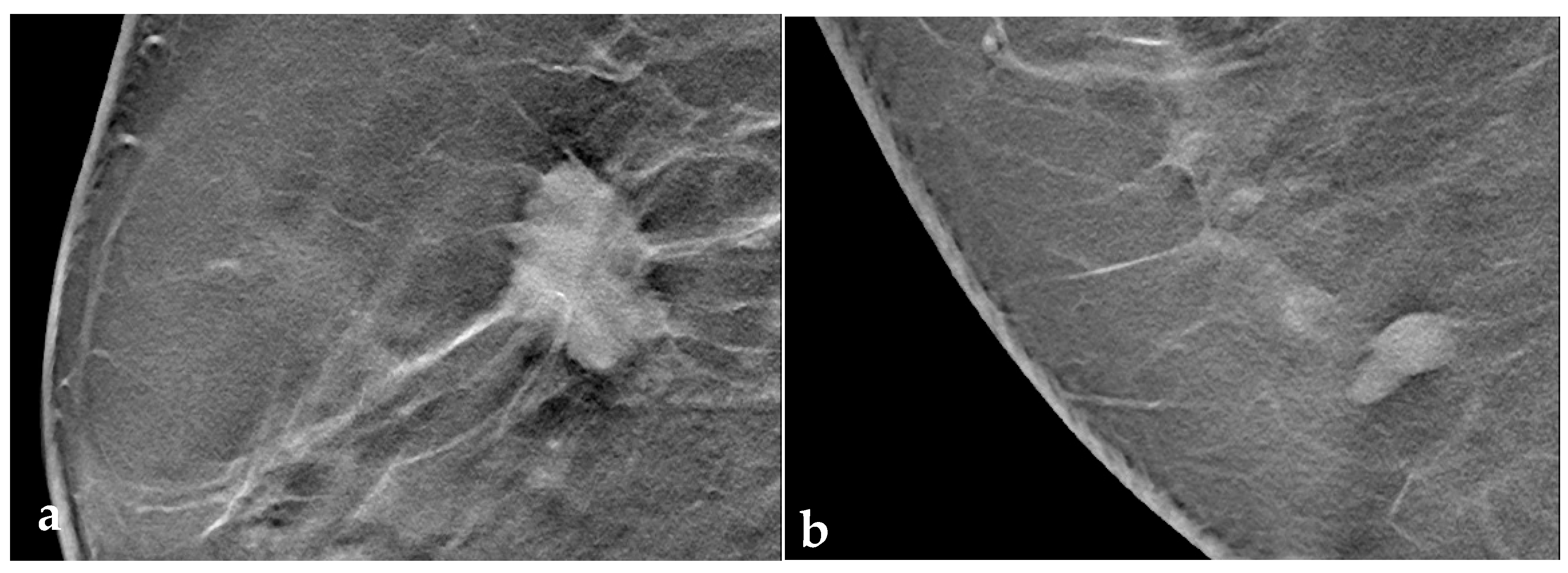
2.4. Image Interpretation and Data Collection
2.5. Core-Needle Biopsy Procedure
2.6. Histologic Evaluation
2.7. Statistics
3. Results
3.1. Patient Characteristics
3.2. Tumor Characteristics
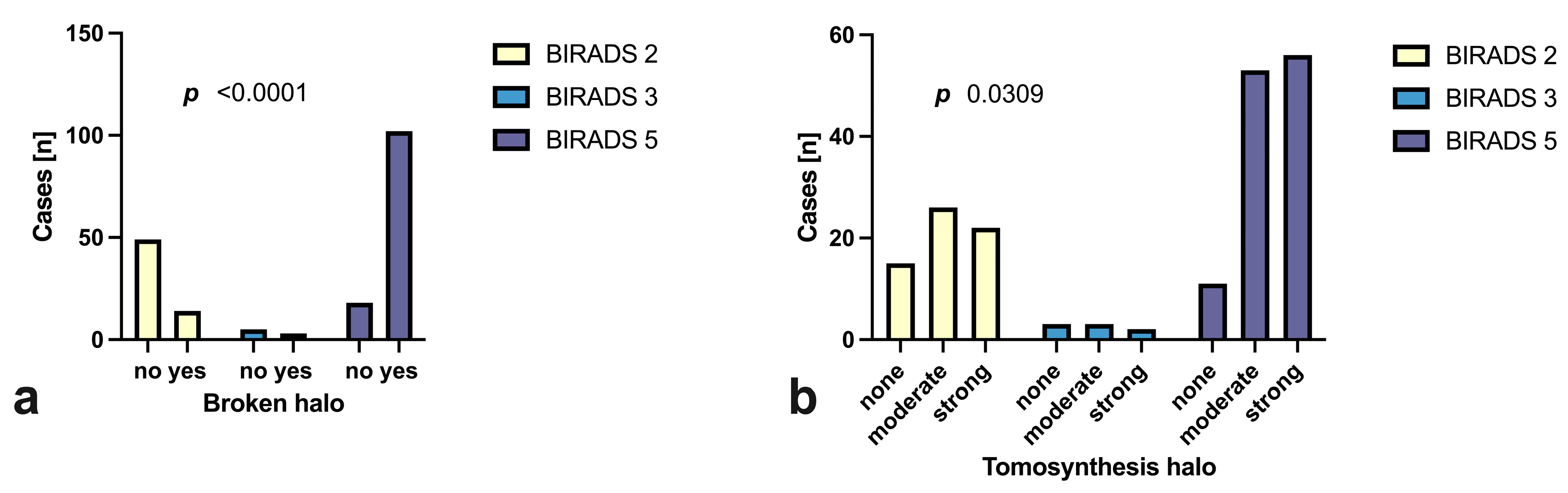
3.3. Tomosynthesis Evaluation
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IC | interval cancer |
| CI | confidence interval |
| ACR | American College of Radiology |
| IBC | interval breast cancer |
| NST | no special type |
| ILC | invasive lobular carcinoma |
| DCIS | ductal carcinoma in situ |
| LN | lymph node |
| RIS | radiology information systems |
| PGMI | Perfect, Good, Moderate, Inadequate |
| MLO | mediolateral oblique |
References
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast Cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- Uematsu, T. The Emerging Role of Breast Tomosynthesis. Breast Cancer 2013, 20, 204–212. [Google Scholar] [CrossRef]
- Hassan, R.M.; Almalki, Y.E.; Basha, M.A.A.; Alduraibi, S.K.; Aboualkheir, M.; Almushayti, Z.A.; Aldhilan, A.S.; Aly, S.A.; Alshamy, A.A. The Impact of Adding Digital Breast Tomosynthesis to BI-RADS Categorization of Mammographically Equivocal Breast Lesions. Diagnostics 2023, 13, 1423. [Google Scholar] [CrossRef]
- Friedewald, S.M.; Rafferty, E.A.; Rose, S.L.; Durand, M.A.; Plecha, D.M.; Greenberg, J.S.; Hayes, M.K.; Copit, D.S.; Carlson, K.L.; Cink, T.M.; et al. Breast Cancer Screening Using Tomosynthesis in Combination with Digital Mammography. JAMA 2014, 311, 2499–2507. [Google Scholar] [CrossRef]
- Ciatto, S.; Houssami, N.; Bernardi, D.; Caumo, F.; Pellegrini, M.; Brunelli, S.; Tuttobene, P.; Bricolo, P.; Fantò, C.; Valentini, M.; et al. Integration of 3D Digital Mammography with Tomosynthesis for Population Breast-Cancer Screening (STORM): A Prospective Comparison Study. Lancet Oncol. 2013, 14, 583–589. [Google Scholar] [CrossRef]
- Andersson, I.; Ikeda, D.M.; Zackrisson, S.; Ruschin, M.; Svahn, T.; Timberg, P.; Tingberg, A. Breast Tomosynthesis and Digital Mammography: A Comparison of Breast Cancer Visibility and BIRADS Classification in a Population of Cancers with Subtle Mammographic Findings. Eur. Radiol. 2008, 18, 2817–2825. [Google Scholar] [CrossRef]
- Teertstra, H.J.; Loo, C.E.; van den Bosch, M.A.A.J.; van Tinteren, H.; Rutgers, E.J.T.; Muller, S.H.; Gilhuijs, K.G.A. Breast Tomosynthesis in Clinical Practice: Initial Results. Eur. Radiol. 2010, 20, 16–24. [Google Scholar] [CrossRef]
- Berment, H.; Becette, V.; Mohallem, M.; Ferreira, F.; Chérel, P. Masses in Mammography: What Are the Underlying Anatomopathological Lesions? Diagn. Interv. Imaging 2014, 95, 124–133. [Google Scholar] [CrossRef]
- Liberman, L.; Abramson, A.F.; Squires, F.B.; Glassman, J.R.; Morris, E.A.; Dershaw, D.D. The Breast Imaging Reporting and Data System: Positive Predictive Value of Mammographic Features and Final Assessment Categories. AJR Am. J. Roentgenol. 1998, 171, 35–40. [Google Scholar] [CrossRef]
- Schrading, S.; Kuhl, C.K. Mammographic, US, and MR Imaging Phenotypes of Familial Breast Cancer. Radiology 2008, 246, 58–70. [Google Scholar] [CrossRef]
- Kaas, R.; Kroger, R.; Hendriks, J.H.C.L.; Besnard, A.P.E.; Koops, W.; Pameijer, F.A.; Prevoo, W.; Loo, C.E.; Muller, S.H. The Significance of Circumscribed Malignant Mammographic Masses in the Surveillance of BRCA 1/2 Gene Mutation Carriers. Eur. Radiol. 2004, 14, 1647–1653. [Google Scholar] [CrossRef]
- Nakashima, K.; Uematsu, T.; Itoh, T.; Takahashi, K.; Nishimura, S.; Hayashi, T.; Sugino, T. Comparison of Visibility of Circumscribed Masses on Digital Breast Tomosynthesis (DBT) and 2D Mammography: Are Circumscribed Masses Better Visualized and Assured of Being Benign on DBT? Eur. Radiol. 2017, 27, 570–577. [Google Scholar] [CrossRef]
- Sánchez-Camacho González-Carrato, M.P.; Romero Castellano, C.; Aguilar Angulo, P.M.; Cruz Hernández, L.M.; Sánchez Casado, M.; Ruiz Martín, J.; Fraile Alonso, I.; Pinto Varela, J.M.; Martínez-Vizcaíno, V. Diagnostic Value of Halo Sign in Young Women (Aged 45 to 49 Years) in a Breast Screening Programme with Synthesized 2D Mammography. Br. J. Radiol. 2018, 91, 20180444. [Google Scholar] [CrossRef]
- Cupples, T.E.; Eklund, G.W.; Cardenosa, G. Mammographic Halo Sign Revisited. Radiology 1996, 199, 105–108. [Google Scholar] [CrossRef]
- Sujlana, P.S.; Mahesh, M.; Vedantham, S.; Harvey, S.C.; Mullen, L.A.; Woods, R.W. Digital Breast Tomosynthesis: Image Acquisition Principles and Artifacts. Clin. Imaging 2019, 55, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Söderman, C.; Johnsson, Å.A.; Vikgren, J.; Norrlund, R.R.; Molnar, D.; Svalkvist, A.; Månsson, L.G.; Båth, M. Influence of the In-Plane Artefact in Chest Tomosynthesis on Pulmonary Nodule Size Measurements. Radiat. Prot. Dosim. 2016, 169, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Santner, T.; Santner, W.; Gutzeit, A. Effect of Image Quality and Motivation of Radiographer Teams in Mammography after Dedicated Training and the Use of an Evaluation Tool like PGMI. Radiography 2021, 27, 1124–1129. [Google Scholar] [CrossRef]
- American College of Radiology. ACR BI-RADS Atlas: Breast Imaging Reporting and Data System; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- EFSUMB Newsletter Guidelines for Ultrasound Guided Breast Biopsy. Ultraschall Med. Eur. J. Ultrasound 2005, 26, 241–244. [CrossRef]
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumours, Breast Tumours, 5th ed.; World Health Organization: Geneva, Switzerland, 2019; Volume 2. [Google Scholar]
- Wang, Y.; Ikeda, D.M.; Narasimhan, B.; Longacre, T.A.; Bleicher, R.J.; Pal, S.; Jackman, R.J.; Jeffrey, S.S. Estrogen Receptor-Negative Invasive Breast Cancer: Imaging Features of Tumors with and without Human Epidermal Growth Factor Receptor Type 2 Overexpression. Radiology 2008, 246, 367–375. [Google Scholar] [CrossRef]
- Wolfe, J.N. Xeroradiography of the Breast. Prog. Clin. Biol. Res. 1977, 12, 213–221. [Google Scholar] [PubMed]
- Gordenne, W.H.; Malchair, F.L. Mach Bands in Mammography. Radiology 1988, 169, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sun, J.; Guo, F.; Nanding, A.; Li, Q.; Jiang, D. Focus on the Predictive Value of Subclassification of Extratumoral Structural Abnormalities for Malignant Nonspiculate and Noncalcified Masses on Digital Mammography. Front. Genet. 2022, 13. [Google Scholar] [CrossRef]
- Woods, R.W.; Oliphant, L.; Shinki, K.; Page, D.; Shavlik, J.; Burnside, E. Validation of Results from Knowledge Discovery: Mass Density as a Predictor of Breast Cancer. J. Digit. Imaging 2010, 23, 554–561. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soysal, S.D.; Tzankov, A.; Muenst, S.E. Role of the Tumor Microenvironment in Breast Cancer. Pathobiology 2015, 82, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.S.; Almeida, C.R.; Helguero, L.A.; Duarte, I.F. Metabolic Crosstalk in the Breast Cancer Microenvironment. Eur. J. Cancer 2019, 121, 154–171. [Google Scholar] [CrossRef]
- Sofopoulos, M.; Fortis, S.P.; Vaxevanis, C.K.; Sotiriadou, N.N.; Arnogiannaki, N.; Ardavanis, A.; Vlachodimitropoulos, D.; Perez, S.A.; Baxevanis, C.N. The Prognostic Significance of Peritumoral Tertiary Lymphoid Structures in Breast Cancer. Cancer Immunol. Immunother. 2019, 68, 1733–1745. [Google Scholar] [CrossRef]
- Martinez, J.; Smith, P.C. The Dynamic Interaction between Extracellular Matrix Remodeling and Breast Tumor Progression. Cells 2021, 10, 1046. [Google Scholar] [CrossRef]
- Zhou, W.; Sollie, T.; Tot, T.; Pinder, S.E.; Amini, R.-M.; Blomqvist, C.; Fjällskog, M.-L.; Christensson, G.; Abdsaleh, S.; Wärnberg, F. Breast Cancer with Neoductgenesis: Histopathological Criteria and Its Correlation with Mammographic and Tumour Features. Int. J. Breast Cancer 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Jing, J.; Zhao, Y.; Li, H.; Zhang, S.; Shi, Z. Prognostic Value of Vascular Endothelial Growth Factor in Breast Cancer. Oncologist 2000, 5 (Suppl. S1), 566–568. [Google Scholar] [CrossRef]
- Tirada, N.; Li, G.; Dreizin, D.; Robinson, L.; Khorjekar, G.; Dromi, S.; Ernst, T. Digital Breast Tomosynthesis: Physics, Artifacts, and Quality Control Considerations. Radiographics 2019, 39, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Moore, R.H.; Kopans, D.B. Voting Strategy for Artifact Reduction in Digital Breast Tomosynthesis. Med. Phys. 2006, 33, 2461–2471. [Google Scholar] [CrossRef] [PubMed]

| Overall (%, n) | Histological Diagnosis (%, n) | Grade (%, n) | |
|---|---|---|---|
| BI-RADS 2 | 33.0 (63) | Mastopathy: 39.1 (18) | |
| Fibroadenoma: 39.1 (18) | |||
| Focal mastitis/inflammatory: 19.6% (9) | |||
| Complicated cyst: 17.9% (10) | |||
| Other: 12.5% (7) | |||
| BI-RADS 3 | 4.2 (8) | Phyllodes tumor: 75.0% (6) | |
| Atypical ductal hyperplasia (ADH): 25.0% (2) | |||
| BI-RADS 5 | 62.8 (120) | No special type (NST): 80.8% (97) | Grade 1: 16.5 (16) |
| Grade 2: 59.8 (58) | |||
| Grade 3: 23.7 (23) | |||
| Invasive lobular carcinoma (ILC): 6.7% (8) | Grade 1: 0.0 (0) | ||
| Grade 2: 87.5 (7) | |||
| Grade 3: 12.5 (1) | |||
| Ductal carcinoma in situ (DCIS): 4.2% (5) | low-grade: 20.0 (1) | ||
| high-grade: 80.0 (4) | |||
| Other: 8.3% (10) | Grade 1: 50.0 (5) | ||
| Grade 2: 40.0 (4) | |||
| Grade 3: 10.0 (1) |
| Halo Visibility: % * (n) | Broken Halo Sign: % * (n) | |||
|---|---|---|---|---|
| BI-RADS 2/3 | no halo: | 25.4 | (18) | 39.4 (21) |
| weak: | 33.8 | (24) | ||
| strong: | 40.8 | (29) | ||
| ∑ | 100 | (71) | ||
| BI-RADS 5 | no halo: | 13.3 | (16) | 89.4 (93) |
| weak: | 44.2 | (53) | ||
| strong: | 42.5 | (51) | ||
| ∑ | 100 | (120) | ||
| Regression Coefficient B | Standardized Error (SE) | |||||
|---|---|---|---|---|---|---|
| p-Value | OR | Lower | Upper | |||
| Architectural distortion (yes) | 1.251 | 0.497 | 0.012 | 3.493 | 1.319 | 9.247 |
| Maximum size | −0.029 | 0.018 | 0.107 | 0.971 | 0.937 | 1.006 |
| Maximum halo depth | 0.054 | 0.061 | 0.375 | 1.056 | 0.937 | 1.190 |
| Broken halo (yes) | 1.846 | 0.471 | 0.000 | 6.331 | 2.513 | 15.952 |
| Conspicuous margin (yes) | 1.702 | 0.618 | 0.006 | 5.485 | 1.635 | 18.400 |
| Shape (irregular) | 0.997 | 0.571 | 0.081 | 2.709 | 0.885 | 8.296 |
| Regression Coefficient B | Standardized Error (SE) | |||||
|---|---|---|---|---|---|---|
| p-Value | OR | Lower | Upper | |||
| Architectural distortion (yes) | 1.059 | 0.627 | 0.092 | 2.882 | 0.843 | 9.860 |
| Maximum size | −0.041 | 0.024 | 0.092 | 0.960 | 0.916 | 1.007 |
| Maximum halo depth | 0.010 | 0.102 | 0.920 | 1.010 | 0.828 | 1.233 |
| Broken halo (yes) | 1.653 | 0.769 | 0.031 | 5.224 | 1.158 | 23.564 |
| Margin (diffuse) | 2.025 | 1.178 | 0.086 | 7.578 | 0.753 | 76.239 |
| Shape (irregular) | 1.190 | 0.856 | 0.164 | 3.286 | 0.614 | 17.590 |
| Predictor | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Likelihood Ratio | CCR (%) | p-Value |
|---|---|---|---|---|---|---|---|
| Max. tumor diameter (cut-off 10.8 mm) | 88.3 (81.4 to 92.9) | 26.8 (17.9 to 38.1) | 67.1 (59.4 to 73.9) | 57.6 (40.7 to 72.8) | 1.21 | 65.5 | 0.0100 |
| Architectural distortion | 72.5 (63.9 to 79.7) | 78.9 (68.0 to 86.8) | 85.3 (77.2 to 90.9) | 62.9 (52.6 to 72.6) | 3.43 | 74.9 | <0.0001 |
| Tumor surface (lobulated, diffuse, serrated) | 96.7 (91.7 to 98.7) | 46.5 (35.4 to 58.0) | 75.3 (68.9 to 81.5) | 89.2 (75.3 to 95.7) | 1.81 | 78.0 | <0.0001 |
| Broken halo sign | 85.0 (77.5 to 90.3) | 76.1 (65.0 to 84.5) | 85.7 (78.3 to 90.9) | 75.00 (63.9 to 83.6) | 3.55 | 81.7 | <0.0001 |
| Broken halo + architectural distortion | 65.8 (57.0 to 73.7) | 90.1 (81.0 to 95.1) | 91.9 (84.1 to 96.0) | 61.0 (51.4 to 69.7) | 6.677 | 74.9 | <0.0001 |
| Broken halo + conspicuous tumor surface (lobulated, diffuse, serrated) | 83.3 (75.7 to 88.9) | 78.9 (68.0 to 86.8) | 87.0 (79.6 to 91.9) | 73.7 (62.8 to 82.3) | 3.94 | 81.7 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deeg, J.; Swoboda, M.; Egle, D.; Wieser, V.; Soleiman, A.; Ladenhauf, V.; Galijasevic, M.; Amort, B.; Haushammer, S.; Daniaux, M.; et al. The Tomosynthesis Broken Halo Sign: Diagnostic Utility for the Classification of Newly Diagnosed Breast Tumors. Tomography 2023, 9, 1987-1998. https://doi.org/10.3390/tomography9060155
Deeg J, Swoboda M, Egle D, Wieser V, Soleiman A, Ladenhauf V, Galijasevic M, Amort B, Haushammer S, Daniaux M, et al. The Tomosynthesis Broken Halo Sign: Diagnostic Utility for the Classification of Newly Diagnosed Breast Tumors. Tomography. 2023; 9(6):1987-1998. https://doi.org/10.3390/tomography9060155
Chicago/Turabian StyleDeeg, Johannes, Michael Swoboda, Daniel Egle, Verena Wieser, Afschin Soleiman, Valentin Ladenhauf, Malik Galijasevic, Birgit Amort, Silke Haushammer, Martin Daniaux, and et al. 2023. "The Tomosynthesis Broken Halo Sign: Diagnostic Utility for the Classification of Newly Diagnosed Breast Tumors" Tomography 9, no. 6: 1987-1998. https://doi.org/10.3390/tomography9060155
APA StyleDeeg, J., Swoboda, M., Egle, D., Wieser, V., Soleiman, A., Ladenhauf, V., Galijasevic, M., Amort, B., Haushammer, S., Daniaux, M., & Gruber, L. (2023). The Tomosynthesis Broken Halo Sign: Diagnostic Utility for the Classification of Newly Diagnosed Breast Tumors. Tomography, 9(6), 1987-1998. https://doi.org/10.3390/tomography9060155






