Using Brain Tumor MRI Structured Reporting to Quantify the Impact of Imaging on Brain Tumor Boards
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Demographics
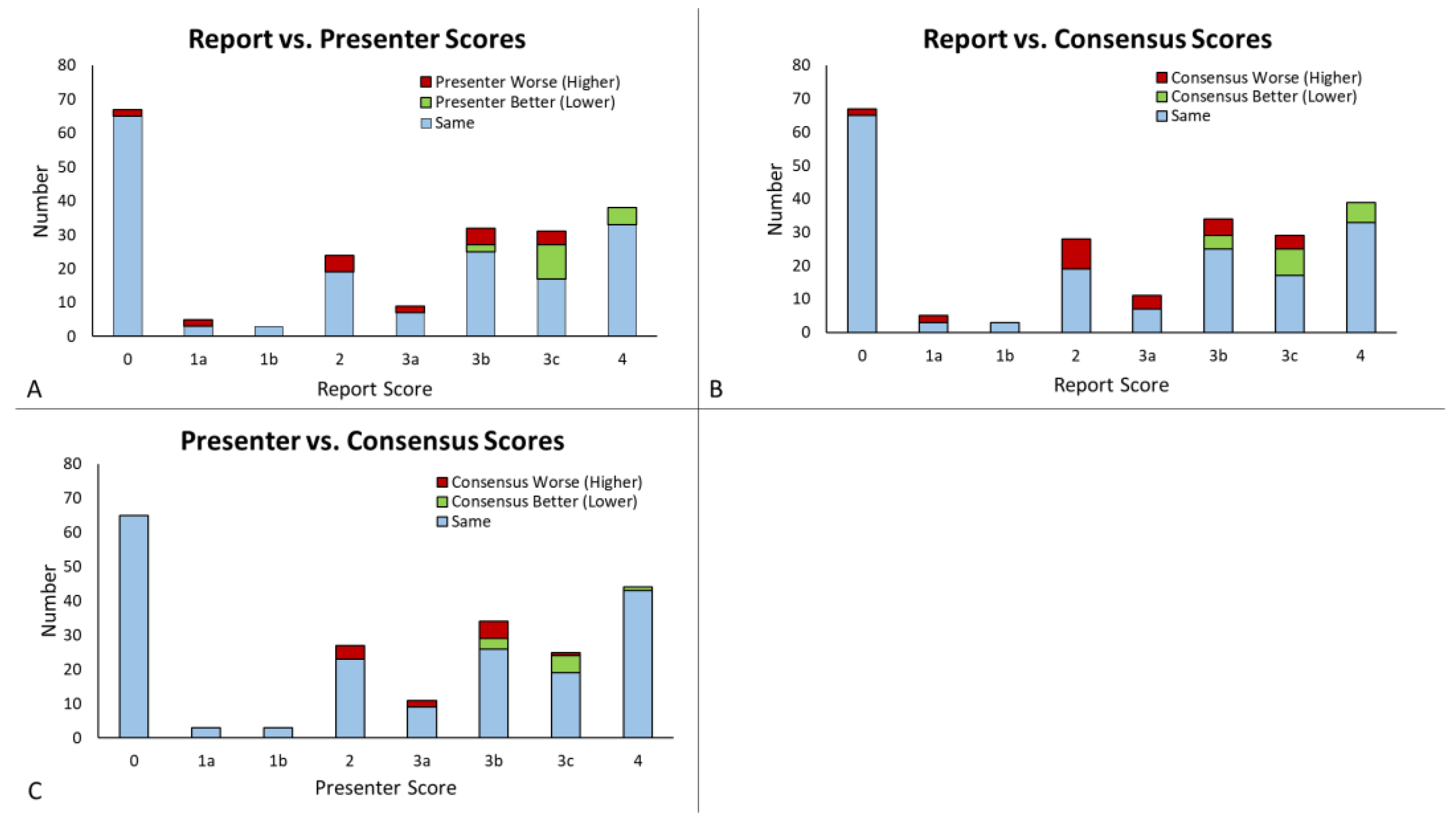
3.2. Score Comparison and Concordance
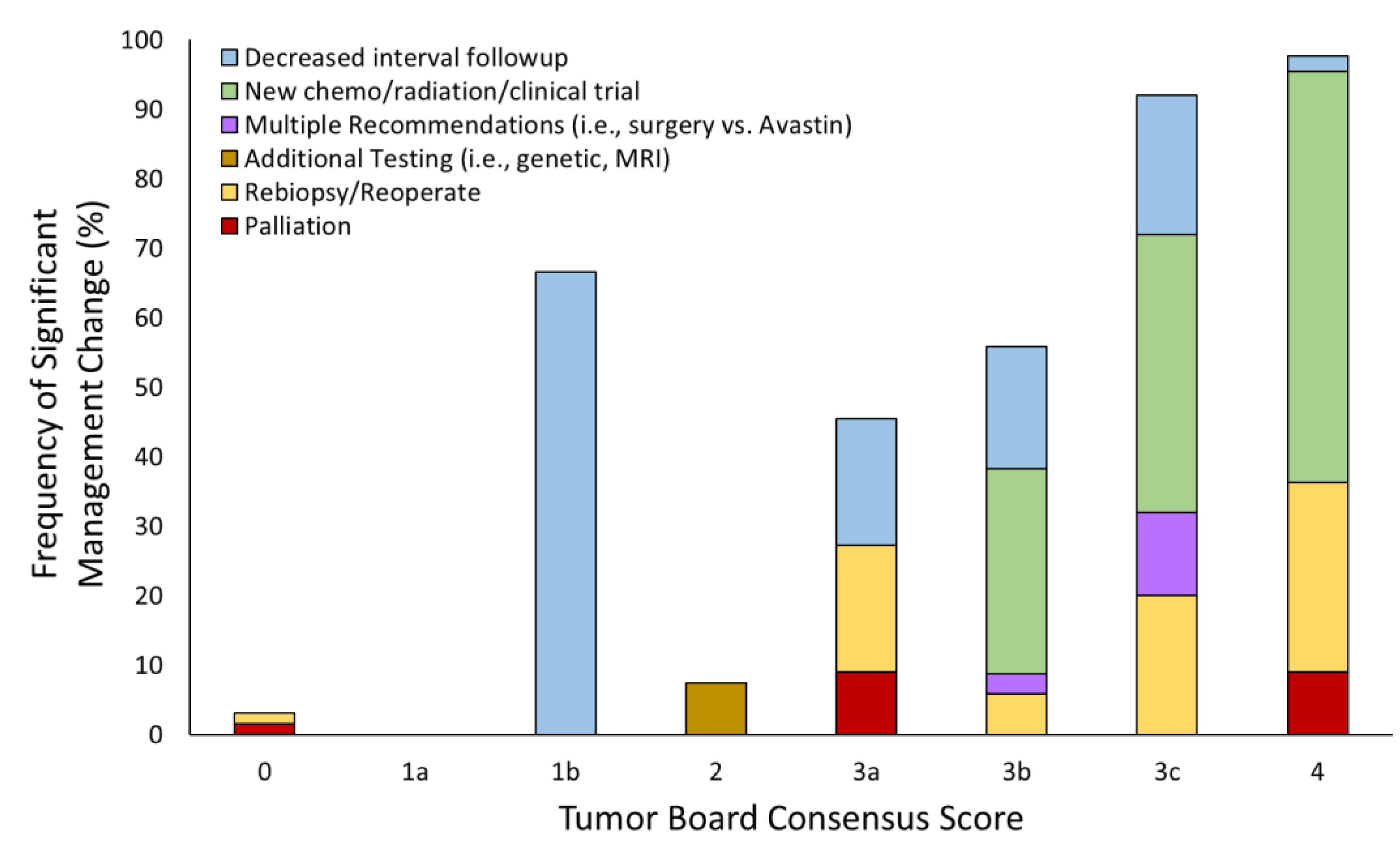
3.3. Tumor Board Management Decisions
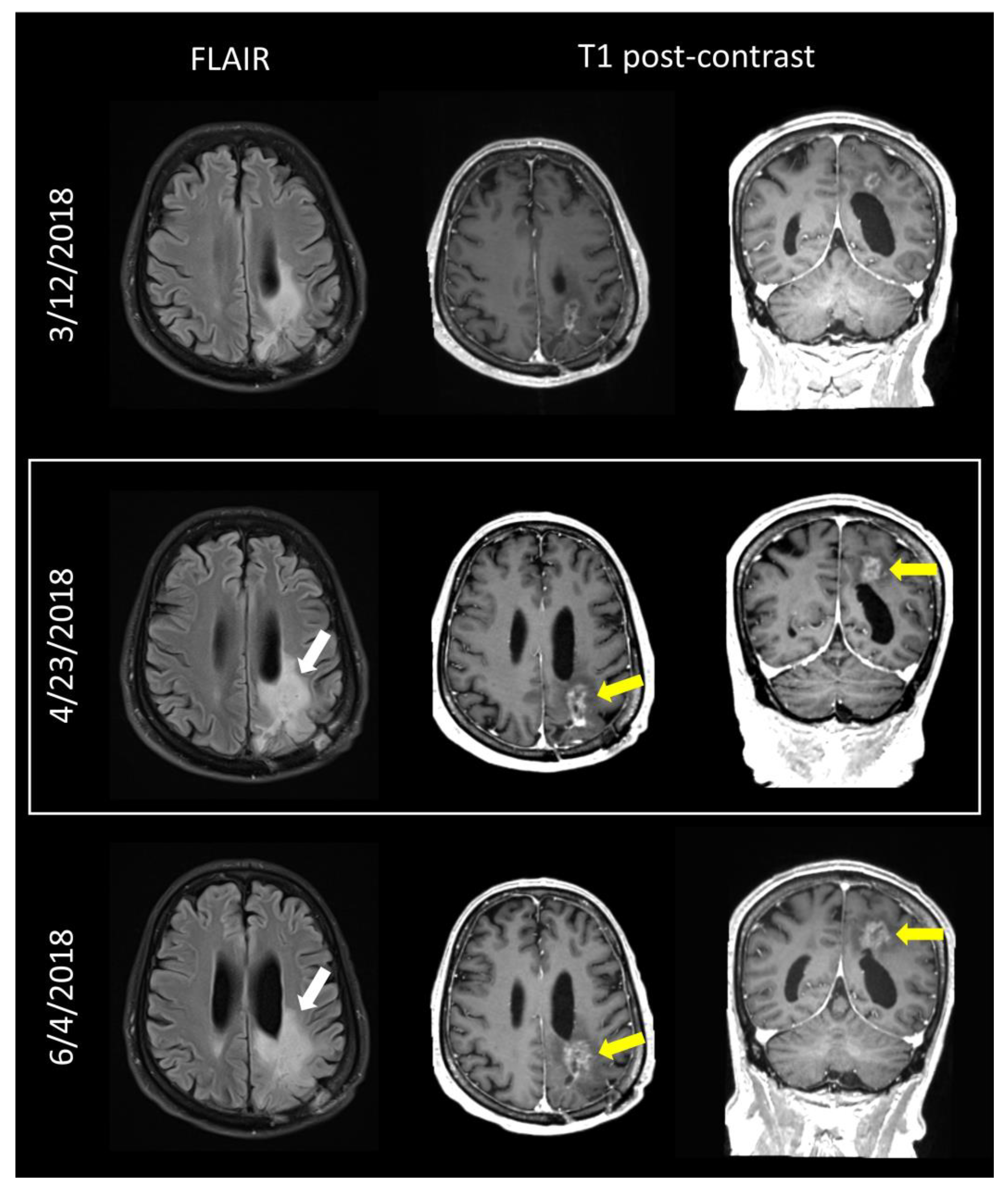
3.4. Clinical Implementation and Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Cancer Institute. Definition of Tumor Board Review. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/tumor-board-review (accessed on 25 February 2023).
- Liu, J.C.; Kaplon, A.; Blackman, E.; Miyamoto, C.; Savior, D.; Ragin, C. The impact of the multidisciplinary tumor board on head and neck cancer outcomes. Laryngoscope 2020, 130, 946–950. [Google Scholar] [CrossRef]
- AlFarhan, H.A.; Algwaiz, G.F.; Alzahrani, H.A.; Alsuhaibani, R.S.; Alolayan, A.; Abdelhafiz, N.; Ali, Y.; Boghdadly, S.; Jazieh, A.R. Impact of GI Tumor Board on Patient Management and Adherence to Guidelines. J. Glob. Oncol. 2018, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Forrest, L.M.; McMillan, D.C.; McArdle, C.S.; Dunlop, D.J. An evaluation of the impact of a multidisciplinary team, in a single centre, on treatment and survival in patients with inoperable non-small-cell lung cancer. Br. J. Cancer 2005, 93, 977–978. [Google Scholar] [CrossRef] [PubMed]
- Gatcliffe, T.A.; Coleman, R.L. Tumor board: More than treatment planning—A 1-year prospective survey. J. Cancer Educ. 2008, 23, 235–237. [Google Scholar] [CrossRef]
- Cohen, P.; Tan, A.L.; Penman, A. The multidisciplinary tumor conference in gynecologic oncology--does it alter management? Int. J. Gynecol. Cancer 2009, 19, 1470–1472. [Google Scholar] [CrossRef] [PubMed]
- Santoso, J.T.; Schwertner, B.; Coleman, R.L.; Hannigan, E.V. Tumor board in gynecologic oncology. Int. J. Gynecol. Cancer 2004, 14, 206–209. [Google Scholar] [CrossRef]
- Lutterbach, J.; Pagenstecher, A.; Spreer, J.; Hetzel, A.; Velthoven, V.; Nikkhah, G.; Frommhold, H.; Volk, B.; Schumacher, M.; Lucking, C.; et al. The brain tumor board: Lessons to be learned from an interdisciplinary conference. Onkologie 2005, 28, 22–26. [Google Scholar] [CrossRef]
- Petty, J.K.; Vetto, J.T. Beyond doughnuts: Tumor board recommendations influence patient care. J. Cancer Educ. 2002, 17, 97–100. [Google Scholar] [CrossRef]
- Blazeby, J.M.; Wilson, L.; Metcalfe, C.; Nicklin, J.; English, R.; Donovan, J.L. Analysis of clinical decision-making in multi-disciplinary cancer teams. Ann. Oncol. 2006, 17, 457–460. [Google Scholar] [CrossRef]
- Newman, E.A.; Guest, A.B.; Helvie, M.A.; Roubidoux, M.A.; Chang, A.E.; Kleer, C.G.; Diehl, K.M.; Cimmino, V.M.; Pierce, L.; Hayes, D.; et al. Changes in surgical management resulting from case review at a breast cancer multidisciplinary tumor board. Cancer 2006, 107, 2346–2351. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Xu, J.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2009–2013. Neuro Oncol. 2016, 18, v1–v75. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Ostrom, Q.T.; Kruchko, C.; Patil, N.; Tihan, T.; Cioffi, G.; Fuchs, H.E.; Waite, K.A.; Jemal, A.; Siegel, R.L.; et al. Brain and other central nervous system tumor statistics, 2021. CA Cancer J. Clin. 2021, 71, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhang, X.; Gao, C.; Jiang, S.; Wu, H.; Liu, Z.; Dou, T. Burden and trends of brain and central nervous system cancer from 1990 to 2019 at the global, regional, and country levels. Arch. Public Health 2022, 80, 209. [Google Scholar] [CrossRef]
- Brown, N.F.; Ottaviani, D.; Tazare, J.; Gregson, J.; Kitchen, N.; Brandner, S.; Fersht, N.; Mulholland, P. Survival Outcomes and Prognostic Factors in Glioblastoma. Cancers 2022, 14, 3161. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.S.; Hanbidge, A.; Wilson, S.R. Radiology reports: Examining radiologist and clinician preferences regarding style and content. AJR Am. J. Roentgenol. 2001, 176, 591–598. [Google Scholar] [CrossRef]
- Bosmans, J.M.; Weyler, J.J.; Parizel, P.M. Structure and content of radiology reports, a quantitative and qualitative study in eight medical centers. Eur. J. Radiol. 2009, 72, 354–358. [Google Scholar] [CrossRef]
- Ganeshan, D.; Duong, P.T.; Probyn, L.; Lenchik, L.; McArthur, T.A.; Retrouvey, M.; Ghobadi, E.H.; Desouches, S.L.; Pastel, D.; Francis, I.R. Structured Reporting in Radiology. Acad. Radiol. 2018, 25, 66–73. [Google Scholar] [CrossRef]
- Lazarus, E.; Mainiero, M.B.; Schepps, B.; Koelliker, S.L.; Livingston, L.S. BI-RADS lexicon for US and mammography: Interobserver variability and positive predictive value. Radiology 2006, 239, 385–391. [Google Scholar] [CrossRef]
- Aiken, A.H.; Farley, A.; Baugnon, K.L.; Corey, A.; El-Deiry, M.; Duszak, R.; Beitler, J.; Hudgins, P.A. Implementation of a Novel Surveillance Template for Head and Neck Cancer: Neck Imaging Reporting and Data System (NI-RADS). J. Am. Coll. Radiol. 2016, 13, 743–746.e741. [Google Scholar] [CrossRef]
- Weinberg, B.D.; Gore, A.; Shu, H.G.; Olson, J.J.; Duszak, R.; Voloschin, A.D.; Hoch, M.J. Management-Based Structured Reporting of Posttreatment Glioma Response with the Brain Tumor Reporting and Data System. J. Am. Coll. Radiol. 2018, 15, 767–771. [Google Scholar] [CrossRef]
- Gore, A.; Hoch, M.J.; Shu, H.G.; Olson, J.J.; Voloschin, A.D.; Weinberg, B.D. Institutional Implementation of a Structured Reporting System: Our Experience with the Brain Tumor Reporting and Data System. Acad. Radiol. 2019, 26, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Weinberg, B.D.; Hu, R.; Saindane, A.; Mullins, M.; Allen, J.; Hoch, M.J. Quantitative Improvement in Brain Tumor MRI Through Structured Reporting (BT-RADS). Acad. Radiol. 2020, 27, 780–784. [Google Scholar] [CrossRef]
- Weinberg, B.D.; Hoch, M.J. Brain Tumor-Reporting and Data System. Available online: www.btrads.com (accessed on 3 March 2020).
- Kim, S.; Hoch, M.J.; Cooper, M.E.; Gore, A.; Weinberg, B.D. Using a Website to Teach a Structured Reporting System, the Brain Tumor Reporting and Data System. Curr. Probl. Diagn. Radiol. 2021, 50, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Wheless, S.A.; McKinney, K.A.; Zanation, A.M. A prospective study of the clinical impact of a multidisciplinary head and neck tumor board. Otolaryngol.—Head Neck Surg. 2010, 143, 650–654. [Google Scholar] [CrossRef]
- Kim, S.; Hoch, M.J.; Peng, L.; Somasundaram, A.; Chen, Z.; Weinberg, B.D. A brain tumor reporting and data system to optimize imaging surveillance and prognostication in high-grade gliomas. J. Neuroimaging 2022, 32, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, Y.; Wu, X.; Pan, Y.; Zhou, D.; Zhang, H.; Chen, Y.; Zhao, J.; Mo, Z.; Huang, B. Adding DSC PWI and DWI to BT-RADS can help identify postoperative recurrence in patients with high-grade gliomas. J. Neurooncol. 2020, 146, 363–371. [Google Scholar] [CrossRef]
- Sooriakumaran, P.; Dick, J.A.; Thompson, A.C.; Morley, R. The central urology multidisciplinary team—Is it time to change the referral criteria? An audit of practice in a district general hospital in London. Ann. R. Coll. Surg. Engl. 2009, 91, 700–702. [Google Scholar] [CrossRef]

| Score | Title | Subscore | Description |
|---|---|---|---|
| 0 | Not scored | New baseline, incomplete study, or otherwise unable to categorize | |
| 1 | Imaging improving | 1a—Improvement | Improvement in imaging findings suspected to reflect decreasing tumor burden and/or improving treatment effect |
| 1b—Medication effect | Improvement in imaging findings potentially due to effect from medications such as increasing steroids or initiating Avastin | ||
| 2 | No change | No appreciable change from the prior | |
| 3 | Imaging worsening | 3a—Favor treatment effect | Worsening imaging findings favored to represent treatment effects, including radiation therapy and medications |
| 3b—Indeterminate | Worsening imaging findings favored to represent an indeterminate mix of treatment effect and tumor worsening | ||
| 3c—Favor tumor progression | Worsening imaging findings favored to represent increasing burden of tumor | ||
| 4 | Imaging worsening | Worsening imaging findings highly suspicious for tumor progression |
| Category | Number (%) |
|---|---|
| Patients | 130 |
| Age Range | 23–84 years |
| Age Mean | 55 years |
| Age Median | 57 years |
| Tumors | |
| Glioblastoma | 63 (48.5) |
| Anaplastic astrocytoma (grade 3) | 16 (12.3) |
| Oligodendroglioma (grade 2) | 12 (9.2) |
| Astrocytoma (grade 2) | 11 (8.5) |
| Anaplastic oligodendroglioma (grade 3) | 10 (7.7) |
| Gliosarcoma | 4 (3.1) |
| Other | |
| Presumed glioma (no biopsy) | 6 (4.6) |
| Anaplastic pleomorphic xanthoastrocytoma | 1 (0.8) |
| Medulloblastoma | 1 (0.8) |
| Grade 1 glioneural tumor, G1 | 1 (0.8) |
| Other diffuse astrocytoma | 1 (0.8) |
| Ependymoma | 1 (0.8) |
| Diffuse midline glioma | 1 (0.8) |
| Atypical liponeurocytoma | 1 (0.8) |
| Grade 1 glioma | 1 (0.8) |
| Category | Number (%) |
|---|---|
| Total Cases | 212 |
| Clinical follow-up within 90 days | 184 (86.8) |
| Clinicians implemented TB recommendations | 155 (84.2) |
| Clinicians did not implement TB recommendations | 26 (14.1) |
| New chemotherapy instead of TB recommendation | 12 (5.7) |
| Decreased interval follow-up instead of TB recommendation | 7 (3.3) |
| Continue follow-up/no change instead of TB recommendation | 3 (1.4) |
| Palliative care instead of TB recommendation | 2 (0.9) |
| Clinical trial instead of TB recommendation | 1 (0.5) |
| Re-operate/re-biopsy instead of TB recommendation | 1 (0.5) |
| Patient changed provider after clinical visit | 3 (1.6) |
| Clinical follow-up >90 days | 18 (8.5) |
| No follow-up present after tumor board | 10 (4.7) |
| No follow-up due to death following tumor board | 4 (1.9) |
| TB Consensus Score | # Biopsied (% Biopsied of All Similar Consensus Scores) | Percent of Biopsies with >50% Tumor Proportion |
|---|---|---|
| 0 | 1 (1.5) | 100 |
| 2 | 1 (3.7) | 100 |
| 3a | 1 (9.1) | 100 |
| 3b | 1 (2.9) | 50 |
| 3c | 2 (8.0) | 67 |
| 4 | 8 (18.2) | 89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abidi, S.A.; Hoch, M.J.; Hu, R.; Sadigh, G.; Voloschin, A.; Olson, J.J.; Shu, H.-K.G.; Neill, S.G.; Weinberg, B.D. Using Brain Tumor MRI Structured Reporting to Quantify the Impact of Imaging on Brain Tumor Boards. Tomography 2023, 9, 859-870. https://doi.org/10.3390/tomography9020070
Abidi SA, Hoch MJ, Hu R, Sadigh G, Voloschin A, Olson JJ, Shu H-KG, Neill SG, Weinberg BD. Using Brain Tumor MRI Structured Reporting to Quantify the Impact of Imaging on Brain Tumor Boards. Tomography. 2023; 9(2):859-870. https://doi.org/10.3390/tomography9020070
Chicago/Turabian StyleAbidi, Syed A., Michael J. Hoch, Ranliang Hu, Gelareh Sadigh, Alfredo Voloschin, Jeffrey J. Olson, Hui-Kuo G. Shu, Stewart G. Neill, and Brent D. Weinberg. 2023. "Using Brain Tumor MRI Structured Reporting to Quantify the Impact of Imaging on Brain Tumor Boards" Tomography 9, no. 2: 859-870. https://doi.org/10.3390/tomography9020070
APA StyleAbidi, S. A., Hoch, M. J., Hu, R., Sadigh, G., Voloschin, A., Olson, J. J., Shu, H.-K. G., Neill, S. G., & Weinberg, B. D. (2023). Using Brain Tumor MRI Structured Reporting to Quantify the Impact of Imaging on Brain Tumor Boards. Tomography, 9(2), 859-870. https://doi.org/10.3390/tomography9020070








