COVID-19 Pneumonia with Migratory Pattern in Agammaglobulinemic Patients: A Report of Two Cases and Review of Literature
Abstract
1. Introduction
2. Case Descriptions
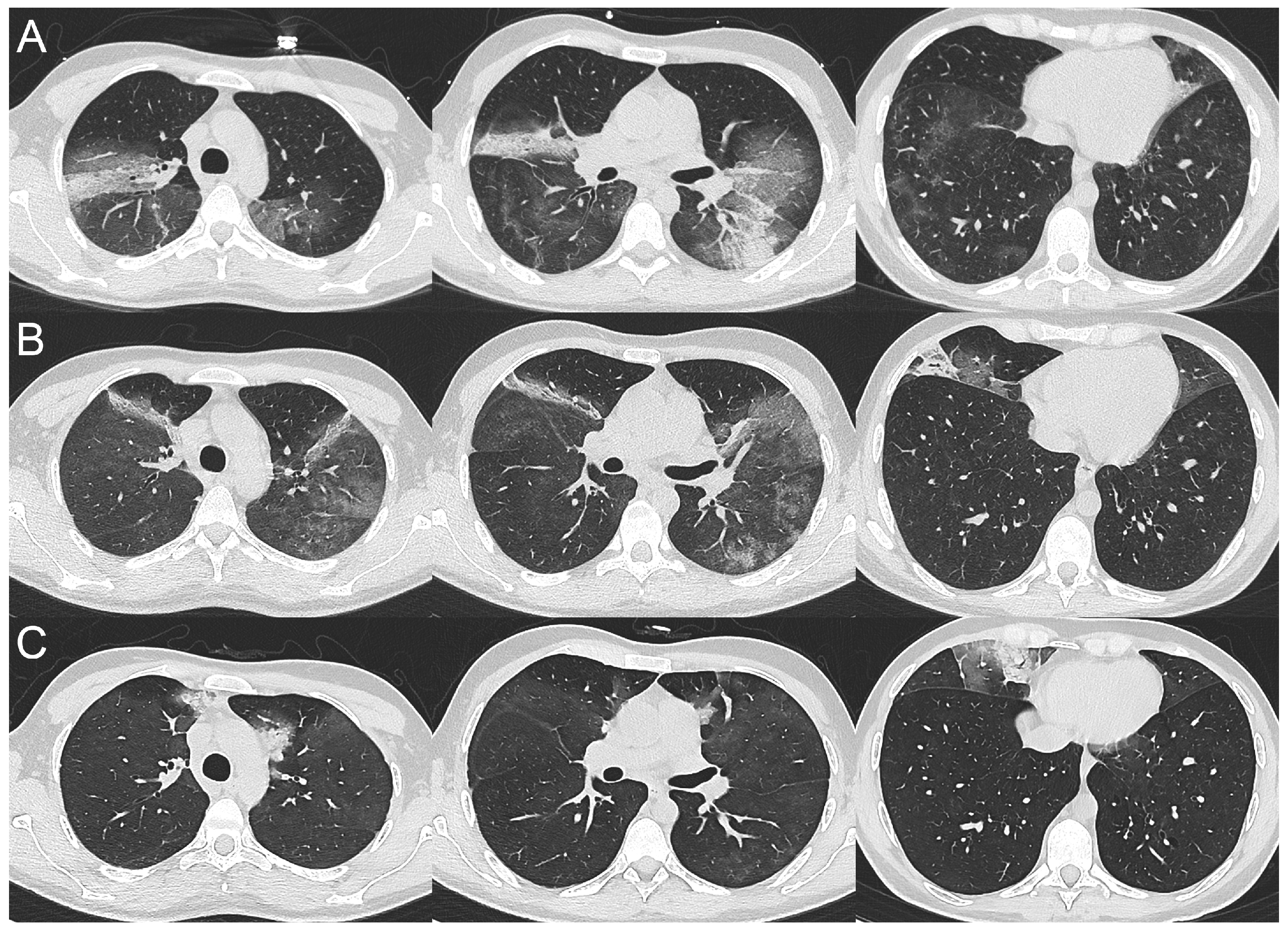
2.1. Case 1
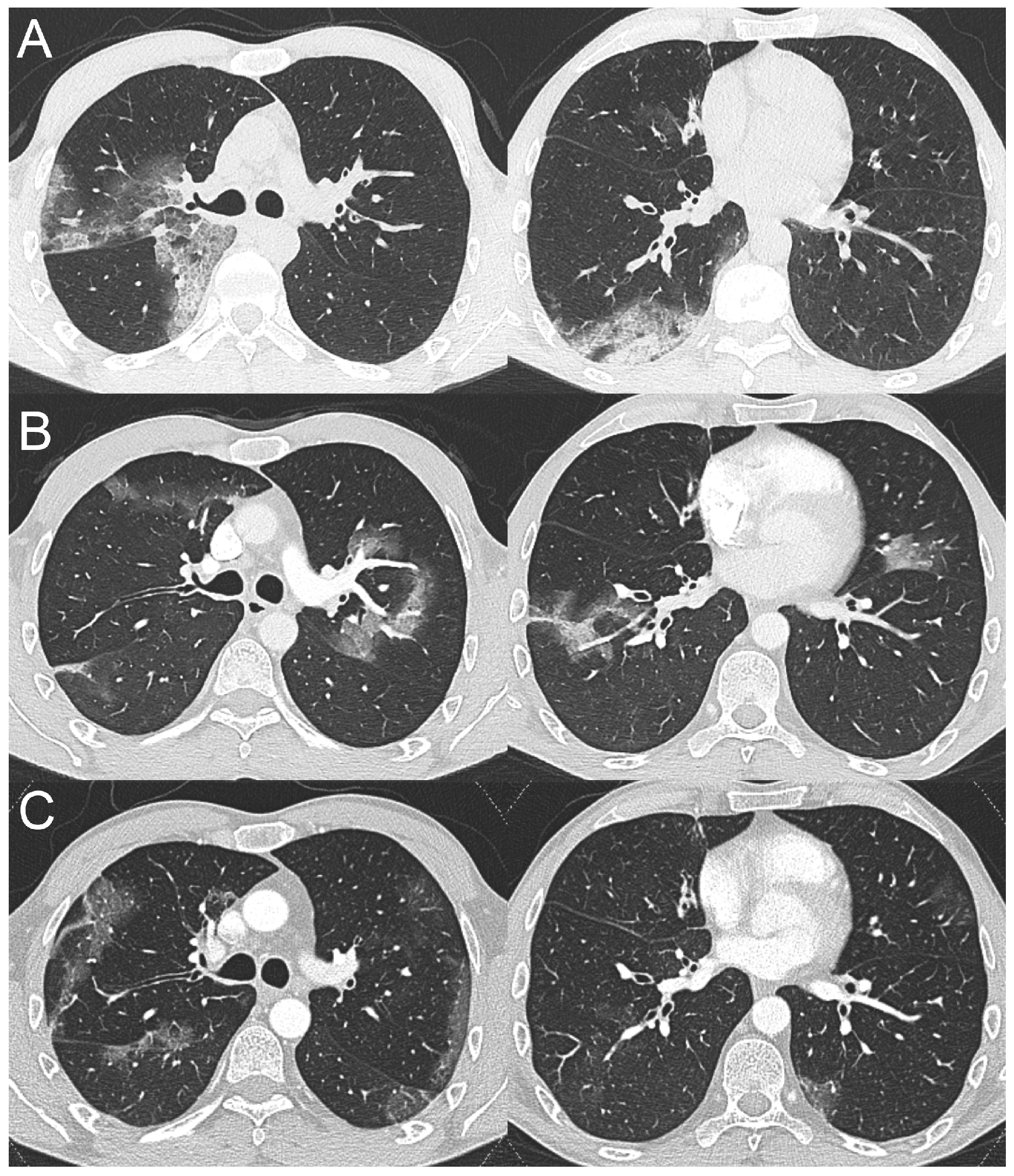
2.2. Case 2
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Coronavirus. Available online: https://www.who.int/health-topics/coronavirus#tab=tab_1 (accessed on 25 November 2021).
- Kwee, T.C.; Kwee, R.M. Chest CT in COVID-19: What the Radiologist Needs to Know. Radiographics 2022, 42, E32. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.J.A.; Kwee, T.C.; Yakar, D.; Hope, M.D.; Kwee, R.M. Chest CT Imaging Signature of Coronavirus Disease 2019 Infection: In Pursuit of the Scientific Evidence. Chest 2020, 158, 1885–1895. [Google Scholar] [CrossRef] [PubMed]
- Pontone, G.; Scafuri, S.; Mancini, M.E.; Agalbato, C.; Guglielmo, M.; Baggiano, A.; Muscogiuri, G.; Fusini, L.; Andreini, D.; Mushtaq, S.; et al. Role of computed tomography in COVID-19. J. Cardiovasc. Comput. Tomogr. 2021, 15, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, A.I.; Singanayagam, A. Immunosuppression for hyperinflammation in COVID-19: A double-edged sword? Lancet 2020, 395, 1111. [Google Scholar] [CrossRef]
- Suri, D.; Rawat, A.; Singh, S. X-linked Agammaglobulinemia. Indian J. Pediatr. 2016, 83, 331–337. [Google Scholar] [CrossRef]
- Shillitoe, B.; Gennery, A. X-Linked Agammaglobulinaemia: Outcomes in the modern era. Clin. Immunol. 2017, 183, 54–62. [Google Scholar] [CrossRef]
- EPIDEMIA COVID-1; Istituto Superiore di Sanità (ISS): Rome, Italy, 5 March 2021; (Aggiornamento Nazionale: ore 12:00, 3 Marzo Febbraio 2021); Available online: https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_3-marzo-2021.pdf (accessed on 25 November 2021).
- Eurostat. The Statistical Office of the European Union Statistics. Available online: https://ec.europa.eu/eurostat/web/cities/data/database (accessed on 25 November 2021).
- Rizzi, M.; Castelli, F.; Latronico, N.; Focá, E. SARS-CoV-2 invades the West. How to face a COVID-19 epidemic in Lombardy, Northern Italy? Infez. Med. 2020, 28, 133–134. [Google Scholar] [PubMed]
- Salehi, S.; Abedi, A.; Balakrishnan, S.; Gholamrezanezhad, A. Coronavirus Disease 2019 (COVID-19): A Systematic Review of Imaging Findings in 919 Patients. AJR Am. J. Roentgenol. 2020, 215, 87–93. [Google Scholar] [CrossRef]
- Pan, F.; Ye, T.; Sun, P.; Gui, S.; Liang, B.; Li, L.; Zheng, D.; Wang, J.; Hesketh, R.L.; Yang, L.; et al. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology 2020, 295, 715–721. [Google Scholar] [CrossRef]
- Pan, Y.; Guan, H.; Zhou, S.; Wang, Y.; Li, Q.; Zhu, T.; Hu, Q.; Xia, L. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): A study of 63 patients in Wuhan, China. Eur. Radiol. 2020, 30, 3306–3309. [Google Scholar] [CrossRef]
- Guan, C.S.; Lv, Z.B.; Yan, S.; Du, Y.N.; Chen, H.; Wei, L.G.; Xie, R.M.; Chen, B.D. Imaging Features of Coronavirus disease 2019 (COVID-19): Evaluation on Thin-Section CT. Acad. Radiol. 2020, 27, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Fields, B.K.K.; Demirjian, N.L.; Dadgar, H.; Gholamrezanezhad, A. Imaging of COVID-19: CT, MRI, and PET. Semin. Nucl. Med. 2021, 51, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Belsky, J.A.; Tullius, B.P.; Lamb, M.G.; Sayegh, R.; Stanek, J.R.; Auletta, J.J. COVID-19 in immunocompromised patients: A systematic review of cancer, hematopoietic cell and solid organ transplant patients. J. Infect. 2021, 82, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Soresina, A.; Moratto, D.; Chiarini, M.; Paolillo, C.; Baresi, G.; Focà, E.; Bezzi, M.; Baronio, B.; Giacomelli, M.; Badolato, R. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover. Pediatr. Allergy Immunol. 2020, 31, 565–569. [Google Scholar] [CrossRef]
- Hovey, J.G.; Tolbert, D.; Howell, D. Burton’s Agammaglobulinemia and COVID-19. Cureus 2020, 12, e11701, published correction appear in Cureus 2021, 13, c43. [Google Scholar] [CrossRef]
- Nyström, K.; Hjorth, M.; Fust, R.; Nilsdotter-Augustinsson, Å.; Larsson, M.; Niward, K.; Nyström, S. Specific T-cell responses for guiding treatment with convalescent plasma in severe COVID-19 and humoral immunodeficiency: A case report. BMC Infect. Dis. 2022, 22, 362. [Google Scholar] [CrossRef] [PubMed]
- Rise, N.; Touborg, T.; Lundsted, D.H.; Dalager-Pedersen, M.; Mogensen, T.H. Case report: Evolution of pulmonary manifestations and virological markers in critical COVID-19 infection in Bruton’s agammaglobulinemia. Front. Immunol. 2022, 13, 1057065. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Reed, J.C.; Liu, S.T.H.; Ho, H.E.; Lopes, J.P.; Ramsey, N.B.; Waqar, O.; Rahman, F.; Aberg, J.A.; Bouvier, N.M. Three patients with X-linked agammaglobulinemia hospitalized for COVID-19 improved with convalescent plasma. J. Allergy Clin. Immunol. Pract. 2020, 8, 3594–3596.e3. [Google Scholar] [CrossRef] [PubMed]
- Almontasheri, A.; Al-Husayni, F.; Alsuraihi, A.K.; Binyahib, H.; Albanna, A.S. The Clinical Course of COVID-19 Pneumonia in a 19-Year-Old Man on Intravenous Immunoglobulin Replacement Therapy for X-Linked Agammaglobulinemia. Am. J. Case Rep. 2021, 22, e929447. [Google Scholar] [CrossRef]
- Iaboni, A.; Wong, N.; Betschel, S.D. A Patient with X-Linked Agammaglobulinemia and COVID-19 Infection Treated with Remdesivir and Convalescent Plasma. J. Clin. Immunol. 2021, 41, 923–925. [Google Scholar] [CrossRef]
- Mira, E.; Yarce, O.A.; Ortega, C.; Fernández, S.; Pascual, N.M.; Gómez, C.; Alvarez, M.A.; Molina, I.J.; Lama, R.; Santamaria, M. Rapid recovery of a SARS-CoV-2-infected X-linked agammaglobulinemia patient after infusion of COVID-19 convalescent plasma. J. Allergy Clin. Immunol. Pract. 2020, 8, 2793–2795. [Google Scholar] [CrossRef]
- Devassikutty, F.M.; Jain, A.; Edavazhippurath, A.; Joseph, M.C.; Peedikayil, M.M.T.; Scaria, V.; Sandhya, P.; Govindaraj, G.M. X-Linked Agammaglobulinemia and COVID-19: Two Case Reports and Review of Literature. Pediatr. Allergy Immunol. Pulmonol. 2021, 34, 115–118. [Google Scholar] [CrossRef]
- Marcus, N.; Frizinsky, S.; Hagin, D.; Ovadia, A.; Hanna, S.; Farkash, M.; Maoz-Segal, R.; Agmon-Levin, N.; Broides, A.; Nahum, A.; et al. Minor Clinical Impact of COVID-19 Pandemic on Patients With Primary Immunodeficiency in Israel. Front. Immunol. 2021, 11, 614086. [Google Scholar] [CrossRef] [PubMed]
- Palomba, E.; Carrabba, M.; Zuglian, G.; Alagna, L.; Saltini, P.; Fortina, V.; Hu, C.; Bandera, A.; Fabio, G.; Gori, A.; et al. Treatment of SARS-CoV-2 relapse with remdesivir and neutralizing antibodies cocktail in a patient with X-linked agammaglobulinaemia. Int. J. Infect. Dis. 2021, 110, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Drzymalla, E.; Green, R.F.; Knuth, M.; Khoury, M.J.; Dotson, W.D.; Gundlapalli, A. COVID-19-related health outcomes in people with primary immunodeficiency: A systematic review. Clin. Immunol. 2022, 243, 109097. [Google Scholar] [CrossRef]
- Cordier, J.F. Cryptogenic organising pneumonia. Eur. Respir. J. 2006, 28, 422–446. [Google Scholar] [CrossRef] [PubMed]
- Drakopanagiotakis, F.; Polychronopoulos, V.; Judson, M.A. Organizing pneumonia. Am. J. Med. Sci. 2008, 335, 34–39. [Google Scholar] [CrossRef]
- Asai, N.; Yokoi, T.; Nishiyama, N.; Koizumi, Y.; Sakanashi, D.; Kato, H.; Hagihara, M.; Suematsu, H.; Yamagishi, Y.; Mikamo, H. Secondary organizing pneumonia following viral pneumonia caused by severe influenza B: A case report and literature reviews. BMC Infect. Dis. 2017, 17, 572. [Google Scholar] [CrossRef]
- Simões, J.P.; Alves Ferreira, A.R.; Almeida, P.M.; Trigueiros, F.; Braz, A.; Inácio, J.R.; Medeiros, F.C.; Braz, S.; Pais de Lacerda, A. Organizing pneumonia and COVID-19: A report of two cases. Respir. Med. Case Rep. 2021, 32, 101359. [Google Scholar] [CrossRef] [PubMed]
- Pogatchnik, B.P.; Swenson, K.E.; Sharifi, H.; Bedi, H.; Berry, G.J.; Guo, H.H. Radiology-Pathology Correlation Demonstrating Organizing Pneumonia in a Patient Who Recovered from COVID-19. Am. J. Respir. Crit. Care Med. 2020, 202, 598–599. [Google Scholar] [CrossRef]
- Funk, G.C.; Nell, C.; Pokieser, W.; Thaler, B.; Rainer, G.; Valipour, A. Organizing pneumonia following Covid19 pneumonia. Wien. Klin. Wochenschr. 2021, 133, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Santana, A.N.C.; Melo, F.X.; Xavier, F.D.; Amado, V.M. Migratory pulmonary infiltrates in a patient with COVID-19 and lymphoma. J. Bras. Pneumol. 2021, 47, e20200528. [Google Scholar] [CrossRef] [PubMed]
- John, T.M.; Malek, A.E.; Mulanovich, V.E.; Adachi, J.A.; Raad, I.I.; Hamilton, A.R.; Shpall, E.J.; Rezvani, K.; Aitken, S.L.; Jain, N. Migratory Pulmonary Infiltrates in a Patient With COVID-19 Infection and the Role of Corticosteroids. Mayo Clin. Proc. 2020, 95, 2038–2040. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degli Antoni, M.; Crosato, V.; Pennati, F.; Borghesi, A.; Cristini, G.; Allegri, R.; Capone, S.; Bergamasco, A.; Soresina, A.; Badolato, R.; et al. COVID-19 Pneumonia with Migratory Pattern in Agammaglobulinemic Patients: A Report of Two Cases and Review of Literature. Tomography 2023, 9, 894-900. https://doi.org/10.3390/tomography9030073
Degli Antoni M, Crosato V, Pennati F, Borghesi A, Cristini G, Allegri R, Capone S, Bergamasco A, Soresina A, Badolato R, et al. COVID-19 Pneumonia with Migratory Pattern in Agammaglobulinemic Patients: A Report of Two Cases and Review of Literature. Tomography. 2023; 9(3):894-900. https://doi.org/10.3390/tomography9030073
Chicago/Turabian StyleDegli Antoni, Melania, Verena Crosato, Francesca Pennati, Andrea Borghesi, Graziella Cristini, Roberto Allegri, Susanna Capone, Alberto Bergamasco, Annarosa Soresina, Raffaele Badolato, and et al. 2023. "COVID-19 Pneumonia with Migratory Pattern in Agammaglobulinemic Patients: A Report of Two Cases and Review of Literature" Tomography 9, no. 3: 894-900. https://doi.org/10.3390/tomography9030073
APA StyleDegli Antoni, M., Crosato, V., Pennati, F., Borghesi, A., Cristini, G., Allegri, R., Capone, S., Bergamasco, A., Soresina, A., Badolato, R., Maroldi, R., Quiros-Roldan, E., Matteelli, A., Castelli, F., & Focà, E. (2023). COVID-19 Pneumonia with Migratory Pattern in Agammaglobulinemic Patients: A Report of Two Cases and Review of Literature. Tomography, 9(3), 894-900. https://doi.org/10.3390/tomography9030073







