Intraoperative Fluorophores: An Update on 5-Aminolevulinic Acid and Sodium Fluorescein in Resection of Tumors of the Central Nervous System and Metastatic Lesions—A Systematic Review and Meta-Analysis
Abstract
:1. Introduction/Background
1.1. 5-Aminolevulinic Acid
1.2. Sodium Fluorescein
2. Objectives
3. Methods
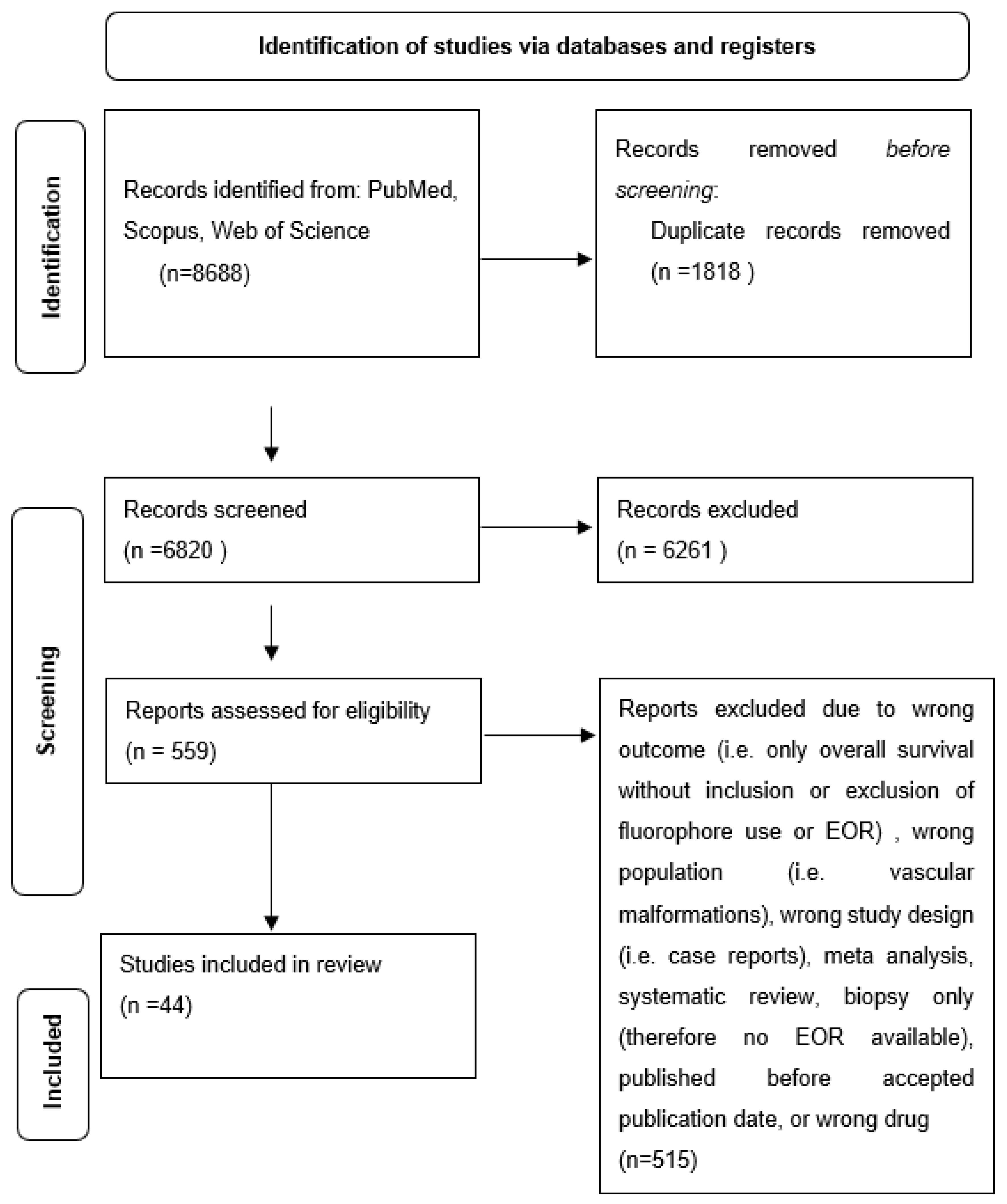
3.1. Literature Search
3.2. Study Selection
3.3. Data Extraction
3.4. Statistical Analysis
4. Results
4.1. Review Characteristics
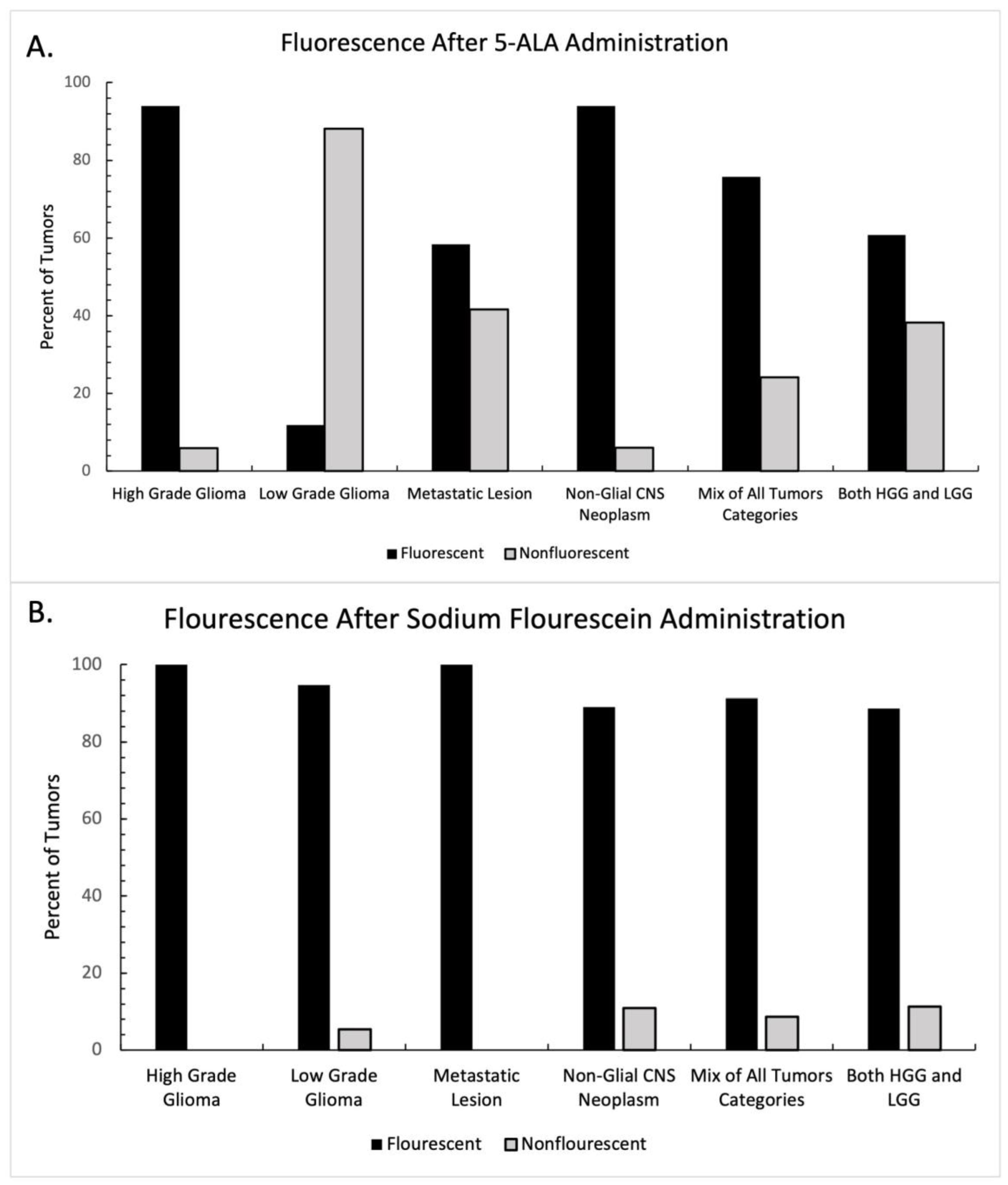
4.2. Intraoperative Fluorescence
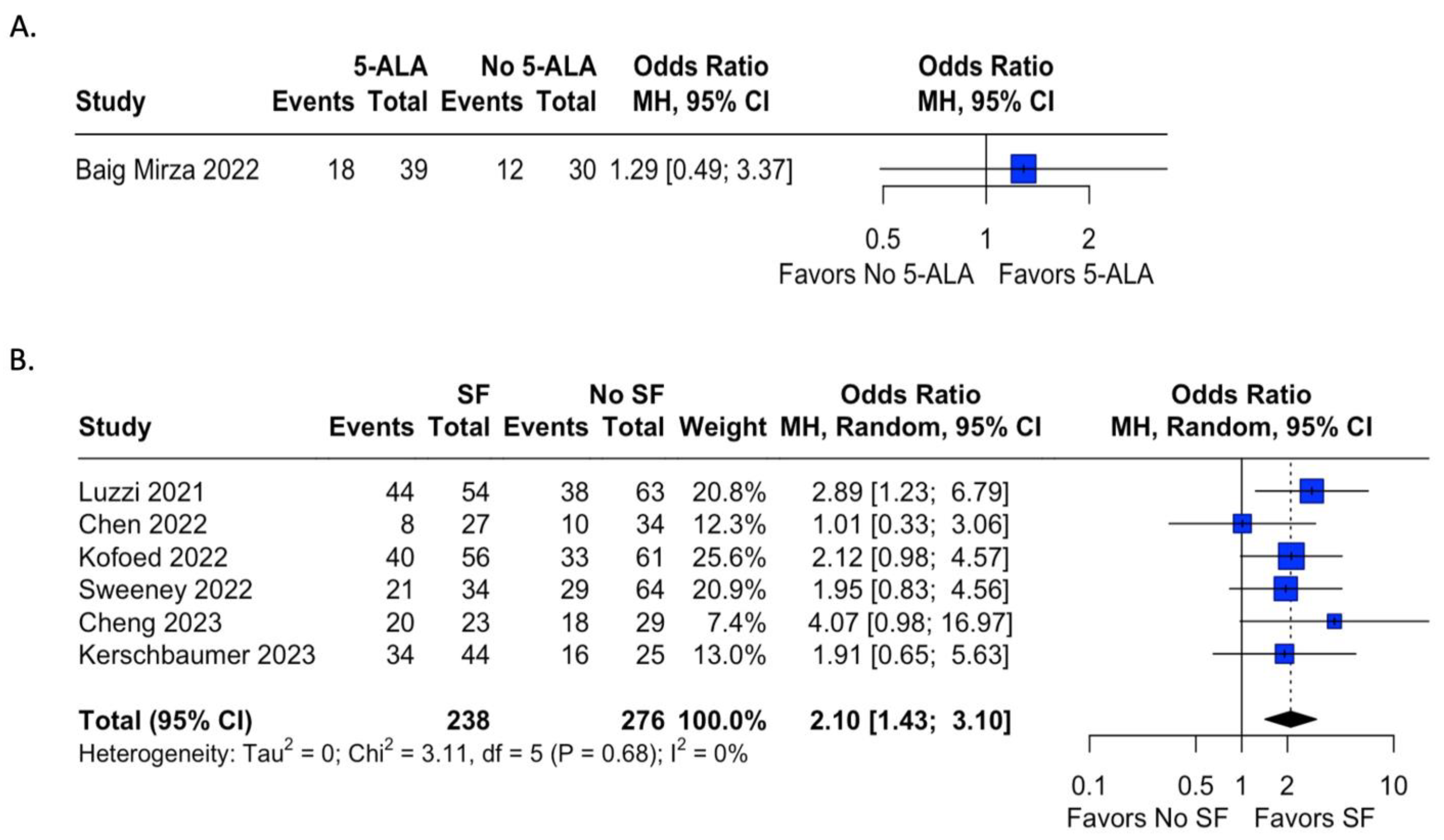
4.3. Extent of Resection
4.4. Fluorophore Safety
5. Discussion
| Author | Year | Type of Study | Fluorophore Used | Tumors Visualized (# of Visualization/Total Number Resected) | Type of Tumor | EOR with Fluorophore (#GTR/Total Number Resected) | EOR in Control Group (#GTR/Total Number Resected) | Adverse Events | Survival Benefit of Fluorophore |
|---|---|---|---|---|---|---|---|---|---|
| Alcazar et al. [46] | 2023 | Retrospective Case Series | Fluorescein | 3/3 | Other Non-Glial CNS Neoplasm (Various) | 3/3 | -- | None | -- |
| Baig Mirza et al. [57] | 2022 | Retrospective Cohort Study | 5-ALA | 28/39 | HGG (Grade III Anaplastic Astrocytoma) | 18/39 | 12/30 | 3 Major Adverse Events | No difference |
| Baig Mirza et al. [58] | 2021 | Retrospective Cohort Study | 5-ALA | -- | HGG (GBM) | 120/253 | -- | -- | Improved compared to non-5-ALA guided surgery |
| Batalov et al. [74] | 2021 | Retrospective Case Series | 5-ALA | 62/75 | HGG & LGG | -- | -- | -- | -- |
| Bettag et al. [35] | 2022 | Retrospective Case Series | 5-ALA | 22/26 | Metastatic Lesions | -- | -- | -- | -- |
| Cardali et al. [53] | 2022 | Retrospective Case Series | Fluorescein | 13/14 | Mixed Tumors (Various) | 11/14 | -- | None | -- |
| Certo et al. [39] | 2021 | Prospective Case Series | 5-ALA | 68/68 | HGG (GBM/Gliosarcoma) | 64/68 | -- | -- | -- |
| Chen et al. [50] | 2022 | Retrospective Cohort Study | Fluorescein | 27/27 | Other Non-Glial CNS Neoplasm (Medulloblastoma) | 8/27 | 10/34 | None | No difference |
| Cheng et al. [49] | 2023 | Retrospective Cohort Study | Fluorescein | 23/23 | Metastatic Lesions | 20/23 | 18/29 | None | Improved with Fluorescein-guided surgery |
| da Silva et al. [75] | 2022 | Retrospective Case Series | 5-ALA | 195/255 | Mixed Tumors (Various) | -- | -- | 1 Major Adverse Event | -- |
| de Laurentis et al. [54] | 2022 | Retrospective Case Series | Fluorescein | 35/45 | Mixed Tumors (Various) | -- | -- | -- | -- |
| Falco et al. [48] | 2023 | Retrospective Case Series | Fluorescein | 93/93 | HGG (GBM) | 77/93 | -- | None | -- |
| Falco et al. [41] | 2022 | Retrospective Case Series | Fluorescein | 41/44 | LGG (Pilocytic Astrocytoma) | 24/39 | -- | None | -- |
| Falco et al. [42] | 2022 | Retrospective Case Series | Fluorescein | 12/12 | Other Non-Glial CNS Neoplasm (Pleiomorphic Xanthoastrocytoma) | 8/12 | -- | None | -- |
| Goryaynov et al. [76] | 2022 | Retrospective Case Series | 5-ALA | 20/34 | HGG & LGG | -- | -- | None | -- |
| Hohne et al. [55] | 2021 | Retrospective Case Series | Fluorescein | 12/12 | Mixed Tumors (Various) | -- | -- | None | -- |
| Hosmann et al. [26] | 2021 | Retrospective Case Series | 5-ALA | 7/52 | LGG | 29/59 | -- | None | -- |
| Ibrahim et al. [62] | 2021 | Retrospective Case Series | 5-ALA | -- | HGG & LGG | 24/40 | -- | One Minor Side Effect | -- |
| Kerschbaumer et al. [72] | 2022 | Retrospective Cohort Study | Fluorescein | -- | Metastatic Lesions | 34/44 | 16/25 | -- | No Difference |
| Kiesel et al. [64] | 2021 | Retrospective Case Series | 5-ALA | 161/163 | HGG (GBM) | 84/94 | -- | None | -- |
| Kofoed et al. [71] | 2022 | Retrospective Case Series | Fluorescein | -- | Metastatic Lesions | 40/56 | 33/61 | -- | Associated With Increased Overall Survival |
| Kutlay et al. [43] | 2021 | Retrospective Case Series | Fluorescein | 18/18 | Mixed Tumors (Various) | 16/18 | -- | None | -- |
| Lavrador et al. [77] | 2023 | Retrospective Case Series | 5-ALA | 6/6 | HGG | -- | -- | -- | -- |
| Luzzi et al. [78] | 2021 | Retrospective Cohort Study | Fluorescein | -- | HGG (GBM + Anaplastic Astrocytoma) | 44/54 | 38/63 | -- | Improved Progression Free Survival, Similar Overall Survival |
| Maragkos et al. [79] | 2021 | Retrospective Case Series | 5-ALA | 16/16 | HGG | -- | -- | None | -- |
| Marhold et al. [31] | 2022 | Retrospective Case Series | 5-ALA | 8/29 | Metastatic Lesions | -- | -- | -- | -- |
| Mercea et al. [30] | 2021 | Retrospective Case Series | 5-ALA | 36/58 | Metastatic Lesions | 17/25 | -- | None | -- |
| Millesi et al. [40] | 2021 | Retrospective Case Series | 5-ALA | 25/31 | Other Non-Glial CNS Neoplasm (Ependymoma/Subependymoma) | 25/31 | -- | None | -- |
| Milos et al. [80] | 2023 | Prospective Case Series | 5-ALA | 5/14 | Mixed Tumors (Various) | 10/14 | -- | None | -- |
| Muscas et al. [81] | 2022 | Retrospective Case Series | 5-ALA | -- | HGG (GBM) | 41/65 | -- | -- | -- |
| Muther et al. [82] | 2022 | Retrospective Case Series | 5-ALA | 81/173 | HGG & LGG | -- | -- | -- | -- |
| Olguner et al. [44] | 2021 | Retrospective Case Series | Fluorescein | 47/49 | Mixed Tumors (Various) | 46/49 | -- | -- | -- |
| Ott et al. [51] | 2022 | Retrospective Case Series | Fluorescein | -- | Mixed Tumors (Various) | 11/14 | -- | None | -- |
| Schebesch et al. [70] | 2022 | Retrospective Case Series | Fluorescein | -- | HGG | -- | -- | None | -- |
| Schupper et al. [63] | 2022 | Prospective Cohort Study | 5-ALA | 69/69 | HGG | -- | -- | 15 minor adverse events | -- |
| Strickland et al. [83] | 2022 | Retrospective Case Series | 5-ALA | 24/30 | HGG (GBM + Anaplastic Astrocytoma + Anaplastic Oligodendroglioma) | 9/21 | -- | None | -- |
| Sun et al. [52] | 2022 | Retrospective Case Series | Fluorescein | 47/59 | Other Non-Glial CNS Neoplasm (Ependymoma) | 56/56 | -- | -- | -- |
| Sweeney et al. [84] | 2022 | Retrospective Cohort Study | Fluorescein | -- | GBM | 21/34 | 29/64 | -- | -- |
| Takeda et al. [85] | 2022 | Retrospective Case Series | 5-ALA | 4/7 | Mixed Tumors (Various) | 6/7 | -- | None | -- |
| Ung et al. [45] | 2022 | Retrospective Case Series | Fluorescein | 12/12 | Mixed Tumors (Various) | -- | -- | None | -- |
| Watts et al. [86] | 2023 | Prospective Case Study | 5-ALA | 85/99 | HGG (GBM) | 75/99 | -- | -- | -- |
| Xue et al. [47] | 2021 | Retrospective Case Series | Fluorescein | 44/50 | HGG + LGG | 41/50 | -- | -- | -- |
| Zeppa et al. * [73] | 2022 | Retrospective Case Series | 5-ALA, Fluorescein | -- | HGG | 18/40 (5-ALA) 21/44 (Fluorescein) | -- | -- | Advantage after concomitant use |
| Zhang et al. [87] | 2022 | Retrospective Case Series | 5-ALA | 10/11 | HGG | -- | -- | None | -- |

6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Cancer Institute. Cancer Stat Facts: Brain and Other Nervous System Cancer. The Surveillance, Epidemiology, and End Results (SEER). Published 2018. Available online: https://seer.cancer.gov/statfacts/html/brain.html (accessed on 3 November 2020).
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Schupper, A.J.; Yong, R.L.; Hadjipanayis, C.G. The Neurosurgeon’s Armamentarium for Gliomas: An Update on Intraoperative Technologies to Improve Extent of Resection. J. Clin. Med. 2021, 10, 236. [Google Scholar] [CrossRef] [PubMed]
- Belykh, E.; Martirosyan, N.L.; Yagmurlu, K.; Miller, E.J.; Eschbacher, J.M.; Izadyyazdanabadi, M.; Bardonova, L.A.; Byvaltsev, V.A.; Nakaji, P.; Preul, M.C. Intraoperative Fluorescence Imaging for Personalized Brain Tumor Resection: Current State and Future Directions. Front. Surg. 2016, 3, 55. [Google Scholar] [CrossRef]
- Schupper, A.J.; Rao, M.; Mohammadi, N.; Baron, R.; Lee, J.Y.K.; Acerbi, F.; Hadjipanayis, C.G. Fluorescence-Guided Surgery: A Review on Timing and Use in Brain Tumor Surgery. Front. Neurol. 2021, 12, 682151. [Google Scholar] [CrossRef]
- Belykh, E.; Shaffer, K.V.; Lin, C.; Byvaltsev, V.A.; Preul, M.C.; Chen, L. Blood-Brain Barrier, Blood-Brain Tumor Barrier, and Fluorescence-Guided Neurosurgical Oncology: Delivering Optical Labels to Brain Tumors. Front. Oncol. 2020, 10, 739. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, G.; Cofano, F.; Salvati, L.F.; Monticelli, M.; Zeppa, P.; Di Perna, G.; Melcarne, A.; Altieri, R.; La Rocca, G.; Sabatino, G.; et al. Fluorescence-Guided Surgery for High-Grade Gliomas: State of the Art and New Perspectives. Technol. Cancer Res. Treat. 2021, 20, 15330338211021605. [Google Scholar] [CrossRef]
- Chanbour, H.; Chotai, S. Review of Intraoperative Adjuncts for Maximal Safe Resection of Gliomas and Its Impact on Outcomes. Cancers 2022, 14, 5705. [Google Scholar] [CrossRef]
- Moore, G.E.; Peyton, W.T.; French, L.A.; Walker, W.W.; Mahajan, P.G.; Dige, N.C.; Vanjare, B.D.; Raza, H.; Hassan, M.; Seo, S.-Y.; et al. The Clinical Use of Fluorescein in Neurosurgery. J. Neurosurg. 1948, 5, 392–398. [Google Scholar] [CrossRef]
- Smith, E.J.; Gohil, K.; Thompson, C.M.; Naik, A.; Hassaneen, W. Fluorescein-Guided Resection of High Grade Gliomas: A Meta-Analysis. World Neurosurg. 2021, 155, 181–188.e7. [Google Scholar] [CrossRef]
- Diaz, R.J.; Dios, R.R.; Hattab, E.M.; Burrell, K.; Rakopoulos, P.; Sabha, N.; Hawkins, C.; Zadeh, G.; Rutka, J.T.; Cohen-Gadol, A.A. Study of the biodistribution of fluorescein in glioma-infiltrated mouse brain and histopathological correlation of intraoperative findings in high-grade gliomas resected under fluorescein fluorescence guidance. J. Neurosurg. 2015, 122, 1360–1369. [Google Scholar] [CrossRef]
- Acerbi, F.; Broggi, M.; Schebesch, K.-M.; Höhne, J.; Cavallo, C.; De Laurentis, C.; Eoli, M.; Anghileri, E.; Servida, M.; Boffano, C.; et al. Fluorescein-Guided Surgery for Resection of High-Grade Gliomas: A Multicentric Prospective Phase II Study (FLUOGLIO). Clin. Cancer Res. 2018, 24, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Chen, B.; Yao, X.; Yang, Y. Outcome comparisons of high-grade glioma resection with or without fluorescein sodium-guidance. Curr. Probl. Cancer 2019, 43, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Koc, K.; Anik, I.; Cabuk, B.; Ceylan, S. Fluorescein sodium-guided surgery in glioblastoma multiforme: A prospective evaluation. Br. J. Neurosurg. 2008, 22, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wang, H.; Ge, P.; Zhao, J.; Li, W.; Gu, H.; Wang, G.; Luo, Y.; Chen, D. Gross Total Resection of Glioma with the Intraoperative Fluorescence-guidance of Fluorescein Sodium. Int. J. Med. Sci. 2012, 9, 708–714. [Google Scholar] [CrossRef]
- Martirosyan, N.L.; Eschbacher, J.M.; Kalani, M.Y.S.; Turner, J.D.; Belykh, E.; Spetzler, R.F.; Nakaji, P.; Preul, M.C.; Lv, A.; Ga, R.; et al. Prospective evaluation of the utility of intraoperative confocal laser endomicroscopy in patients with brain neoplasms using fluorescein sodium: Experience with 74 cases. Neurosurg. Focus 2016, 40, E11. [Google Scholar] [CrossRef]
- Acerbi, F.; Cavallo, C.; Broggi, M.; Cordella, R.; Anghileri, E.; Eoli, M.; Schiariti, M.; Broggi, G.; Ferroli, P. Fluorescein-guided surgery for malignant gliomas: A review. Neurosurg. Rev. 2014, 37, 547–557. [Google Scholar] [CrossRef]
- Dilek, O.; Ihsan, A.; Tulay, H. Anaphylactic reaction after fluorescein sodium administration during intracranial surgery. J. Clin. Neurosci. 2011, 18, 430–431. [Google Scholar] [CrossRef]
- Kuroiwa, T.; Kajimoto, Y.; Ohta, T. Development of a Fluorescein Operative Microscope for use During Malignant Glioma Surgery: A Technical Note and Preliminary Report. Surg. Neurol. 1998, 50, 41–49. [Google Scholar] [CrossRef]
- Wallace, M.B.; Meining, A.; Canto, M.I.; Fockens, P.; Miehlke, S.; Roesch, T.; Lightdale, C.J.; Pohl, H.; Carr-Locke, D.; Löhr, M.; et al. The safety of intravenous fluorescein for confocal laser endomicroscopy in the gastrointestinal tract. Aliment. Pharmacol. Ther. 2010, 31, 548–552. [Google Scholar] [CrossRef]
- Restelli, F.; Bonomo, G.; Monti, E.; Broggi, G.; Acerbi, F.; Broggi, M. Safeness of sodium fluorescein administration in neurosurgery: Case-report of an erroneous very high-dose administration and review of the literature. Brain Spine 2022, 2, 101703. [Google Scholar] [CrossRef]
- Tanahashi, S.; Lida, H.; Dohi, S. An Anaphylactoid Reaction After Administration of Fluorescein Sodium During Neurosurgery. Obstet. Anesth. Dig. 2006, 103, 503. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Jaber, M.; Wölfer, J.; Ewelt, C.; Holling, M.; Hasselblatt, M.; Niederstadt, T.; Zoubi, T.; Weckesser, M.; Stummer, W. The Value of 5-Aminolevulinic Acid in Low-grade Gliomas and High-grade Gliomas Lacking Glioblastoma Imaging Features: An Analysis Based on Fluorescence, Magnetic Resonance Imaging, 18 F-Fluoroethyl Tyrosine Positron Emission Tomography, and Tumor Molecula. Neurosurgery 2016, 78, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Thomas, C. Comment on Hosmann et al. 5-ALA Fluorescence Is a Powerful Prognostic Marker during Surgery of Low-Grade Gliomas (WHO Grade II)—Experience at Two Specialized Centers. Cancers 2021, 13, 2540. Cancers 2021, 13, 5634. [Google Scholar] [CrossRef]
- Widhalm, G.; Kiesel, B.; Woehrer, A.; Traub-Weidinger, T.; Preusser, M.; Marosi, C.; Prayer, D.; Hainfellner, J.A.; Knosp, E.; Wolfsberger, S. 5-Aminolevulinic Acid Induced Fluorescence Is a Powerful Intraoperative Marker for Precise Histopathological Grading of Gliomas with Non-Significant Contrast-Enhancement. PLoS ONE 2013, 8, e76988. [Google Scholar] [CrossRef]
- Ewelt, C.; Floeth, F.W.; Felsberg, J.; Steiger, H.J.; Sabel, M.; Langen, K.-J.; Stoffels, G.; Stummer, W. Finding the anaplastic focus in diffuse gliomas: The value of Gd-DTPA enhanced MRI, FET-PET, and intraoperative, ALA-derived tissue fluorescence. Clin. Neurol. Neurosurg. 2011, 113, 541–547. [Google Scholar] [CrossRef]
- Jaber, M.; Ewelt, C.; Wölfer, J.; Brokinkel, B.; Thomas, C.; Hasselblatt, M.; Grauer, O.; Stummer, W. Is Visible Aminolevulinic Acid-Induced Fluorescence an Independent Biomarker for Prognosis in Histologically Confirmed (World Health Organization 2016) Low-Grade Gliomas? Clin. Neurosurg. 2019, 84, 1214–1224. [Google Scholar] [CrossRef]
- Mercea, P.A.; Mischkulnig, M.; Kiesel, B.; Wadiura, L.I.; Roetzer, T.; Prihoda, R.; Heicappell, P.; Kreminger, J.; Furtner, J.; Woehrer, A.; et al. Prognostic Value of 5-ALA Fluorescence, Tumor Cell Infiltration and Angiogenesis in the Peritumoral Brain Tissue of Brain Metastases. Cancers 2021, 13, 603. [Google Scholar] [CrossRef]
- Marhold, F.; Roetzer-Pejrimovsky, T.; Scheichel, F.; Mercea, P.A.; Mischkulnig, M.; Wadiura, L.I.; Kiesel, B.; Weber, M.; Popadic, B.; Prihoda, R.; et al. Does pigmentation, hemosiderin and blood effect visible 5-ALA fluorescence in cerebral melanoma metastasis? Photodiagnosis Photodyn. Ther. 2022, 39, 102864. [Google Scholar] [CrossRef]
- Kamp, M.A.; Grosser, P.; Felsberg, J.; Slotty, P.J.; Steiger, H.-J.; Reifenberger, G.; Sabel, M. 5-Aminolevulinic acid (5-ALA)-induced fluorescence in intracerebral metastases: A retrospective study. Acta Neurochir. 2012, 154, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Schatlo, B.; Stockhammer, F.; Barrantes-Freer, A.; Bleckmann, A.; Siam, L.; Pukrop, T.; Rohde, V. 5-Aminolevulinic Acid Fluorescence Indicates Perilesional Brain Infiltration in Brain Metastases. World Neurosurg. X 2020, 5, 100069. [Google Scholar] [CrossRef] [PubMed]
- Marbacher, S.; Klinger, E.; Schwyzer, L.; Fischer, I.; Nevzati, E.; Diepers, M.; Roelcke, U.; Fathi, A.-R.; Coluccia, D.; Fandino, J. Use of fluorescence to guide resection or biopsy of primary brain tumors and brain metastases. Neurosurg. Focus 2014, 36, E10. [Google Scholar] [CrossRef] [PubMed]
- Bettag, C.; Hussein, A.; Schatlo, B.; Barrantes-Freer, A.; Abboud, T.; Rohde, V.; Mielke, D. Endoscope-assisted visualization of 5-aminolevulinic acid fluorescence in surgery for brain metastases. J. Neurosurg. 2022, 137, 1650–1655. [Google Scholar] [CrossRef]
- Eljamel, S. 5-ALA Fluorescence Image Guided Resection of Glioblastoma Multiforme: A Meta-Analysis of the Literature. Int. J. Mol. Sci. 2015, 16, 10443–10456. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J.; ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Valle, R.D.; Slof, J.; Galván, J.; Arza, C.; Romariz, C.; Vidal, C. Observational, retrospective study of the effectiveness of 5-aminolevulinic acid in malignant glioma surgery in Spain (The VISIONA study). Neurología 2014, 29, 131–138. [Google Scholar] [CrossRef]
- Certo, F.; Altieri, R.; Maione, M.; Schonauer, C.; Sortino, G.; Fiumanò, G.; Tirrò, E.; Massimino, M.; Broggi, G.; Vigneri, P.; et al. FLAIRectomy in Supramarginal Resection of Glioblastoma Correlates with Clinical Outcome and Survival Analysis: A Prospective, Single Institution, Case Series. Oper. Neurosurg. 2021, 20, 151–163. [Google Scholar] [CrossRef]
- Millesi, M.; Kiesel, B.; Mazanec, V.; Wadiura, L.I.; Wöhrer, A.; Herta, J.; Wolfsberger, S.; Novak, K.; Furtner, J.; Rössler, K.; et al. 5-ALA fluorescence for intraoperative visualization of spinal ependymal tumors and identification of unexpected residual tumor tissue: Experience in 31 patients. J. Neurosurg. Spine 2021, 34, 374–382. [Google Scholar] [CrossRef]
- Falco, J.; Höhne, J.; Broggi, M.; Rubiu, E.; Restelli, F.; Vetrano, I.G.; Schiariti, M.; Mazzapicchi, E.; Bonomo, G.; Ferroli, P.; et al. Fluorescein-guided surgery for the resection of pilocytic astrocytomas: A multicentric retrospective study. Front. Oncol. 2022, 12, 943085. [Google Scholar] [CrossRef]
- Falco, J.; Broggi, M.; Vetrano, I.G.; Rubiu, E.; Schiariti, M.; Restelli, F.; Mazzapicchi, E.; Bonomo, G.; La Corte, E.; Ferroli, P.; et al. Fluorescein sodium in the surgical treatment of pleomorphic xanthoastrocytomas: Results from a retrospective study. Front. Oncol. 2022, 12, 1009769. [Google Scholar] [CrossRef] [PubMed]
- Kutlay, M.; Durmaz, O.; Ozer, I.; Kırık, A.; Yasar, S.; Kural, C.; Temiz, Ç.; Tehli, Ö.; Ezgu, M.C.; Daneyemez, M.; et al. Fluorescein Sodium-Guided Neuroendoscopic Resection of Deep-Seated Malignant Brain Tumors: Preliminary Results of 18 Patients. Oper. Neurosurg. 2020, 20, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Olguner, S.K.; Arslan, A.; Açık, V.; Istemen, I.; Can, M.; Gezercan, Y.; Ökten, A.I. Sodium Fluorescein for Spinal Intradural Tumors. Front. Oncol. 2021, 10, 618579. [Google Scholar] [CrossRef] [PubMed]
- Ung, T.H.; Serva, S.; Chatain, G.P.; Witt, J.-P.; Finn, M. Application of sodium fluorescein for spinal cord lesions: Intraoperative localization for tissue biopsy and surgical resection. Neurosurg. Rev. 2021, 45, 1563–1569. [Google Scholar] [CrossRef]
- Alcazar, P.; Avedillo, A.; Vazquez, S.; Lopez, L.B.; Fustero, D.; Moles, J.; Gonzalez, L.; Orduna, J. The usefulness of intraoperative sodium fluorescein in the surgical treatment of relapsed high-grade brain tumors in pediatric patients. Child’s Nerv. Syst. 2023, 39, 1501–1507. [Google Scholar] [CrossRef]
- Xue, Z.; Kong, L.; Hao, S.; Wang, Y.; Jia, G.; Wu, Z.; Jia, W.; Zhang, J.; Zhang, L. Combined Application of Sodium Fluorescein and Neuronavigation Techniques in the Resection of Brain Gliomas. Front. Neurol. 2021, 12, 747072. [Google Scholar] [CrossRef]
- Falco, J.; Rubiu, E.; Broggi, M.; Farinotti, M.; Vetrano, I.G.; Schiariti, M.; Anghileri, E.; Eoli, M.; Pollo, B.; Moscatelli, M.; et al. Towards an Established Intraoperative Oncological Favorable Tool: Results of Fluorescein-Guided Resection from a Monocentric, Prospective Series of 93 Primary Glioblastoma Patients. J. Clin. Med. 2023, 12, 178. [Google Scholar] [CrossRef]
- Cheng, X.; Chen, J.; Tang, R.; Ruan, J.; Mao, D.; Yang, H. Sodium Fluorescein-Guided Surgery for Resection of Brain Metastases from Lung Cancer: A Consecutive Case Series Study and Literature Review. Cancers 2023, 15, 882. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Zhang, X.-H.; Lin, F.-H.; Li, C.; Jin, J.-T.; Zhou, Z.-H.; Zhu, S.-H.; Cheng, Z.-Q.; Zhong, S.; He, Z.-Q.; et al. The application of fluorescein sodium for the resection of medulloblastoma. J. Neuro-Oncol. 2022, 158, 463–470. [Google Scholar] [CrossRef]
- Ott, C.; Proescholdt, M.; Friedrich, M.; Hoehne, J.; Rosengarth, K.; Schmidt, N.-O.; Schebesch, K.-M. The use of the sodium fluorescein and YELLOW 560 nm filter for the resection of pediatric posterior fossa lesions. Child’s Nerv. Syst. 2022, 39, 1495–1500. [Google Scholar] [CrossRef]
- Sun, Z.; Jing, L.; Fan, Y.; Zhang, H.; Chen, L.; Wang, G.; Sharma, H.S.; Wang, J. Fluorescein-guided surgery for spinal gliomas: Analysis of 220 consecutive cases. Int. Rev. Neurobiol. 2020, 151, 139–154. [Google Scholar] [CrossRef]
- Cardali, S.M.; Ricciardo, G.; Garufi, G.; Raffa, G.; Messineo, F.; Scalia, G.; Conti, A.; Germanò, A. Fluorescein-guided surgery for intradural spinal tumors: A single-center experience. Brain Spine 2022, 2, 100908. [Google Scholar] [CrossRef] [PubMed]
- de Laurentis, C.; Beuriat, P.A.; Bteich, F.; Mottolese, C.; Szathmari, A.; Vinchon, M.; Di Rocco, F. Pediatric Low-Grade Glioma Surgery with Sodium Fluorescein: Efficient Localization for Removal and Association with Intraoperative Pathological Sampling. Diagnostics 2022, 12, 2927. [Google Scholar] [CrossRef] [PubMed]
- Höhne, J.; Schebesch, K.-M.; Zoubaa, S.; Proescholdt, M.; Riemenschneider, M.J.; Schmidt, N.O. Intraoperative imaging of brain tumors with fluorescein: Confocal laser endomicroscopy in neurosurgery. Clinical and user experience. Neurosurg. Focus 2021, 50, E19. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, B.M.; Jeltema, H.-R.; Kruijff, S.; Groen, R.J.M. The application of fluorescence techniques in meningioma surgery—A review. Neurosurg. Rev. 2019, 42, 799–809. [Google Scholar] [CrossRef]
- Mirza, A.B.; Lavrador, J.P.; Christodoulides, I.; Boardman, T.M.; Vastani, A.; Al Banna, Q.; Ahmed, R.; Norman, I.C.F.; Murphy, C.; Devi, S.; et al. 5-Aminolevulinic Acid-Guided Resection in Grade III Tumors—A Comparative Cohort Study. Oper. Neurosurg. 2022, 22, 215–223. [Google Scholar] [CrossRef]
- Mirza, A.B.; Christodoulides, I.; Lavrador, J.P.; Giamouriadis, A.; Vastani, A.; Boardman, T.; Ahmed, R.; Norman, I.; Murphy, C.; Devi, S.; et al. 5-Aminolevulinic acid-guided resection improves the overall survival of patients with glioblastoma—A comparative cohort study of 343 patients. Neuro-Oncol. Adv. 2021, 3, vdab047. [Google Scholar] [CrossRef]
- Yannuzzi, L.A.; Rohrer, K.T.; Tindel, L.J.; Sobel, R.S.; Costanza, M.A.; Shields, W.; Zang, E. Fluorescein Angiography Complication Survey. Ophthalmology 1986, 93, 611–617. [Google Scholar] [CrossRef]
- Kashani, A.H.; Chen, C.-L.; Gahm, J.K.; Zheng, F.; Richter, G.M.; Rosenfeld, P.J.; Shi, Y.; Wang, R.K. Optical coherence tomography angiography: A comprehensive review of current methods and clinical applications. Prog. Retin. Eye Res. 2017, 60, 66–100. [Google Scholar] [CrossRef]
- Senders, J.T.; Muskens, I.S.; Schnoor, R.; Karhade, A.V.; Cote, D.J.; Smith, T.R.; Broekman, M.L.D. Agents for fluorescence-guided glioma surgery: A systematic review of preclinical and clinical results. Acta Neurochir. 2017, 159, 151–167. [Google Scholar] [CrossRef]
- Ibrahim, O.; Hafez, M.A.; Haleem, H.A.; El Maghraby, H. Recent Advances in the Treatment of Gliomas: The Multimodal Care Therapy. Open Access Maced. J. Med. Sci. 2021, 9, 503–508. [Google Scholar] [CrossRef]
- Schupper, A.J.; Baron, R.B.; Cheung, W.; Rodriguez, J.; Kalkanis, S.N.; Chohan, M.O.; Andersen, B.J.; Chamoun, R.; Nahed, B.V.; Zacharia, B.E.; et al. 5-Aminolevulinic acid for enhanced surgical visualization of high-grade gliomas: A prospective, multicenter study. J. Neurosurg. 2022, 136, 1525–1534. [Google Scholar] [CrossRef]
- Kiesel, B.; Wadiura, L.I.; Mischkulnig, M.; Makolli, J.; Sperl, V.; Borkovec, M.; Freund, J.; Lang, A.; Millesi, M.; Berghoff, A.S.; et al. Efficacy, Outcome, and Safety of Elderly Patients with Glioblastoma in the 5-ALA Era: Single Center Experience of More Than 10 Years. Cancers 2021, 13, 6119. [Google Scholar] [CrossRef]
- Goryaynov, S.A.; Widhalm, G.; Goldberg, M.F.; Chelushkin, D.; Spallone, A.; Chernyshov, K.A.; Ryzhova, M.; Pavlova, G.; Revischin, A.; Shishkina, L.; et al. The Role of 5-ALA in Low-Grade Gliomas and the Influence of Antiepileptic Drugs on Intraoperative Fluorescence. Front. Oncol. 2019, 9, 423. [Google Scholar] [CrossRef] [PubMed]
- Wach, J.; Güresir, Á.; Hamed, M.; Vatter, H.; Herrlinger, U.; Güresir, E. Impact of Levetiracetam Treatment on 5-Aminolevulinic Acid Fluorescence Expression in IDH1 Wild-Type Glioblastoma. Cancers 2022, 14, 2134. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.E.; Steele, C.J.; Rovin, R.A.; Belton, R.J.; Winn, R.J. Dexamethasone alone and in combination with desipramine, phenytoin, valproic acid or levetiracetam interferes with 5-ALA-mediated PpIX production and cellular retention in glioblastoma cells. J. Neuro-Oncol. 2016, 127, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Hefti, M.; Albert, I.; Luginbuehl, V. Phenytoin reduces 5-aminolevulinic acid-induced protoporphyrin IX accumulation in malignant glioma cells. J. Neuro-Oncol. 2012, 108, 443–450. [Google Scholar] [CrossRef]
- Wadiura, L.I.; Mischkulnig, M.; Hosmann, A.; Borkovec, M.; Kiesel, B.; Rötzer, T.; Mercea, P.A.; Furtner, J.; Hervey-Jumper, S.; Rössler, K.; et al. Influence of Corticosteroids and Antiepileptic Drugs on Visible 5-Aminolevulinic Acid Fluorescence in a Series of Initially Suspected Low-Grade Gliomas Including World Health Organization Grade II, III, and IV Gliomas. World Neurosurg. 2020, 137, e437–e446. [Google Scholar] [CrossRef]
- Schebesch, K.-M.; Höhne, J.; Rosengarth, K.; Noeva, E.; Schmidt, N.O.; Proescholdt, M. Fluorescein-guided resection of newly diagnosed high-grade glioma: Impact on extent of resection and outcome. Brain Spine 2022, 2, 101690. [Google Scholar] [CrossRef]
- Kofoed, M.S.; Pedersen, C.B.; Schulz, M.K.; Kristensen, B.W.; Hansen, R.W.; Markovic, L.; Halle, B.; Poulsen, F.R. Fluorescein-guided resection of cerebral metastases is associated with greater tumor resection. Acta Neurochir. 2022, 164, 451–457. [Google Scholar] [CrossRef]
- Kerschbaumer, J.; Demetz, M.; Krigers, A.; Pinggera, D.; Spinello, A.; Thomé, C.; Freyschlag, C.F. Mind the gap—The use of sodium fluoresceine for resection of brain metastases to improve the resection rate. Acta Neurochir. 2023, 165, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Zeppa, P.; De Marco, R.; Monticelli, M.; Massara, A.; Bianconi, A.; Di Perna, G.; Crasto, S.G.; Cofano, F.; Melcarne, A.; Lanotte, M.M.; et al. Fluorescence-Guided Surgery in Glioblastoma: 5-ALA, SF or Both? Differences between Fluorescent Dyes in 99 Consecutive Cases. Brain Sci. 2022, 12, 555. [Google Scholar] [CrossRef] [PubMed]
- Batalov, A.I.; Goryaynov, S.A.; Zakharova, N.E.; Solozhentseva, K.D.; Kosyrkova, A.V.; Potapov, A.A.; Pronin, I.N. Prediction of Intraoperative Fluorescence of Brain Gliomas: Correlation between Tumor Blood Flow and the Fluorescence. J. Clin. Med. 2021, 10, 2387. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.B.; Ramina, R.; Neto, M.C.; Machado, G.; Cavalcanti, M.S.; Da Silva, J.F.C. Extending the indications of 5-Aminolevulinic acid for Fluorescence-Guided surgery for different central nervous system tumors: A series of 255 cases in Latin America. Arq. Bras. Neurocir. 2022, 41, e35–e42. [Google Scholar] [CrossRef]
- Goryaynov, S.A.; Buklina, S.B.; Khapov, I.V.; Batalov, A.I.; Potapov, A.A.; Pronin, I.N.; Belyaev, A.U.; Aristov, A.A.; Zhukov, V.U.; Pavlova, G.V.; et al. 5-ALA-guided tumor resection during awake speech mapping in gliomas located in eloquent speech areas: Single-center experience. Front. Oncol. 2022, 12, 940951. [Google Scholar] [CrossRef]
- Lavrador, J.P.; Reisz, Z.; Sibtain, N.; Rajwani, K.; Baig Mirza, A.; Vergani, F.; Gullan, R.; Bhangoo, R.; Ashkan, K.; Bleil, C.; et al. H3 G34-mutant high-grade gliomas: Integrated clinical, imaging and pathological characterisation of a single-centre case series. Acta Neurochir. 2023, 165, 1615–1633. [Google Scholar] [CrossRef]
- Luzzi, S.; Giotta Lucifero, A.; Martinelli, A.; Del Maestro, M.; Savioli, G.; Simoncelli, A.; Lafe, E.; Preda, L.; Galzio, R. Supratentorial high-grade gliomas: Maximal safe anatomical resection guided by augmented reality high-definition fiber tractography and fluorescein. Neurosurg. Focus 2021, 51, E5. [Google Scholar] [CrossRef]
- Maragkos, G.A.; Schüpper, A.J.; Lakomkin, N.; Sideras, P.; Price, G.; Baron, R.; Hamilton, T.; Haider, S.; Lee, I.Y.; Hadjipanayis, C.G.; et al. Fluorescence-Guided High-Grade Glioma Surgery More Than Four Hours After 5-Aminolevulinic Acid Administration. Front. Neurol. 2021, 12, 644804. [Google Scholar] [CrossRef]
- Milos, P.; Haj-Hosseini, N.; Hillman, J.; Wårdell, K. 5-ALA fluorescence in randomly selected pediatric brain tumors assessed by spectroscopy and surgical microscope. Acta Neurochir. 2023, 165, 71–81. [Google Scholar] [CrossRef]
- Muscas, G.; Orlandini, S.; Bonaudo, C.; Dardo, M.; Esposito, A.; Campagnaro, L.; Carrai, R.; Fainardi, E.; Della Puppa, A. Functional outcomes, extent of resection, and bright/vague fluorescence interface in resection of glioblastomas involving the motor pathways assisted by 5-ALA. Acta Neurochir. 2022, 164, 3267–3274. [Google Scholar] [CrossRef]
- Müther, M.; Jaber, M.; Johnson, T.D.; Orringer, D.A.; Stummer, W. A Data-Driven Approach to Predicting 5-Aminolevulinic Acid-Induced Fluorescence and World Health Organization Grade in Newly Diagnosed Diffuse Gliomas. Neurosurgery 2022, 90, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Strickland, B.A.; Wedemeyer, M.; Ruzevick, J.; Micko, A.; Shahrestani, S.; Daneshmand, S.; Shiroishi, M.S.; Hwang, D.H.; Attenello, F.; Chen, T.; et al. 5-Aminolevulinic acid-enhanced fluorescence-guided treatment of high-grade glioma using angled endoscopic blue light visualization: Technical case series with preliminary follow-up. J. Neurosurg. 2022, 137, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, J.F.; Rosoklija, G.; Sheldon, B.L.; Bondoc, M.; Bandlamuri, S.; Adamo, M.A. Comparison of sodium fluorescein and intraoperative ultrasonography in brain tumor resection. J. Clin. Neurosci. 2022, 106, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Takeda, J.; Nonaka, M.; Li, Y.; Isozaki, H.; Kamei, T.; Hashiba, T.; Asai, A. 5-Aminolevulinic acid fluorescence-guided endoscopic surgery for intraventricular tumors. Surg. Neurol. Int. 2022, 13, 302. [Google Scholar] [CrossRef] [PubMed]
- Watts, C.; Dayimu, A.; Matys, T.; Ashkan, K.; Price, S.; Jenkinson, M.D.; Doughton, G.; Mather, C.; Young, G.; Qian, W.; et al. Refining the Intraoperative Identification of Suspected High-Grade Glioma Using a Surgical Fluorescence Biomarker: GALA BIDD Study Report. J. Pers. Med. 2023, 13, 514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jaman, E.; Habib, A.; Ozpinar, A.; Andrews, E.; Amankulor, N.M.; Zinn, P.O. A Novel 5-Aminolevulinic Acid-Enabled Surgical Loupe System-A Consecutive Brain Tumor Series of 11 Cases. Oper. Neurosurg. 2022, 22, 298–304. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, S.; Ivey, N.; Matur, A.; Andaluz, N. Intraoperative Fluorophores: An Update on 5-Aminolevulinic Acid and Sodium Fluorescein in Resection of Tumors of the Central Nervous System and Metastatic Lesions—A Systematic Review and Meta-Analysis. Tomography 2023, 9, 1551-1567. https://doi.org/10.3390/tomography9050124
Shah S, Ivey N, Matur A, Andaluz N. Intraoperative Fluorophores: An Update on 5-Aminolevulinic Acid and Sodium Fluorescein in Resection of Tumors of the Central Nervous System and Metastatic Lesions—A Systematic Review and Meta-Analysis. Tomography. 2023; 9(5):1551-1567. https://doi.org/10.3390/tomography9050124
Chicago/Turabian StyleShah, Sanjit, Natalie Ivey, Abhijith Matur, and Norberto Andaluz. 2023. "Intraoperative Fluorophores: An Update on 5-Aminolevulinic Acid and Sodium Fluorescein in Resection of Tumors of the Central Nervous System and Metastatic Lesions—A Systematic Review and Meta-Analysis" Tomography 9, no. 5: 1551-1567. https://doi.org/10.3390/tomography9050124
APA StyleShah, S., Ivey, N., Matur, A., & Andaluz, N. (2023). Intraoperative Fluorophores: An Update on 5-Aminolevulinic Acid and Sodium Fluorescein in Resection of Tumors of the Central Nervous System and Metastatic Lesions—A Systematic Review and Meta-Analysis. Tomography, 9(5), 1551-1567. https://doi.org/10.3390/tomography9050124





