Applying a Radiation Therapy Volume Analysis Pipeline to Determine the Utility of Spectroscopic MRI-Guided Adaptive Radiation Therapy for Glioblastoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Acquisition and RT Planning
2.2. Registration
2.3. Metabolite Map Coverage
2.4. Overlap Statistics
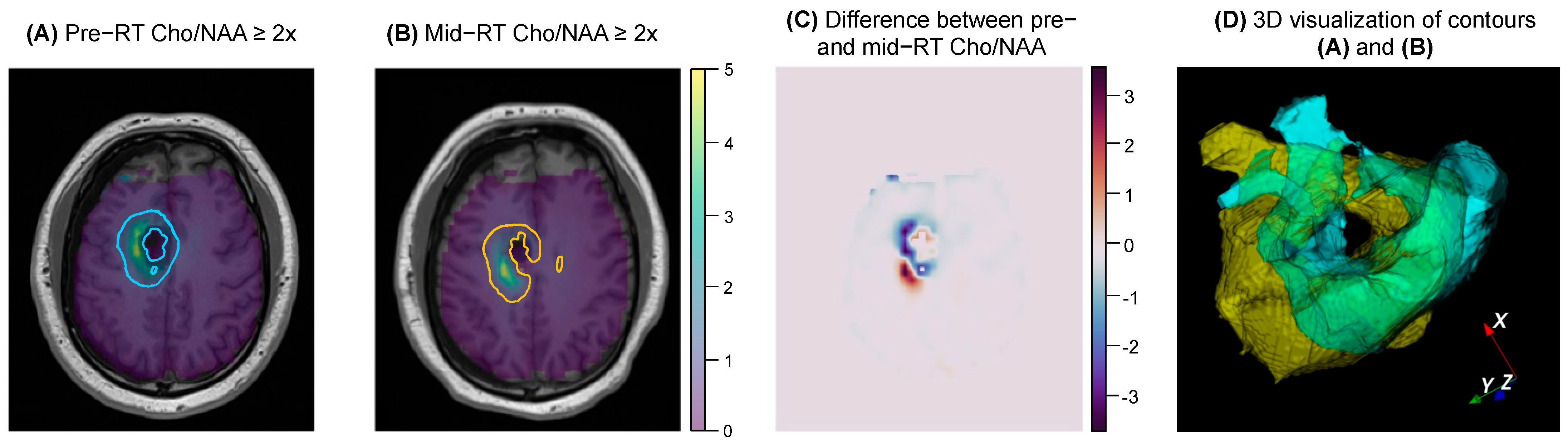
2.5. Visualization
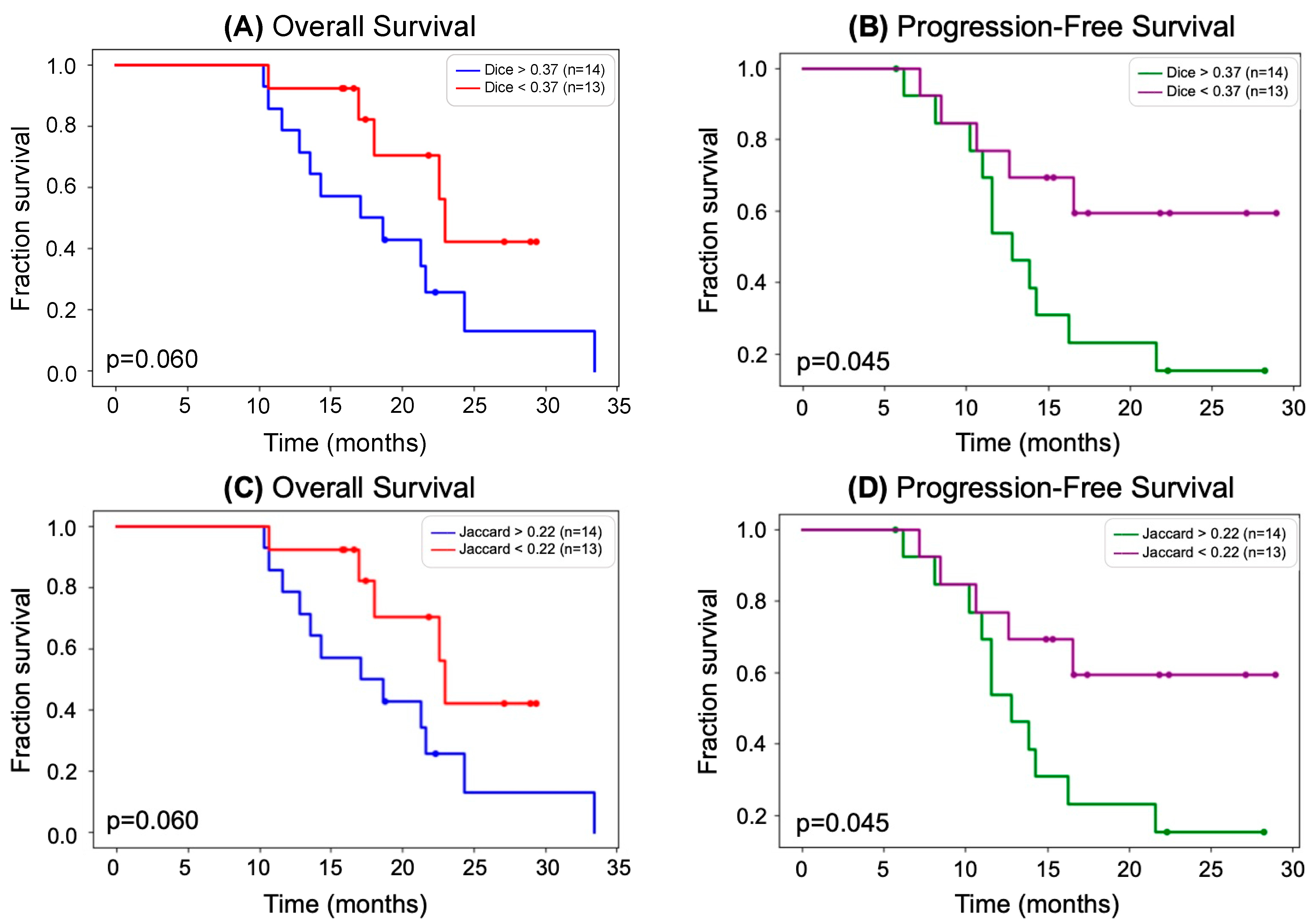
2.6. Statistical Analysis
2.7. Combined Biomarkers
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A Randomized Trial of Bevacizumab for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.A.; Kesari, S.; Steinberg, D.M.; Toms, S.A.; Taylor, L.P.; Lieberman, F.; Silvani, A.; Fink, K.L.; et al. Maintenance Therapy with Tumor-Treating Fields Plus Temozolomide vs. Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA 2015, 314, 2535–2543. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.B.; Jin, W.; Goryawala, M.Z.; Azzam, G.A.; Abramowitz, M.C.; Diwanji, T.; Ivan, M.E.; del Pilar Guillermo Prieto Eibl, M.; de la Fuente, M.I.; Mellon, E.A. Delineation of recurrent glioblastoma by whole brain spectroscopic magnetic resonance imaging. Radiat. Oncol. 2023, 18, 37. [Google Scholar] [CrossRef] [PubMed]
- Pope, W.B.; Young, J.R.; Ellingson, B.M. Advances in MRI Assessment of Gliomas and Response to Anti-VEGF Therapy. Curr. Neurol. Neurosci. Rep. 2011, 11, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, K.; Mizutani, Y.; Hachiya, J. Preliminary evaluation of fluid-attenuated inversion-recovery MR in the diagnosis of intracranial tumors. Am. J. Neuroradiol. 1996, 17, 1081–1086. [Google Scholar]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Goryawala, M.; Saraf-Lavi, E.; Nagornaya, N.; Heros, D.; Komotar, R.; Maudsley, A.A. The Association between Whole-Brain MR Spectroscopy and IDH Mutation Status in Gliomas. J. Neuroimaging 2020, 30, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Goryawala, M.Z.; Sheriff, S.; Maudsley, A.A. Regional distributions of brain glutamate and glutamine in normal subjects. NMR Biomed. 2016, 29, 1108–1116. [Google Scholar] [CrossRef]
- Goryawala, M.Z.; Sheriff, S.; Stoyanova, R.; Maudsley, A.A. Spectral decomposition for resolving partial volume effects in MRSI. Magn. Reson. Med. 2018, 79, 2886–2895. [Google Scholar] [CrossRef]
- Sabati, M.; Sheriff, S.; Gu, M.; Wei, J.; Zhu, H.; Barker, P.B.; Spielman, D.M.; Alger, J.R.; Maudsley, A.A. Multivendor implementation and comparison of volumetric whole-brain echo-planar MR spectroscopic imaging. Magn. Reson. Med. 2014, 74, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Cordova, J.S.; Shu, H.-K.G.; Liang, Z.; Gurbani, S.S.; Cooper, L.A.D.; Holder, C.A.; Olson, J.J.; Kairdolf, B.; Schreibmann, E.; Neill, S.G.; et al. Whole-brain spectroscopic MRI biomarkers identify infiltrating margins in glioblastoma patients. Neuro-Oncology 2016, 18, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Brock, K.K. Adaptive Radiotherapy: Moving into the Future. Semin. Radiat. Oncol. 2019, 29, 181–184. [Google Scholar] [CrossRef]
- Dajani, S.; Hill, V.B.; Kalapurakal, J.A.; Horbinski, C.M.; Nesbit, E.G.; Sachdev, S.; Yalamanchili, A.; Thomas, T.O. Imaging of GBM in the Age of Molecular Markers and MRI Guided Adaptive Radiation Therapy. J. Clin. Med. 2022, 11, 5961. [Google Scholar] [CrossRef]
- Kim, T.G.; Lim, D.H. Interfractional Variation of Radiation Target and Adaptive Radiotherapy for Totally Resected Glioblastoma. J. Korean Med. Sci. 2013, 28, 1233–1237. [Google Scholar] [CrossRef]
- Guevara, B.; Cullison, K.; Maziero, D.; Azzam, G.A.; De La Fuente, M.I.; Brown, K.; Valderrama, A.; Meshman, J.; Breto, A.; Ford, J.C.; et al. Simulated Adaptive Radiotherapy for Shrinking Glioblastoma Resection Cavities on a Hybrid MRI–Linear Accelerator. Cancers 2023, 15, 1555. [Google Scholar] [CrossRef]
- Tseng, C.-L.; Chen, H.; Stewart, J.; Lau, A.Z.; Chan, R.W.; Lawrence, L.S.P.; Myrehaug, S.; Soliman, H.; Detsky, J.; Lim-Fat, M.J.; et al. High grade glioma radiation therapy on a high field 1.5 Tesla MR-Linac—Workflow and initial experience with daily adapt-to-position (ATP) MR guidance: A first report. Front. Oncol. 2022, 12, 1060098. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, W.; Martin, K.M.; Lorensen, W.E. The Visualization Toolkit an Object-Oriented Approach to 3D Graphics; Prentice-Hall, Inc.: Hoboken, NJ, USA, 1998. [Google Scholar]
- Lowekamp, B.C.; Chen, D.T.; Eibanez, L.; Eblezek, D. The Design of SimpleITK. Front. Neuroinform. 2013, 7, 45. [Google Scholar] [CrossRef]
- Mason, D. SU-E-T-33: Pydicom: An Open Source DICOM Library. Med. Phys. 2011, 38, 3493. [Google Scholar] [CrossRef]
- Davidson-Pilon, C. lifelines: Survival analysis in Python. J. Open Source Softw. 2019, 4, 01317. [Google Scholar] [CrossRef]
- Ramesh, K.; A Mellon, E.; Gurbani, S.S.; Weinberg, B.D.; Schreibmann, E.; Sheriff, S.A.; Goryawala, M.; de le Fuente, M.; Eaton, B.R.; Zhong, J.; et al. A multi-institutional pilot clinical trial of spectroscopic MRI-guided radiation dose escalation for newly diagnosed glioblastoma. Neuro-Oncol. Adv. 2022, 4, vdac006. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Gurbani, S.; Weinberg, B.; Cooper, L.; Mellon, E.; Schreibmann, E.; Sheriff, S.; Maudsley, A.; Goryawala, M.; Shu, H.-K.; Shim, H. The Brain Imaging Collaboration Suite (BrICS): A Cloud Platform for Integrating Whole-Brain Spectroscopic MRI into the Radiation Therapy Planning Workflow. Tomography 2019, 5, 184–191. [Google Scholar] [CrossRef]
- Ramesh, K.K.; Huang, V.; Rosenthal, J.; Mellon, E.A.; Goryawala, M.; Barker, P.B.; Gurbani, S.S.; Trivedi, A.G.; Giuffrida, A.S.; Schreibmann, E.; et al. A Novel Approach to Determining Tumor Progression Using a Three-Site Pilot Clinical Trial of Spectroscopic MRI-Guided Radiation Dose Escalation in Glioblastoma. Tomography 2023, 9, 362–374. [Google Scholar] [CrossRef]
- Gurbani, S.S.; Schreibmann, E.; Maudsley, A.A.; Cordova, J.S.; Soher, B.J.; Poptani, H.; Verma, G.; Barker, P.B.; Shim, H.; Cooper, L.A.D. A convolutional neural network to filter artifacts in spectroscopic MRI. Magn. Reson. Med. 2018, 80, 1765–1775. [Google Scholar] [CrossRef]
- Maudsley, A.A.; Darkazanli, A.; Alger, J.R.; Hall, L.O.; Schuff, N.; Studholme, C.; Yu, Y.; Ebel, A.; Frew, A.; Goldgof, D.; et al. Comprehensive processing, display and analysis forin vivo MR spectroscopic imaging. NMR Biomed. 2006, 19, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Maudsley, A.A.; Domenig, C.; Govind, V.; Darkazanli, A.; Studholme, C.; Arheart, K.; Bloomer, C. Mapping of brain metabolite distributions by volumetric proton MR spectroscopic imaging (MRSI). Magn. Reson. Med. 2009, 61, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Maudsley, A.A.; Domenig, C.; Sheriff, S. Reproducibility of serial whole-brain MR Spectroscopic Imaging. NMR Biomed. 2010, 23, 251–256. [Google Scholar] [CrossRef]
- Veenith, T.V.; Mada, M.; Carter, E.; Grossac, J.; Newcombe, V.; Outtrim, J.; Lupson, V.; Nallapareddy, S.; Williams, G.B.; Sheriff, S.; et al. Comparison of Inter Subject Variability and Reproducibility of Whole Brain Proton Spectroscopy. PLoS ONE 2014, 9, e115304. [Google Scholar] [CrossRef]
- Zhang, Y.; Taub, E.; Salibi, N.; Uswatte, G.; Maudsley, A.A.; Sheriff, S.; Womble, B.; Mark, V.W.; Knight, D.C. Comparison of reproducibility of single voxel spectroscopy and whole-brain magnetic resonance spectroscopy imaging at 3T. NMR Biomed. 2018, 31, e3898. [Google Scholar] [CrossRef]
- Tustison, N.J.; Gee, J.C. Introducing Dice, Jaccard, and Other Label Overlap Measures To ITK. Insight J. 2009, 2, 707. [Google Scholar] [CrossRef]
- Huttenlocher, D.P.; Klanderman, G.A.; Rucklidge, W.J. Comparing images using the Hausdorff distance. IEEE Trans. Pattern Anal. Mach. Intell. 1993, 15, 850–863. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistics Notes: Survival probabilities (the Kaplan-Meier method). BMJ 1998, 317, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. The logrank test. BMJ 2004, 328, 1073. [Google Scholar] [CrossRef] [PubMed]
- Binabaj, M.M.; Bahrami, A.; ShahidSales, S.; Joodi, M.; Joudi Mashhad, M.; Hassanian, S.M.; Anvari, K.; Avan, A. The prognostic value of MGMT promoter methylation in glioblastoma: A meta-analysis of clinical trials. J. Cell. Physiol. 2018, 233, 378–386. [Google Scholar] [CrossRef]
- Han, H.; Song, A.W.; Truong, T.K. Integrated parallel reception, excitation, and shimming (iPRES). Magn. Reson. Med. 2013, 70, 241–247. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trivedi, A.G.; Kim, S.H.; Ramesh, K.K.; Giuffrida, A.S.; Weinberg, B.D.; Mellon, E.A.; Kleinberg, L.R.; Barker, P.B.; Han, H.; Shu, H.-K.G.; et al. Applying a Radiation Therapy Volume Analysis Pipeline to Determine the Utility of Spectroscopic MRI-Guided Adaptive Radiation Therapy for Glioblastoma. Tomography 2023, 9, 1052-1061. https://doi.org/10.3390/tomography9030086
Trivedi AG, Kim SH, Ramesh KK, Giuffrida AS, Weinberg BD, Mellon EA, Kleinberg LR, Barker PB, Han H, Shu H-KG, et al. Applying a Radiation Therapy Volume Analysis Pipeline to Determine the Utility of Spectroscopic MRI-Guided Adaptive Radiation Therapy for Glioblastoma. Tomography. 2023; 9(3):1052-1061. https://doi.org/10.3390/tomography9030086
Chicago/Turabian StyleTrivedi, Anuradha G., Su Hyun Kim, Karthik K. Ramesh, Alexander S. Giuffrida, Brent D. Weinberg, Eric A. Mellon, Lawrence R. Kleinberg, Peter B. Barker, Hui Han, Hui-Kuo G. Shu, and et al. 2023. "Applying a Radiation Therapy Volume Analysis Pipeline to Determine the Utility of Spectroscopic MRI-Guided Adaptive Radiation Therapy for Glioblastoma" Tomography 9, no. 3: 1052-1061. https://doi.org/10.3390/tomography9030086
APA StyleTrivedi, A. G., Kim, S. H., Ramesh, K. K., Giuffrida, A. S., Weinberg, B. D., Mellon, E. A., Kleinberg, L. R., Barker, P. B., Han, H., Shu, H.-K. G., Shim, H., & Schreibmann, E. (2023). Applying a Radiation Therapy Volume Analysis Pipeline to Determine the Utility of Spectroscopic MRI-Guided Adaptive Radiation Therapy for Glioblastoma. Tomography, 9(3), 1052-1061. https://doi.org/10.3390/tomography9030086






