The Role of Contrast-Enhanced Ultrasound (CEUS) in the Detection of Neoplastic Portal Vein Thrombosis in Patients with Hepatocellular Carcinoma
Abstract
1. Introduction
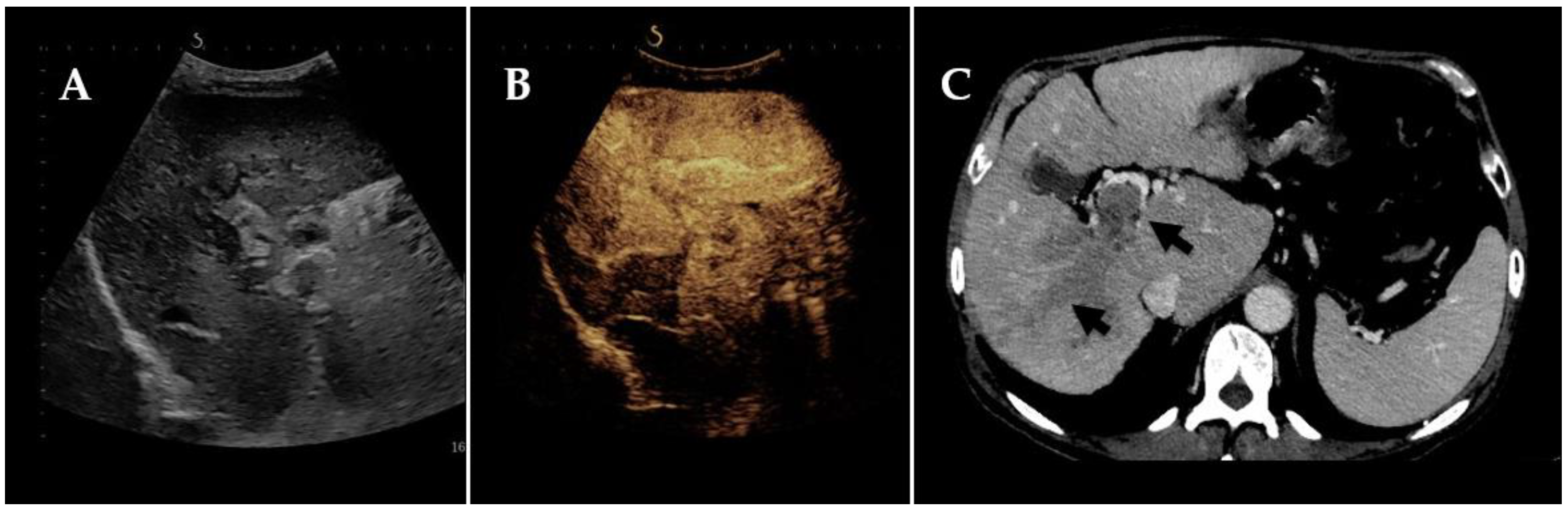
2. Radiological Footsteps towards the Diagnosis of Neoplastic Portal Vein Thrombosis
3. Discussion and Future Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Massarweh, N.N.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control 2017, 24, 1073274817729245. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Chen, Y.S.; Lo, G.H.; Wu, T.C.; Yeh, J.H.; Yeh, M.L.; Dai, C.Y.; Huang, J.F.; Chuang, W.L.; Roberts, L. Resubclassification and clinical management for Barcelona Clinic Liver Cancer Stage C hepatocellular carcinoma. Hepatol. Int. 2021, 15, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- Cerrito, L.; Annichiarico, B.E.; Iezzi, R.; Gasbarrini, A.; Pompili, M.; Ponziani, F.R. Treatment of hepatocellular carcinoma in patients with portal vein tumor thrombosis: Beyond the known frontiers. World J. Gastroenterol. 2019, 25, 4360–4382. [Google Scholar] [CrossRef] [PubMed]
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491. [Google Scholar] [CrossRef]
- Kudo, M.; Chung, H.; Osaki, Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): Its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J. Gastroenterol. 2003, 38, 207–215. [Google Scholar] [CrossRef]
- Chun, Y.H.; Kim, S.U.; Park, J.Y.; Han, K.H.; Chon, C.Y.; Kim, B.K.; Choi, G.H.; Kim, K.S.; Choi, J.S.; Ahn, S.H. Prognostic value of the 7th edition of the AJCC staging system as a clinical staging system in patients with hepatocellular carcinoma. Eur. J. Cancer 2011, 47, 2568–2575. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, L.; Ge, R.L.; Sun, Y.; Wei, G. Survival benefit of surgical treatment for hepatocellular carcinoma with inferior vena cava/right atrium tumor thrombus: Results of a retrospective cohort study. Ann. Surg. Oncol. 2013, 20, 914–922. [Google Scholar] [CrossRef]
- Tarantino, L.; Francica, G.; Sordelli, I.; Esposito, F.; Giorgio, A.; Sorrentino, P.; De Stefano, G.; Sarno, A.D.; Ferraioli, G.; Sperlongano, P. Diagnosis of benign and malignant portal vein thrombosis in cirrhotic patients with hepatocellular carcinoma: Color Doppler US, contrast-enhanced US, and fine-needle biopsy. Abdom. Imaging 2006, 31, 537–544. [Google Scholar] [CrossRef]
- Shi, J.; Lai, E.C.; Li, N.; Guo, W.X.; Xue, J.; Lau, W.Y.; Wu, M.C.; Cheng, S.Q. A new classification for hepatocellular carcinoma with portal vein tumor thrombus. J. Hepatobiliary Pancreat. Sci. 2011, 18, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Wang, K.; Zhang, X.P.; Feng, J.K.; Chai, Z.T.; Guo, W.X.; Shi, J.; Wu, M.C.; Lau, W.Y.; Cheng, S.Q. A new classification for hepatocellular carcinoma with hepatic vein tumor thrombus. Hepatobiliary Surg. Nutr. 2020, 9, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Li, A.J.; Zhou, W.P.; Lin, C.; Lang, X.L.; Wang, Z.G.; Yang, X.Y.; Tang, Q.H.; Tao, R.; Wu, M.C. Surgical treatment of Hepatocellular carcinoma with inferior vena cava tumor thrombus: A new classification for surgical guidance. Hepatobiliary Pancreat. Dis. Int. 2013, 12, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Kokudo, T.; Hasegawa, K.; Yamamoto, S.; Shindoh, J.; Takemura, N.; Aoki, T.; Sakamoto, Y.; Makuuchi, M.; Sugawara, Y.; Kokudo, N. Surgical treatment of hepatocellular carcinoma associated with hepatic vein tumor thrombosis. J. Hepatol. 2014, 61, 583–588. [Google Scholar] [CrossRef]
- Ikai, I.; Arii, S.; Okazaki, M.; Okita, K.; Omata, M.; Kojiro, M.; Takayasu, K.; Nakanuma, Y.; Makuuchi, M.; Matsuyama, Y.; et al. Report of the 17th Nationwide Follow-up Survey of Primary Liver Cancer in Japan. Hepatol. Res. 2007, 37, 676–691. [Google Scholar] [CrossRef]
- Chen, H.; Turon, F.; Hernández-Gea, V.; Fuster, J.; Garcia-Criado, A.; Barrufet, M.; Darnell, A.; Fondevila, C.; Garcia-Valdecasas, J.C.; Garcia-Pagán, J.C. Nontumoral portal vein thrombosis in patients awaiting liver transplantation. Liver Transpl. 2016, 22, 352–365. [Google Scholar] [CrossRef]
- Gadani, S.; Partovi, S.; Levitin, A.; Zerona, N.; Sengupta, S.; D’Amico, G.; Diago Uso, T.; Menon, K.V.N.; Quintini, C. Narrative review of portal vein thrombosis in cirrhosis: Pathophysiology, diagnosis, and management from an interventional radiology perspective. Cardiovasc. Diagn. Ther. 2022, 12, 135–146. [Google Scholar] [CrossRef]
- Webster, G.J.; Burroughs, A.K.; Riordan, S.M. Review article: Portal vein thrombosis—New insights into aetiology and management. Aliment. Pharmacol. Ther. 2005, 1, 1–9. [Google Scholar] [CrossRef]
- Osman, N.M.M.; Samy, L.A.M. Benign and malignant portal venous thrombosis: Multi-modality imaging evaluation. Egypt. J. Radiol. Nucl. Med. 2016, 47, 387–397. [Google Scholar] [CrossRef]
- Piscaglia, F.; Gianstefani, A.; Ravaioli, M.; Golfieri, R.; Cappelli, A.; Giampalma, E.; Sagrini, E.; Imbriaco, G.; Pinna, A.D.; Bolondi, L.; et al. Criteria for diagnosing benign portal vein thrombosis in the assessment of patients with cirrhosis and hepatocellular carcinoma for liver transplantation. Liver Transpl. 2010, 16, 658–667. [Google Scholar] [CrossRef]
- Luca, A.; Caruso, S.; Milazzo, M.; Marrone, G.; Mamone, G.; Crinò, F.; Maruzzelli, L.; Miraglia, R.; Floridia, G.; Vizzini, G. Natural course of extrahepatic nonmalignant partial portal vein thrombosis in patients with cirrhosis. Radiology 2012, 265, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.S.; Lee, J.M.; Yoon, J.H.; Jang, S.; Chung, J.W.; Lee, K.B.; Yi, N.J.; Lee, J.H. How to best detect portal vein tumor thrombosis in patients with hepatocellular carcinoma meeting the Milan criteria: Gadoxetic acid-enhanced MRI versus contrast-enhanced CT. Liver Cancer 2020, 9, 293–307. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.M.; Yoon, J.H.; Lee, D.H.; Lee, K.B.; Han, J.K.; Choi, B.I. Portal vein thrombosis in patients with hepatocellular carcinoma: Diagnostic accuracy of gadoxetic acid-enhanced MR imaging. Radiology 2016, 279, 773–783. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sofia, C.; Cattafi, A.; Silipigni, S.; Pitrone, P.; Carerj, M.L.; Marino, M.A.; Pitrone, A.; Ascenti, G. Portal vein thrombosis in patients with chronic liver diseases: From conventional to quantitative imaging. Eur. J. Radiol. 2021, 142, 109859. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, L.; Ambrosino, P.; Di Minno, M.N. Contrast-enhanced ultrasound in differentiating malignant from benign portal vein thrombosis in hepatocellular carcinoma. World J. Gastroenterol. 2015, 21, 9457–9460. [Google Scholar] [CrossRef]
- Rossi, S.; Rosa, L.; Ravetta, V.; Cascina, A.; Quaretti, P.; Azzaretti, A.; Scagnelli, P.; Tinelli, C.; Dionigi, P.; Calliada, F. Contrast-enhanced versus conventional and color Doppler sonography for the detection of thrombosis of the portal and hepatic venous systems. AJR Am. J. Roentgenol. 2006, 186, 763–773. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, J.; Zhang, C.; Song, Y.; Huang, P. Contrast-enhanced ultrasound for the characterization of portal vein thrombosis vs tumor-in-vein in HCC patients: A systematic review and meta-analysis. Eur. Radiol. 2020, 30, 2871–2880. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. Electronic address: Easloffice@easloffice.eu, EASL Clinical Practice Guidelines: Vascular diseases of the liver. J. Hepatol. 2016, 64, 179–202. [Google Scholar] [CrossRef]
- Claudon, M.; Dietrich, C.; Choi, B.; Cosgrove, D.; Kudo, M.; Nolsøe, C.P.; Piscaglia, F.; Wilson, S.R.; Barr, R.G.; Chammas, M.C.; et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver–update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med. Stuttg. Ger. 2013, 34, 11–29. [Google Scholar] [CrossRef]
- Tublin, M.E.; Dodd, G.D., 3rd; Baron, R.L. Benign and malignant portal vein thrombosis: Differentiation by CT characteristics. AJR Am. J. Roentgenol. 1997, 168, 719–723. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, J.; Cheng, C.; Liu, Q.; Sun, G.; Zuo, C. The role of 18F-FDG PET/CT in differentiating malignant from benign portal vein thrombosis. Abdom. Imaging 2014, 39, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Rammohan, A.; Jeswanth, S.; Sukumar, R.; Anand, L.; Kumar, P.S.; Srinivasan, U.P.; Ravi, R.; Ravichandran, P. Percutaneous ultrasound-guided fine-needle aspiration of portal vein thrombi as a diagnostic and staging technique for hepatocellular carcinoma. Abdom. Imaging 2013, 38, 1057–1060. [Google Scholar] [CrossRef] [PubMed]
- Pozniak, M.A.; Baus, K.M. Hepatofugal arterial signal in the main portal vein: An indicator of intravascular tumor spread. Radiology 1991, 180, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Numata, K.; Okazaki, H.; Nakamura, S.; Inoue, S.; Takamura, Y. Diagnosis of portal vein thrombosis in patients with hepatocellular carcinoma: Efficacy of color Doppler sonography compared with angiography. AJR Am. J. Roentgenol. 1993, 160, 1279–1283. [Google Scholar] [CrossRef]
- Lencioni, R.; Caramella, D.; Sanguinetti, F.; Battolla, L.; Falaschi, F.; Bartolozzi, C. Portal vein thrombosis after percutaneous ethanol injection for hepatocellular carcinoma: Value of color Doppler sonography in distinguishing chemical and tumor thrombi. AJR Am. J. Roentgenol. 1995, 164, 1125–1130. [Google Scholar] [CrossRef][Green Version]
- Ricci, P.; Cantisani, V.; Biancari, F.; Drud, F.M.; Coniglio, M.; Di Filippo, A.; Fasoli, F.; Passariello, R. Contrast-enhanced color Doppler US in malignant portal vein thrombosis. Acta Radiol. 2000, 41, 470–473. [Google Scholar] [CrossRef]
- Marshall, M.M.; Beese, R.C.; Muiesan, P.; Sarma, D.I.; O’Grady, J.; Sidhu, P.S. Assessment of portal venous system patency in the liver transplant candidate: A prospective study comparing ultrasound, microbubble-enhanced colour Doppler ultrasound, with arteriography and surgery. Clin. Radiol. 2002, 57, 377–383. [Google Scholar] [CrossRef]
- Chammas, M.C.; Oliveira, A.C.; Ávilla, M.J.D.; Moraes, P.H.; Takahashi, M.S. Characterization of Malignant Portal Vein Thrombosis with Contrast-Enhanced Ultrasonography. Ultrasound Med. Biol. 2019, 45, 50–55. [Google Scholar] [CrossRef]
- Raza, S.A.; Jang, H.J.; Kim, T.K. Differentiating malignant from benign thrombosis in hepatocellular carcinoma: Contrast-enhanced ultrasound. Abdom. Imaging 2014, 39, 153–161. [Google Scholar] [CrossRef]
- Song, Z.Z.; Huang, M.; Jiang, T.A.; Zhao, Q.Y.; Yao, L.; Mou, Y.; Zhao, J.K.; Ao, J.Y.; Chen, F.; Chen, Y. Diagnosis of portal vein thrombosis discontinued with liver tumors in patients with liver cirrhosis and tumors by contrast-enhanced US: A pilot study. Eur. J. Radiol. 2010, 75, 185–188. [Google Scholar] [CrossRef]
- Rossi, S.; Ghittoni, G.; Ravetta, V.; Torello Viera, F.; Rosa, L.; Serassi, M.; Scabini, M.; Vercelli, A.; Tinelli, C.; Dal Bello, B.; et al. Contrast-enhanced ultrasonography and spiral computed tomography in the detection and characterization of portal vein thrombosis complicating hepatocellular carcinoma. Eur. Radiol. 2008, 18, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, P.; D’Angelo, S.; Tarantino, L.; Ferbo, U.; Bracigliano, A.; Vecchione, R. Contrast-enhanced sonography versus biopsy for the differential diagnosis of thrombosis in hepatocellular carcinoma patients. World J. Gastroenterol. 2009, 15, 2245–2251. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Chen, Z.B.; Zhao, S.F.; Li, X.; Zhous, T.; Peng, Y.; Hu, Q.Q.; Lu, D.M.; Li, H.; Liu, J.J.; et al. The clinical value of contrast-enhanced ultrasound and quantitative analysis parameters in the diagnosis and classification of portal vein tumor thrombus. Int. J. Clin. Exp. Med. 2016, 9, 13466–13474. [Google Scholar]
- Kwon, J.H.; Yoo, S.H.; Nam, S.W.; Lee, Y.J.; Shin, Y.R. Diagnostic Role of Contrast-enhanced Ultrasound in the Discrimination of Malignant Portal Vein Thrombosis in Patients With Hepatocellular Carcinoma. Anticancer Res. 2020, 40, 4351–4363. [Google Scholar] [CrossRef]
- Sparchez, Z.; Radu, P.; Zaharia, T.; Kacso, G.; Diaconu, B.; Grigorescu, I.; Badea, R. B-mode and contrast enhanced ultrasound guided biopsy of portal vein thrombosis. Value in the diagnosis of occult hepatocellular carcinoma in liver cirrhosis. Med. Ultrason. 2010, 12, 286–294. [Google Scholar]
- Burciu, C.; Șirli, R.; Bende, F.; Fofiu, R.; Popescu, A.; Sporea, I.; Ghiuchici, A.M.; Miuțescu, B.; Dănilă, M. Usefulness of Imaging and Biological Tools for the Characterization of Portal Vein Thrombosis in Hepatocellular Carcinoma. Diagnostics 2022, 12, 1145. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, G.; Lu, M.D.; Xie, X.; Kuang, M.; Wang, W.; Xu, Z.; Lin, M.; Chen, L. Role of portal vein tumor thrombosis in quantitative perfusion analysis of contrast-enhanced ultrasound of hepatocellular carcinoma. Ultrasound Med. Biol. 2015, 41, 1277–1286. [Google Scholar] [CrossRef]
- Burns, P.N. Contrast ultrasound technology. In Contrast-Enhanced Ultrasound of Liver Diseases; Solbiati, L., Martegani, A., Leen, E., Correas, J.-M., Burns, P.N., Becker, D., Eds.; Springer: Milan, Italy, 2002; pp. 1–19. [Google Scholar]
- Gardeur, D.; Lautrou, J.; Millard, J.C.; Berger, N.; Metzger, J. Pharmacokinetics of contrast media: Ex-perimental results in dog and man with CT implication. J. Comput. Assist. Tomogr. 1980, 4, 178–185. [Google Scholar] [CrossRef]
- Wilson, S.R.; Kim, T.K.; Jang, H.J.; Burns, P.N. Enhancement patterns of focal liver masses: Discordance between contrast-enhanced sonography and con-trast-enhanced CT and MRI. AJR 2007, 189, W7–W12. [Google Scholar] [CrossRef]
| Patients | Imaging Techniques | Sensitivity | Specificity | Accuracy | PPV | NPV | |
|---|---|---|---|---|---|---|---|
| Ricci et al. [36] | 56 (46 benign PVT 16 malignant PVT) | CDUS CEUS | 57% 94% | 95% 100% | NA | NA | NA |
| Lencioni et al. [35] | 19 (6 benign PVT; 13 malignant PVT) | CDUS | 92% | 100% | 95% | NA | NA |
| Tanaka et al. [34] | 18 PVT 22 no PVT | CDUS | 89% | 100% | 96% | NA | NA |
| Tarantino et al. [10] | 54 | CEUS CDUS FNB | 88% 20% 76% | 100% 100% 100% | 92.5% 50% 78.7% | 100% 100% 100% | 83.3% 42.5% 33.3% |
| Chammas et al. [38] | 43 (21 benign PVT; 22 malignant PVT) | CEUS CDUS | 90.9% 9.1% | 100% 100% | 95.3% 53.4% | NA | NA |
| Song et al. [40] | 17 | CEUS | 100% | 66.7% | 93.3% | NA | NA |
| Raza et al. [39] | 50 | CEUS | 100% | 83–92% | NA | 95–97% | 100% |
| Rossi et al. [41] | 50 | US CDUS CEUS CT | 86.4% 54.3% 98% 67.6% | - - 100% 60% | NA | NA | NA |
| Li et al. [43] | 93 | CEUS CT | 97.8% 96.7% | 90.2% 86.4% | 100% 97.7% | NA | NA |
| Kwon et al. [44] | 49 | CEUS DWI-MRI | 91.4% 90.6% | 100% 100% | 93.9% 93.2% | 100% 100% | 82.4% 80% |
| Sorrentino et al. [42] | 186 | CEUS FNB | 89.6% 89.6% | 100% 100% | ND | 100% 100% | 89.2% 89.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerrito, L.; Ainora, M.E.; Di Francesco, S.; Galasso, L.; Gasbarrini, A.; Zocco, M.A. The Role of Contrast-Enhanced Ultrasound (CEUS) in the Detection of Neoplastic Portal Vein Thrombosis in Patients with Hepatocellular Carcinoma. Tomography 2023, 9, 1976-1986. https://doi.org/10.3390/tomography9050154
Cerrito L, Ainora ME, Di Francesco S, Galasso L, Gasbarrini A, Zocco MA. The Role of Contrast-Enhanced Ultrasound (CEUS) in the Detection of Neoplastic Portal Vein Thrombosis in Patients with Hepatocellular Carcinoma. Tomography. 2023; 9(5):1976-1986. https://doi.org/10.3390/tomography9050154
Chicago/Turabian StyleCerrito, Lucia, Maria Elena Ainora, Silvino Di Francesco, Linda Galasso, Antonio Gasbarrini, and Maria Assunta Zocco. 2023. "The Role of Contrast-Enhanced Ultrasound (CEUS) in the Detection of Neoplastic Portal Vein Thrombosis in Patients with Hepatocellular Carcinoma" Tomography 9, no. 5: 1976-1986. https://doi.org/10.3390/tomography9050154
APA StyleCerrito, L., Ainora, M. E., Di Francesco, S., Galasso, L., Gasbarrini, A., & Zocco, M. A. (2023). The Role of Contrast-Enhanced Ultrasound (CEUS) in the Detection of Neoplastic Portal Vein Thrombosis in Patients with Hepatocellular Carcinoma. Tomography, 9(5), 1976-1986. https://doi.org/10.3390/tomography9050154






