An Online Repository for Pre-Clinical Imaging Protocols (PIPs)
Abstract
:1. Introduction
2. Methods
2.1. Identification of the Possible Use Cases
- Disseminating a new material/method combination: A new injectable reporter/imaging method was developed, and the authors want early adopters, particularly at other institutions or globally, to be more successful in their early pilot experiments. Thus, a new PIP was written and published.
- Documenting an imaging process for testing new drugs: A robust method was developed for proving target engagement for a class of drugs. A pre-clinical imaging protocol (PIP) was written, thus novel and emerging drugs in the same class can be tested for superior target engagement in vivo.
- Reporting new instrument tolerances: New tighter instrument tolerances such as resolution were developed that enable new applications. A PIP was written and uploaded for others to adjust tolerances.
- Empowering the community of researchers: Many research protocols have reasonable similarities but not identity, e.g., similar animal models; tumor locations; and assumed mechanisms of action such as cytotoxic treatment. A detailed PIP could be used by the research community to jumpstart their new investigations.
- Improving serial study designs: A community of non-imaging researchers have used invasive methods to assess therapeutic efficacy and have relied on group comparisons (e.g., treated vs. control groups). They hypothesized that greater sensitivity or specificity can be achieved through a non-invasive serial study. A published PIP provides a reasonable estimate of “precision” of a relevant QIB, which was useful in their serial study design. These estimates of precision were particularly helpful for order of magnitude power analysis (size of N).
- Accelerating clinical translation: It is anticipated that the inclusion of additional important details in PIPs (e.g., details of phantoms; analysis methods/stats; animal model, contrast agents) may enable translational researchers to more quickly and effectively design companion imaging trials.
- Providing a sustainable historical record: PIPs could provide detailed long-term records of the lab’s own investigations, which are useful to incoming and established members of the laboratory.
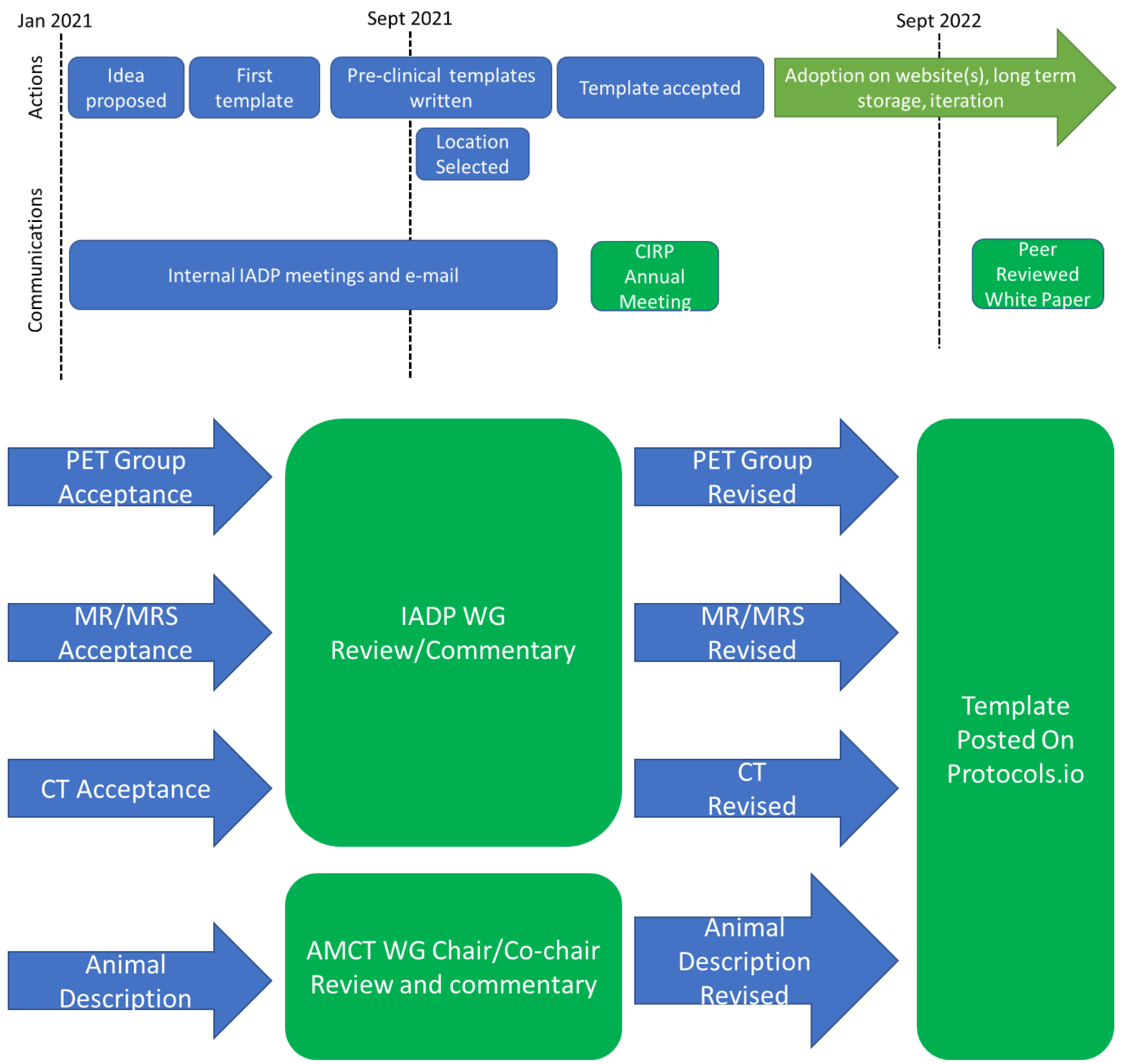
2.2. Development Process and Vetting
3. Developmental Boundaries and Constraints
3.1. Top Level Boundaries and Constraints
3.2. Template Location Sustainability Requirements and Constraints
4. Results
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Errington, T.M.; Mathur, M.; Soderberg, C.K.; Denis, A.; Perfito, N.; Iorns, E.; Nosek, B.A. Investigating the replicability of preclinical cancer biology. Elife 2021, 10, e71601. [Google Scholar] [CrossRef] [PubMed]
- Hutson, M. Artificial intelligence faces reproducibility crisis. Science 2018, 359, 725–726. [Google Scholar] [CrossRef] [PubMed]
- Baker, M. Over half of psychology studies fail reproducibility test. Nature 2015, 27, 1–3. [Google Scholar] [CrossRef]
- Canada, C. Lunit AI Solution for Radiology Receives Health Canada Nod for Commercial Use; MIT Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Paquier, Z.; Chao, S.L.; Bregni, G.; Sanchez, A.V.; Guiot, T.; Dhont, J.; Gulyban, A.; Levillain, H.; Sclafani, F.; Reynaert, N.; et al. Pre-trial quality assurance of diffusion-weighted MRI for radiomic analysis and the role of harmonisation. Phys. Med. 2022, 103, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Boss, M.A.; Snyder, B.S.; Kim, E.; Flamini, D.; Englander, S.; Sundaram, K.M.; Gumpeni, N.; Palmer, S.L.; Choi, H.; Froemming, A.T.; et al. Repeatability and Reproducibility Assessment of the Apparent Diffusion Coefficient in the Prostate: A Trial of the ECOG-ACRIN Research Group (ACRIN 6701). J. Magn. Reson. Imaging 2022, 56, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Avila, R.S.; Fain, S.B.; Hatt, C.; Armato, I.I.I.S.G.; Mulshine, J.L.; Gierada, D.; Silva, M.; Lynch, D.A.; Hoffman, E.A.; Ranallo, F.N.; et al. QIBA guidance: Computed tomography imaging for COVID-19 quantitative imaging applications. Clin. Imaging 2021, 77, 151–157. [Google Scholar] [CrossRef] [PubMed]
- QIBA Profiles. [Cited 2023 01/02/2023]; Profiles Published Here Have Completed One of the QIBA Profile Stages of Development and Been Approved by Their BIOMARKER Committee. Available online: https://qibawiki.rsna.org/index.php/Profiles (accessed on 20 March 2023).
- Raunig, D.L.; Pennello, G.A.; Delfino, J.G.; Buckler, A.J.; Hall, T.J.; Guimaraes, A.R.; Wang, X.; Huang, E.P.; Barnhart, H.X.; deSouza, N.; et al. Multiparametric Quantitative Imaging Biomarker as a Multivariate Descriptor of Health: A Roadmap. Acad. Radiol. 2022, 30, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Kessler, L.G.; Barnhart, H.X.; Buckler, A.J.; Choudhury, K.R.; Kondratovich, M.V.; Toledano, A.; Guimaraes, A.R.; Filice, R.; Zhang, Z.; Sullivan, D.C.; et al. The emerging science of quantitative imaging biomarkers terminology and definitions for scientific studies and regulatory submissions. Stat. Methods Med. Res. 2015, 24, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Shukla-Dave, A.; Obuchowski, N.A.; Chenevert, T.L.; Jambawalikar, S.; Schwartz, L.H.; Malyarenko, D.; Huang, W.; Noworolski, S.M.; Young, R.J.; Shiroishi, M.S.; et al. Quantitative imaging biomarkers alliance (QIBA) recommendations for improved precision of DWI and DCE-MRI derived biomarkers in multicenter oncology trials. J. Magn. Reson. Imaging 2019, 49, e101–e121. [Google Scholar] [CrossRef] [PubMed]
- Kinahan, P.E.; Perlman, E.S.; Sunderland, J.J.; Subramaniam, R.; Wollenweber, S.D.; Turkington, T.G.; Lodge, M.A.; Boellaard, R.; Obuchowski, N.A.; Wahl, R.L. The QIBA Profile for FDG PET/CT as an Imaging Biomarker Measuring Response to Cancer Therapy. Radiology 2020, 294, 647–657. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. Clinical Trial Imaging Endpoint Process Standards Guidance for Industry. United States Food and Drug Administration. Available online: https://www.fda.gov/media/81172/download (accessed on 20 March 2023).

| Suggested Hosting Location | Advantages |
| MICAD | Website already exists |
| CIRP HUB | Website already exists |
| NCI hosting | Could be publicly accessible and built for purpose |
| Journals Articles—SI or appendix | System exists, protocols are publicly accessible, funding included in page charges |
| STAR protocols & manuscripts | Website: already exists, is publicly accessible, used in biology |
| Github | Website: already exists, publicly accessible, non-for profit, free |
| Protocols.io | Website: already exists, publicly accessible, non-for profit, free, can reference PLOS one, leveraged by PDX consortium |
| Suggested Hosting Location | Challenges |
| MICAD | No funding, time, or personnel to update site |
| CIRP HUB | Website is small and not publicly accessible |
| NCI hosting | No funding, time, or personnel to create site |
| Journals Articles—SI or appendix | Cannot update or sunset protocols, template adoption may be difficult |
| STAR protocols & manuscripts | Cannot update or sunset protocols, template adoption may be difficult, need journal to adopt templates and template updates |
| Github | Solution primarily used in software development, may need to adapt format for biological protocols |
| Protocols.io | Currently free but may be moving to paid subscription model |
| Suggested Hosting Location | Consensus Action |
| MICAD | Not selected |
| CIRP HUB | Not selected |
| NCI hosting | Not selected |
| Journals Articles—SI or appendix | Not selected |
| STAR protocols & manuscripts | Not selected |
| Github | Selected as backup |
| Protocols.io | Selected as preferred site |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gammon, S.T.; Cohen, A.S.; Lehnert, A.L.; Sullivan, D.C.; Malyarenko, D.; Manning, H.C.; Hormuth, D.A.; Daldrup-Link, H.E.; An, H.; Quirk, J.D.; et al. An Online Repository for Pre-Clinical Imaging Protocols (PIPs). Tomography 2023, 9, 750-758. https://doi.org/10.3390/tomography9020060
Gammon ST, Cohen AS, Lehnert AL, Sullivan DC, Malyarenko D, Manning HC, Hormuth DA, Daldrup-Link HE, An H, Quirk JD, et al. An Online Repository for Pre-Clinical Imaging Protocols (PIPs). Tomography. 2023; 9(2):750-758. https://doi.org/10.3390/tomography9020060
Chicago/Turabian StyleGammon, Seth T., Allison S. Cohen, Adrienne L. Lehnert, Daniel C. Sullivan, Dariya Malyarenko, Henry Charles Manning, David A. Hormuth, Heike E. Daldrup-Link, Hongyu An, James D. Quirk, and et al. 2023. "An Online Repository for Pre-Clinical Imaging Protocols (PIPs)" Tomography 9, no. 2: 750-758. https://doi.org/10.3390/tomography9020060
APA StyleGammon, S. T., Cohen, A. S., Lehnert, A. L., Sullivan, D. C., Malyarenko, D., Manning, H. C., Hormuth, D. A., Daldrup-Link, H. E., An, H., Quirk, J. D., Shoghi, K., Pagel, M. D., Kinahan, P. E., Miyaoka, R. S., Houghton, A. M., Lewis, M. T., Larson, P., Sriram, R., Blocker, S. J., ... Chenevert, T. L. (2023). An Online Repository for Pre-Clinical Imaging Protocols (PIPs). Tomography, 9(2), 750-758. https://doi.org/10.3390/tomography9020060










