Abstract
Introduction: Termed the “silent epidemic,” traumatic brain injury (TBI) is one of the greatest global contributors not only to post-traumatic death but also to post-traumatic long-term disability. This systematic review and meta-analysis aims to specifically evaluate the prognostic value of features on initial imaging completed within 24 h of arrival in adult patients with TBI. Method: The authors followed the PRISMA 2020 checklist for systematic review and meta-analysis design and reporting. Comprehensive searches of the Medline and Embase databases were carried out. Two independent readers extracted the following demographic, clinical and imaging information using a predetermined data abstraction form. Statistics were performed using Revman 5.4.1 and R version 4.2.0. For pooled data in meta-analysis, forest plots for sensitivity and specificity were created to calculate the diagnostic odds ratio (DOR). Summary receiver operating characteristic (SROC) curves were generated using a bivariate model, and diagnostic accuracy was determined using pooled sensitivity and specificity as well as the area under the receiver operator characteristic curve (AUC). Results: There were 10,733 patients over the 19 studies. Overall, most of the studies included had high levels of bias in multiple, particularly when it came to selection bias in patient sampling, bias in controlling for confounders, and reporting bias, such as in reporting missing data. Only subdural hematoma (SDH) and mortality in all TBI patients had both an AUC with 95% CI not crossing 0.5 and a DOR with 95% CI not crossing 1, at 0.593 (95% CI: 0.556–0.725) and 2.755 (95% CI: 1.474–5.148), respectively. Conclusion: In meta-analysis, only SDH with mortality in all TBI patients had a moderate but significant association. Given the small number of studies, additional research focused on initial imaging, particularly for imaging modalities other than NECT, is required in order to confirm the findings of our meta-analysis and to further evaluate the association of imaging findings and outcome.
1. Introduction
Termed the “silent epidemic,” traumatic brain injury (TBI) is one of the greatest global contributors not only to post-traumatic death but also to post-traumatic long-term disability in multiple domains ranging from neurological and physical to behavioral and psychosocial [1,2,3]. On a per capita basis, the United States and Canada together demonstrate the highest incidence of TBI in the world at 1299 cases per 100,000 people [1]. It is estimated that 1.1% of the American population, or roughly 3.1 million people, are living with long-term disability post-TBI associated with a lifetime economic cost of $76.5 billion in 2010 US dollars [3,4]. Despite this, the highest healthcare and socioeconomic burden is seen in resource-poor and lower-income countries [1].
Since its adoption around 50 years ago, non-enhanced computed tomography (NECT) of the head has become an indispensable and widely available imaging tool that can accurately and promptly diagnose intracranial injuries post-TBI including intracranial hemorrhage (ICH), mass effect and herniation, midline shift, and cranial fractures [5]. To aid in predicting outcomes in TBI patients based on NECT findings, classification systems such as the Marshall score (in 1991) and the Rotterdam CT score in (2005) have been developed [6,7]. Multivariate models combining both clinical and imaging findings such as the International Mission on Prognosis and Analysis of Clinical Trials in TBI (IMPACT) also attempt to prognosticate, but they are not widely used clinically and are designed for guiding clinical trials [8]. In addition to NECT, other CT techniques such as CT perfusion (CTP) [9], and additional imaging modalities such as positron emission tomography (PET) [10], single-photon emission computed tomography (SPECT) [11], transcranial doppler (TCD) ultrasound (US) [12], and magnetic resonance imaging (MRI) [13], have also been used to evaluate the extent of intracranial injuries post-TBI and to aid in prognostication.
This systematic review and meta-analysis aims to specifically evaluate the prognostic value of features on initial imaging completed within 24 h of arrival in adult patients with TBI.
2. Methods
The authors followed the PRISMA 2020 checklist for systematic review and meta-analysis design and reporting [14]. Comprehensive searches of the Medline and Embase databases were carried out by an experienced librarian (JL) using the OVID platform to identify relevant studies for inclusion. A combination of keyword and MeSH or EMTREE subject headings was used.
The search was limited to articles published in peer-reviewed journals in English from inception to September 2020. All imaging modalities were eligible for inclusion. Aside from reviews, editorials and case reports, all study types and designs were included. Studies were excluded if (1) there were no patients with TBI OR (2) initial imaging was not performed within 24 h of arrival to emergency department (ED) OR (3) there was no follow-up or patient outcome measures reported OR (4) the mechanism of insult was non-traumatic OR (5) patients less than 18 years of age were included OR (6) the study sample size was fewer than five individuals.
Three independent screeners extracted the following information using a predetermined data abstraction form: study characteristics, patient demographics, information regarding patient initial clinical presentation when available (e.g., Glasgow coma score (GCS), pupil reactivity and evenness, vital signs), imaging modality characteristics, associated imaging findings (e.g., ICH, midline shift), and follow-up and associated patient outcomes related to study imaging findings.
2.1. Statistics
Statistics were performed using Revman 5.4.1 (Cochrane, London, UK) and R version 4.2.0 (R Foundation for Statistical Computing, Vienna, Austria) [15,16]. Each imaging finding and associated outcome measure had 2 × 2 tables created to calculate the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). For pooled data in meta-analysis, forest plots for sensitivity and specificity were created to calculate the diagnostic odds ratio (DOR). Summary receiver operating characteristic (SROC) curves were generated using a bivariate model, and diagnostic accuracy was determined using pooled sensitivity and specificity as well as the area under the receiver operator characteristic curve (AUC). The R program “mada” was used to generate the SROC curves. AUC and 95% confidence intervals (CI) were calculated using a parametric bootstraps method [17]. Study heterogeneities were measured by Higgins’ I2.
2.2. Assessing Bias
Bias was assessed using the domains of the ROBINS-I tool (Cochrane, London, UK) [18]. Two authors independently assessed each domain for high, unclear, and low risk of bias in each included study. Disagreements were resolved with consensus. Inclusion of only studies with full publication in peer-reviewed journals was done to maintain the highest possible quality of evidence.
3. Results
The study selection process is shown in Figure 1. The initial search yielded 4586 results, and 4027 unique articles after duplicates were removed. Of the original 109 articles, only 19 studies were eligible for inclusion within our systematic review [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. A summary of the articles included can be found in Table 1. In total, there were 10,733 patients over the 19 studies. The risk of bias analysis is shown in Figure 2. Overall, most of the included studies had high levels of bias in multiple, particularly when it came to selection bias in patient sampling, bias in controlling for confounders, and reporting bias, such as in reporting missing data.
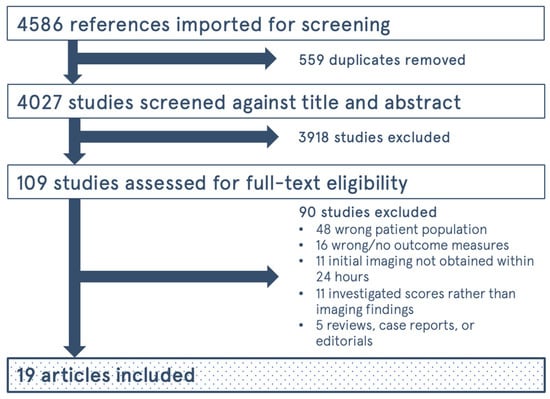
Figure 1.
Flow Diagram for study selection process.

Table 1.
Study characteristics.
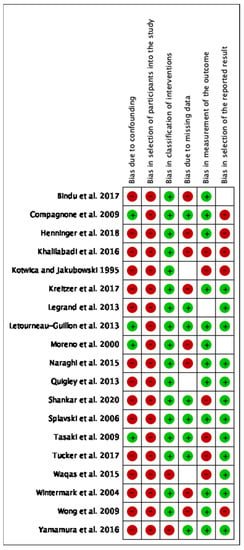
Figure 2.
Risk of bias analysis for the included studies [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. Red indicates high risk of bias, blank indicates unclear risk of bias, and green indicates low risk of bias.
Imaging modalities that were investigated included NECT, CTP, CTA, and TCD. Patient outcome measures and follow-up intervals varied widely, but most of the included studies used the Glasgow Outcome Scale (GOS) as their primary patient outcome measure in follow-up, with a GOS 1–3 commonly interpreted as an unfavorable outcome and a GOS 4–5 interpreted as a favorable outcome. Only data involving the association between imaging features and patient outcome measures were recorded. In certain studies, the authors report imaging features as demographic information but ultimately did not provide data or analysis on their association with patient outcome measures. These were not included in analysis. Some studies, in addition to researching the association between imaging findings and patient outcome measures, also investigated imaging finding associations with non-outcome-related findings including biochemical and laboratory data, such as lactate levels, and relationships with other imaging findings. These were also not included. Isolated relationships between imaging and patient clinical features, such as CTP findings with intracranial hypertension, were not recorded unless an associated patient outcome measure was also analyzed.
The association between imaging findings and select patient outcome measures is summarized in Table 2. In general, the presence of subarachnoid hemorrhage (SAH), midline shift, cerebral edema, and basal cistern effacement are associated with poorer outcomes in TBI. Other types of ICH were not associated with worse outcomes, especially after consideration of confounders in multivariate analysis. Kotwica and Jakubowski 1995 found that the presence of subdural hematoma (SDH) was associated with worse outcomes and epidural hematoma (EDH) was associated with better outcomes in patients presenting with GCS 3 [23]. While the presence of diffuse axonal injury (DAI) on CT was associated with worse outcomes in comparison to the absence of DAI on CT, predominantly hemorrhagic DAI on CT was associated with better outcomes compared to predominantly non-hemorrhagic DAI [21].

Table 2.
Imaging findings and their association with patient outcomes.
In CTP, decreased cerebral blood flow (CBF) and cerebral blood volume (CBV) prognosticated poorer outcomes and higher mortality [19,30,35]. Increased pulsatility index (PI) and decreased middle cerebral artery (MCA) velocity on TCD were associated with poorer outcomes, as was increased optic nerve sheath diameter (ONSD) [25,27,31]. Increased ONSD was not associated with worse outcomes in a subpopulation consisting of those requiring decompressive craniectomy [34]. Regarding findings on CT angiography (CTA), Naraghi et al. 2015 found that findings on CTA only changed management for one of their 132 patients and concluded that the examination is unnecessary as an initial investigation for TBI [28]. Alternatively, Letourneau-Guillon et al. 2013 found that arterial extravasation predicted the need for surgical evacuation and in-hospital mortality [26].
The data for six studies [22,23,25,33,34,36] could be pooled for meta-analysis. Figure 3 provides the forest plots for sensitivity and specificity for the meta-analysis data.
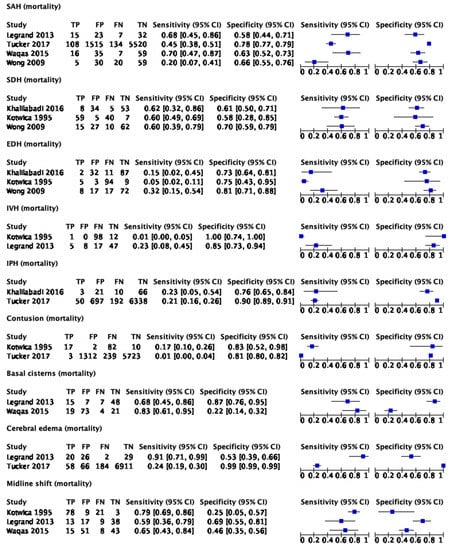
Figure 3.
Forest plots for imaging findings and mortality in all TBI patients [22,23,25,33,34,36]. TP: true positive; FP: false positive; FN: false negative; TN: true negative.
The results of Figure 3 were used to generate the SROC curves shown in Figure 4. The summary estimate represents the mean sensitivity and 1-specificity (false positive rate) of the pooled data for each imaging finding with an associated 95% confidence region outlined by the thin black line. Using the SROC curves, the diagnostic odds ratio (DOR) and the area under the receiver operator characteristic curve (AUC) were calculated along with their 95% confidence intervals (CI).
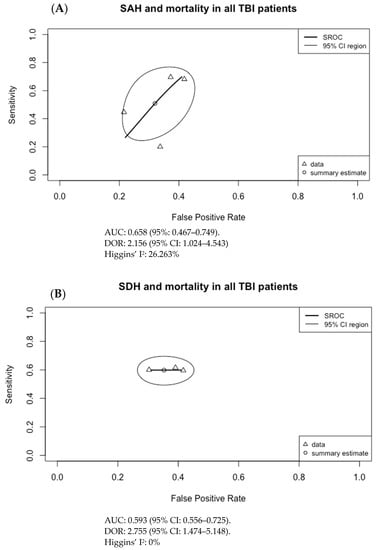
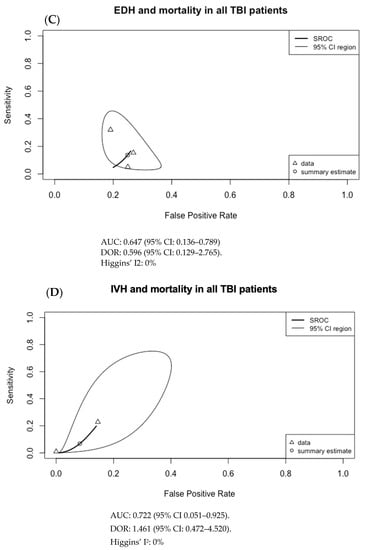
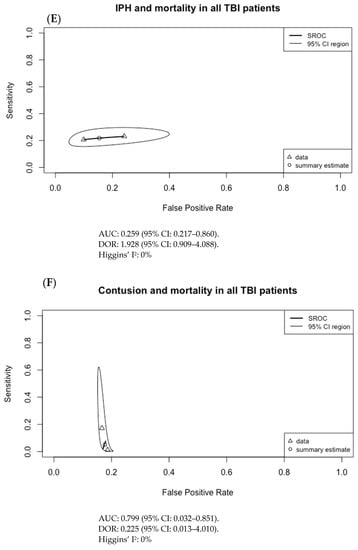
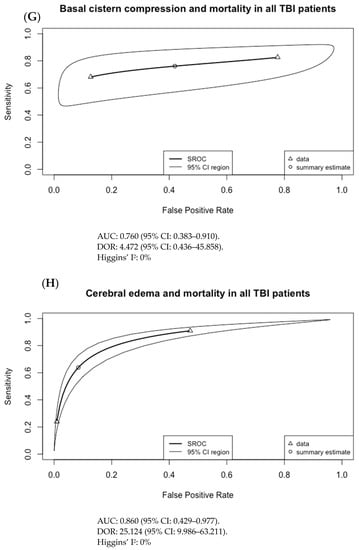
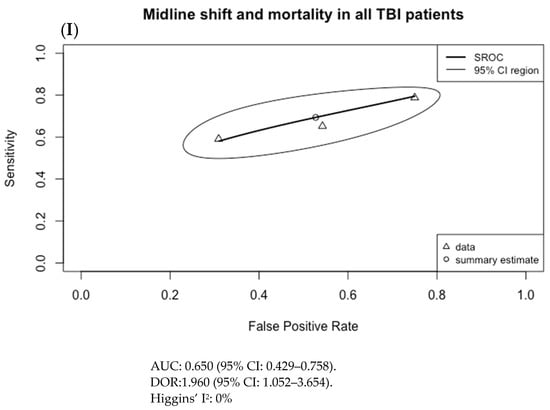
Figure 4.
Summary ROC curves (SROC; bivariate model) for mortality in adult TBI patients for (A) subarachnoid hemorrhage (SAH), (B) subdural hematoma (SDH), (C) epidural hematoma (EDH), (D) intraventricular hemorrhage (IVH), (E) intraparenchymal hematoma (IPH), (F) cerebral contusion, (G) basal cistern compression, (H) cerebral edema, and (I) midline shift. Only SDH had both an AUC with 95% CI not crossing 0.5 and a DOR with 95% CI not crossing 1, at 0.593 (95% CI: 0.556–0.725) and 2.755 (95% CI: 1.474–5.148), respectively.
4. Discussion
To our knowledge, this is the first systematic review and meta-analysis of initial imaging findings undertaken within 24 h post-presentation and their ability to prognosticate mortality in TBI patients. With NECT, the findings of our systematic review are generally in keeping with prior observations that a midline shift greater than 5 mm, effacement of the basal cisterns, and SAH are associated with increased mortality [6]. The findings of decreased MCA velocity and PI are also concordant with a prior systemic review and meta-analysis on TCD and outcomes in TBI performed by Fatima et al. 2019 [12].
For the pooled data, only SDH with mortality in adult TBI patients had a moderate but significant association with AUC of 0.593 (95% CI: 0.556–0.725) and DOR of 2.755 (95% CI: 1.474–5.148). No other imaging finding had both a statistically significant AUC and DOR. Although a sensitivity analysis was not pursued, this result may be secondary to the inclusion and exclusion criteria of requiring all patients to be 18 years of age or older, and for imaging to be done within 24 h of presentation to ED. The result can also be secondary to selection bias in the pooled data and to significant heterogeneity in the included studies, particularly regarding outcome measures and the patient population studied.
Our study focused solely on imaging characteristics without consideration of clinical findings such as decreased GCS and absence of pupillary reflex. Despite this, we believe that imaging features by themselves are valuable in prognostication as they are the only direct way to assess underlying anatomy and physiology and because of their wider availability, improved techniques, and faster scan times. Although prior multivariate models combining clinical, biochemical, and imaging findings have been created, no model is validated for routine clinical use and prior systematic reviews and meta-analyses evaluating various multivariate prognostic models have concluded that the majority have poor external validation and quality [38,39,40].
There were several limitations to our review. All the included studies were observational, most provided experiences at a single centre, and there was significant heterogeneity in the studied imaging findings and outcome measures. There were a limited number of articles included that studied modalities that were not NECT, including only three studies regarding CTP and two involving TCD. Additionally, no studies investigated MRI and associated findings. MRI is a widely used modality for assessing the extent of traumatic brain injury and is significantly more sensitive than CT in the detection of cerebral injuries such as hemorrhagic cortical contusions and white matter shearing lesions, as well as in evaluating the temporal course of intracranial hemorrhage [5]. Similarly, there were no studies that used nuclear medicine examinations such as Tc-99m HMPAO cerebral perfusion SPECT [11]. The reasoning is favored secondary to their more limited availability and longer examination times; this means it would have been logistically difficult and unfeasible for TBI patients to have these examinations within 24 h of presentation as the care team would have been focused on resuscitation and stabilization. Furthermore, MRI is generally considered superior to CT at least 48–72 h post-TBI and in the subacute, chronic, and remote phases of injury, making scanning less than 24 h post-presentation even less likely [5,41]. Shankar et al. 2020 demonstrated high sensitivity and specificity, as well as high negative and positive predictive value in CTP’s ability to prognosticate in-hospital mortality [30]. However, it was the only study of its kind included in our study and its data could not be used for meta-analysis. Finally, our study did not assess for improvements in scan speed, increased resolution, and improved techniques that could have aided in more accurate imaging diagnosis.
5. Conclusions
In conclusion, initial imaging findings of cerebral edema, midline shift, and SAH, the presence of decreased CBF and CBV, decreased MCA velocity and increased PI, and increased ONSD are all associated with mortality and unfavorable outcomes in TBI patients based on the available literature. In meta-analysis, only SDH with mortality in adult TBI patients had a moderate but significant association with AUC of 0.593 (95% CI: 0.556–0.725) and DOR of 2.755 (95% CI: 1.474–5.148). Given the small number of studies, additional research focused on initial imaging, particularly for imaging modalities other than NECT, is required in order to confirm the findings of our meta-analysis and to further evaluate the association between imaging findings and outcomes.
Author Contributions
Conceptualization, J.S., D.B., S.R.A. and H.Y.; methodology J.S., J.L. and H.Y.; software, H.Y.; validation, J.S., D.B., S.R.A. and H.Y.; formal analysis, J.S. and H.Y.; data curation, J.S. and H.Y.; writing—original draft preparation, J.S. and H.Y.; writing—review and editing, J.S. and H.Y.; visualization, H.Y.; supervision, J.S.; project administration, J.S. and J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study as it uses publicly available documents and involves minimal risk.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ACA | anterior cerebral artery |
| ARDS | acute respiratory distress syndrome |
| AUC | area under (the receiver operating characteristic) curve |
| CBF | cerebral blood flow |
| CBV | cerebral blood volume |
| CI | confidence interval |
| CT | computed tomography |
| CTA | CT angiography |
| CTP | CT perfusion |
| ED | emergency department |
| EDH | epidural hematoma |
| FU | follow-up |
| GCS | Glasgow Coma Score |
| GOS | Glasgow Outcome Scale |
| ICH | intracranial hemorrhage |
| ICP | intracranial pressure |
| ICU | intensive care unit |
| IPH | intraparenchymal hematoma |
| ISS | injury severity score |
| IVH | intraventricular hemorrhage |
| LR | likelihood ratio |
| MCA | middle cerebral artery |
| MRI | magnetic resonance imaging |
| MTT | mean transit time |
| NECT | non-enhanced CT |
| NPV | negative predictive value |
| ONSD | optic nerve sheath diameter |
| PET | positron emission tomography |
| PCA | posterior cerebral artery |
| PI | pulsatility index |
| PPV | positive predictive value |
| ROC | receiver operating characteristic |
| SAH | subarachnoid hemorrhage |
| SDH | subdural hematoma |
| SPECT | single-photon emission computed tomography |
| SROC | summary receiver operating characteristic |
| TBI | traumatic brain injury |
References
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.-C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2019, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.Y.; Lee, A.Y.W. Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic Targets. Front. Cell. Neurosci. 2019, 13, 528. [Google Scholar] [CrossRef] [PubMed]
- Zaloshnja, E.; Miller, T.; Langlois, J.A.; Selassie, A.W. Prevalence of Long-Term Disability From Traumatic Brain Injury in the Civilian Population of the United States, 2005. J. Head Trauma Rehabil. 2008, 23, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Seifert, J. Incidence and economic burden of injuries in the United States. J. Epidemiol. Community Health 2007, 61, 926. [Google Scholar] [CrossRef]
- Lee, B.; Newberg, A. Neuroimaging in Traumatic Brain Imaging. NeuroRx 2005, 2, 372–383. [Google Scholar] [CrossRef]
- Marshall, L.F.; Marshall, S.B.; Klauber, M.R.; Clark, M.V.B.; Eisenberg, H.M.; Jane, J.A.; Luerssen, T.G.; Marmarou, A.; Foulkes, M.A. A new classification of head injury based on computerized tomography. J. Neurosurg. 1991, 75, S14–S20. [Google Scholar] [CrossRef]
- Maas, A.I.; Hukkelhoven, C.W.; Marshall, L.F.; Steyerberg, E.W. Prediction of Outcome in Traumatic Brain Injury with Computed Tomographic Characteristics: A Comparison between the Computed Tomographic Classification and Combinations of Computed Tomographic Predictors. Neurosurgery 2005, 57, 1173–1182. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; A Mushkudiani, N.; Perel, P.; Butcher, I.; Lu, J.; McHugh, G.S.; Murray, G.; Marmarou, A.; Roberts, I.; Habbema, J.D.F.; et al. Predicting Outcome after Traumatic Brain Injury: Development and International Validation of Prognostic Scores Based on Admission Characteristics. PLoS Med. 2008, 5, e165. [Google Scholar] [CrossRef]
- Bendinelli, C.; Cooper, S.; Abel, C.; Balogh, A.; Balogh, Z.J. Perfusion Computed Tomography in Traumatic Brain Injury. Traumatic Brain Injury-Pathobiology, Advanced Diagnostics and Acute Management. IntechOpen. 2018. Available online: https://www.intechopen.com/books/traumatic-brain-injury-pathobiology-advanced-diagnostics-and-acute-management/perfusion-computed-tomography-in-traumatic-brain-injury (accessed on 21 January 2021).
- Byrnes, K.R.; Wilson, C.M.; Brabazon, F.; Von Leden, R.; Jurgens, J.S.; Oakes, T.R.; Selwyn, R.G. FDG-PET imaging in mild traumatic brain injury: A critical review. Front. Neuroenerget. 2014, 5, 13. [Google Scholar] [CrossRef]
- Donohoe, K.J.; Agrawal, G.; Frey, K.A.; Gerbaudo, V.H.; Mariani, G.; Nagel, J.S.; Shulkin, B.L.; Stabin, M.G.; Stokes, M.K. SNM Practice Guideline for Brain Death Scintigraphy 2.0. J. Nucl. Med. Technol. 2012, 40, 198–203. [Google Scholar] [CrossRef]
- Fatima, N.; Shuaib, A.; Chughtai, T.; Ayyad, A.; Saqqur, M. The role of transcranial doppler in traumatic brain injury: A systemic review and meta-analysis. Asian J. Neurosurg. 2019, 14, 626–633. [Google Scholar] [CrossRef]
- Haghbayan, H.; Boutin, A.; Laflamme, M.; Lauzier, F.; Shemilt, M.; Moore, L.; Zarychanski, R.; Douville, V.; Fergusson, D.; Turgeon, A.F. The Prognostic Value of MRI in Moderate and Severe Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Crit. Care Med. 2017, 45, e1280–e1288. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- RevMan. 2022. Available online: https://training.cochrane.org/online-learning/core-software/revman (accessed on 24 May 2021).
- R: The R Project for Statistical Computing. 2022. Available online: https://www.r-project.org/ (accessed on 3 June 2021).
- Noma, H.; Matsushima, Y.; Ishii, R. Confidence interval for the AUC of SROC curve and some related methods using bootstrap for meta-analysis of diagnostic accuracy studies. Commun. Stat. Case Stud. Data Anal. Appl. 2021, 7, 344–358. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Bindu, T.S.; Vyas, S.; Khandelwal, N.; Bhatia, V.; Dhandapani, S.; Kumar, A.; Ahuja, C.K. Role of whole-brain computed tomography perfusion in head injury patients to predict outcome. Indian J. Radiol. Imaging 2017, 27, 268–273. [Google Scholar] [CrossRef]
- Compagnone, C.; d’Avella, D.; Servadei, F.; Angileri, F.F.; Brambilla, G.; Conti, C.; Cristofori, L.; Delfini, R.; Denaro, L.; Ducati, A.; et al. Patients with moderate head injury: A prospective multicenter study of 315 patients. Neurosurgery 2009, 64, 690–696, discussion 696–697. [Google Scholar] [CrossRef]
- Henninger, N.; Compton, R.A.; Khan, M.W.; Carandang, R.; Hall, W.; Muehlschlegel, S. “Don’t lose hope early”: Hemorrhagic diffuse axonal injury on head computed tomography is not associated with poor outcome in moderate to severe traumatic brain injury patients. J. Trauma Inj. Infect. Crit. Care 2018, 84, 473–482. [Google Scholar] [CrossRef]
- Khalilabadi, A.J.; Shahraki, M.K.; Rezaeifard, R.; Jafari, R.; Zarooei, J.M.; Salarzaei, M.; Zaare, M.A. The Relationship between CT scan Findings, Level of Consciousness and Outcome Score in Patients with Traumatic Brain Hemorrhage. Pharm. Lett. 2016, 8, 140–144. [Google Scholar]
- Kotwica, Z.; Jakubowski, J.K. Head-injured adult patients with GCS of 3 on admission--who have a chance to survive? Acta Neurochir. 1995, 133, 56–59. [Google Scholar] [CrossRef]
- Kreitzer, N.; Hart, K.; Lindsell, C.J.; Betham, B.; Gozal, Y.; Andaluz, N.O.; Lyons, M.S.; Bonomo, J.; Adeoye, O. Factors associated with adverse outcomes in patients with traumatic intracranial hemorrhage and Glasgow Coma Scale of 15. Am. J. Emerg. Med. 2017, 35, 875–880. [Google Scholar] [CrossRef]
- Legrand, A.; Jeanjean, P.; Delanghe, F.; Peltier, J.; Lecat, B.; Dupont, H. Estimation of optic nerve sheath diameter on an initial brain computed tomography scan can contribute prognostic information in traumatic brain injury patients. Crit. Care 2013, 17, R61. [Google Scholar] [CrossRef]
- Letourneau-Guillon, L.; Huynh, T.; Jakobovic, R.; Milwid, R.; Symons, S.; Aviv, R. Traumatic Intracranial Hematomas: Prognostic Value of Contrast Extravasation. Am. J. Neuroradiol. 2012, 34, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.A.; Mesalles, E.; Gener, J.; Tomasa, A.; Ley, A.; Roca, J.; Fernández-Llamazares, J. Evaluating the outcome of severe head injury with transcranial Doppler ultrasonography. Neurosurg. Focus 2000, 8, e8. [Google Scholar] [CrossRef]
- Naraghi, L.; Larentzakis, A.; Chang, Y.; Duhaime, A.C.; Kaafarani, H.; Yeh, D.D.; King, D.R.; de Moya, M.A.; Velmahos, G.C. Is CT Angiography of the Head Useful in the Management of Traumatic Brain Injury? J. Am. Coll. Surg. 2015, 220, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Quigley, M.R.; Chew, B.G.; Swartz, C.E.; Wilberger, J.E. The clinical significance of isolated traumatic subarachnoid hemorrhage. J. Trauma Inj. Infect. Crit. Care 2013, 74, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Shankar, J.J.S.; Green, R.; Virani, K.; Wong, H.; Eddy, K.; Vandorpe, R. Admission Perfusion CT for Classifying Early In-Hospital Mortality of Patients With Severe Traumatic Brain Injury: A Pilot Study. Am. J. Roentgenol. 2020, 214, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Splavski, B.; Radanović, B.; Vranković, D.; Has, B.; Mužević, D.; Jančuljak, D.; Legcević, J. Transcranial Doppler ultrasonography as an early outcome forecaster following severe brain injury. Br. J. Neurosurg. 2006, 20, 386–390. [Google Scholar] [CrossRef]
- Tasaki, O.; Shiozaki, T.; Hamasaki, T.; Kajino, K.; Nakae, H.; Tanaka, H.; Shimazu, T.; Sugimoto, H. Prognostic Indicators and Outcome Prediction Model for Severe Traumatic Brain Injury. J. Trauma Inj. Infect. Crit. Care 2009, 66, 304–308. [Google Scholar] [CrossRef]
- Tucker, B.; Aston, J.; Dines, M.; Caraman, E.; Yacyshyn, M.; McCarthy, M.; Olson, J.E. Early Brain Edema is a Predictor of In-Hospital Mortality in Traumatic Brain Injury. J. Emerg. Med. 2017, 53, 18–29. [Google Scholar] [CrossRef]
- Waqas, M.; Bakhshi, S.K.; Shamim, M.S.; Anwar, S. Radiological prognostication in patients with head trauma requiring decompressive craniectomy: Analysis of optic nerve sheath diameter and Rotterdam CT Scoring System. J. Neuroradiol. 2016, 43, 25–30. [Google Scholar] [CrossRef]
- Wintermark, M.; van Melle, G.; Schnyder, P.; Revelly, J.-P.; Porchet, F.; Regli, L.; Meuli, R.; Maeder, P.; Chioléro, R. Admission Perfusion CT: Prognostic Value in Patients with Severe Head Trauma. Radiology 2004, 232, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.K.; Tang, B.Y.H.; Yeung, J.H.H.; Collins, G.; Rainer, T.; Ng, S.C.; Poon, W.S. Traumatic intracerebral haemorrhage: Is the CT pattern related to outcome? Br. J. Neurosurg. 2009, 23, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, H.; Morioka, T.; Yamamoto, T.; Mizobata, Y. Head computed tomographic measurement as a predictor of outcome in patients with subdural hematoma with cerebral edema. Scand. J. Trauma Resusc. Emerg. Med. 2016, 24, 83. [Google Scholar] [CrossRef]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.J.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, K.N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Husson, E.; Ribbers, G.; Son, A.W.-V.; Verhagen, A.; Stam, H. Prognosis of six-month functioning after moderate to severe traumatic brain injury: A systematic review of prospective cohort studies. J. Rehabil. Med. 2010, 42, 425–436. [Google Scholar] [CrossRef]
- Perel, P.; Edwards, P.; Wentz, R.; Roberts, I. Systematic review of prognostic models in traumatic brain injury. BMC Med. Inform. Decis. Mak. 2006, 6, 38. [Google Scholar] [CrossRef]
- Kelly, A.B.; Zimmerman, R.D.; Snow, R.B.; E Gandy, S.; A Heier, L.; Deck, M.D. Head trauma: Comparison of MR and CT--experience in 100 patients. Am. J. Neuroradiol. 1988, 9, 699–708. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).