Evaluation of Anterior and Posterior Corneal Higher Order Aberrations for the Detection of Keratoconus and Suspect Keratoconus
Abstract
1. Introduction
2. Material and Methods
2.1. Study Participants
2.2. Mean Outcomes Measures
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Anterior and Posterior Corneal HOAs Data
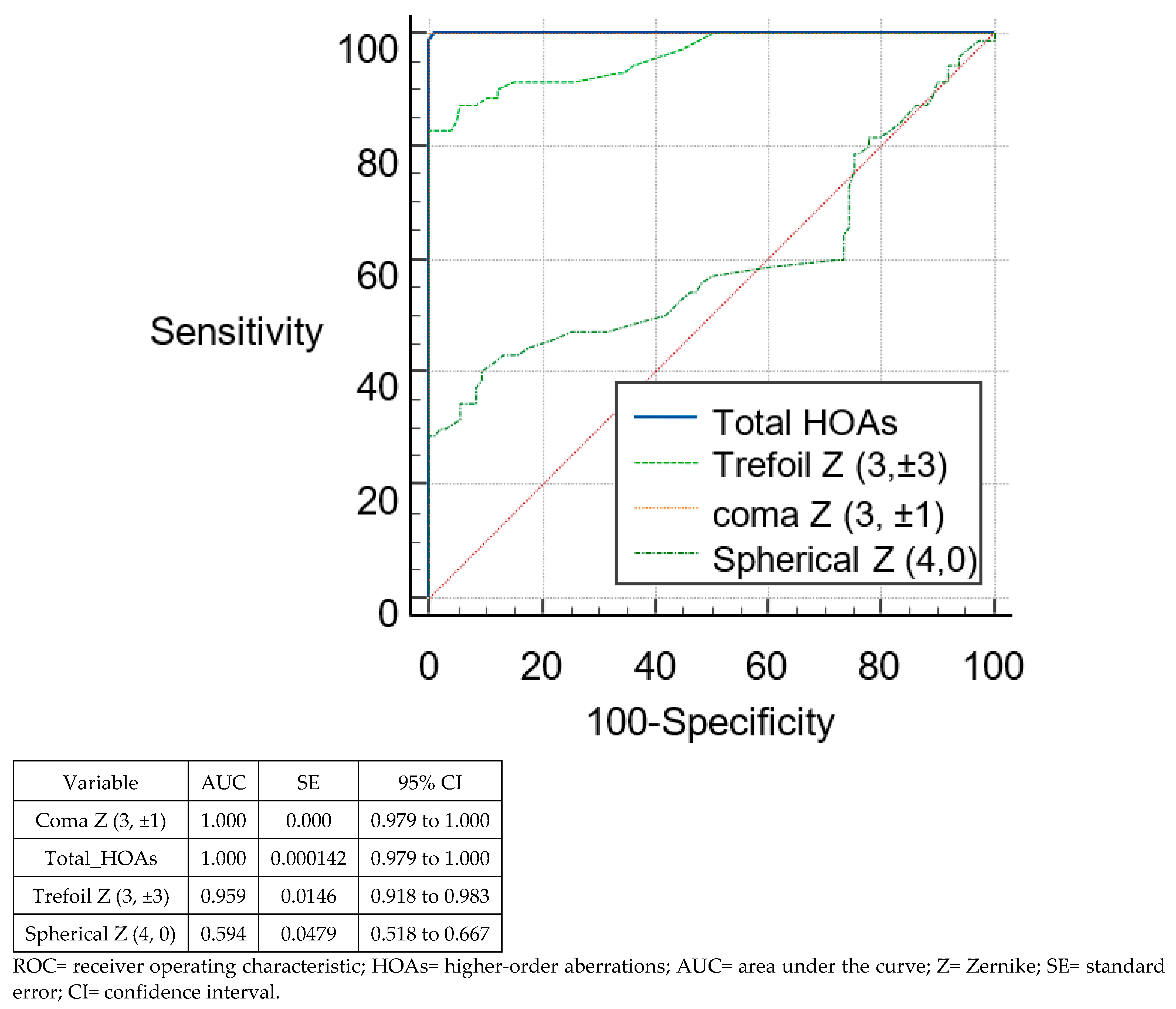
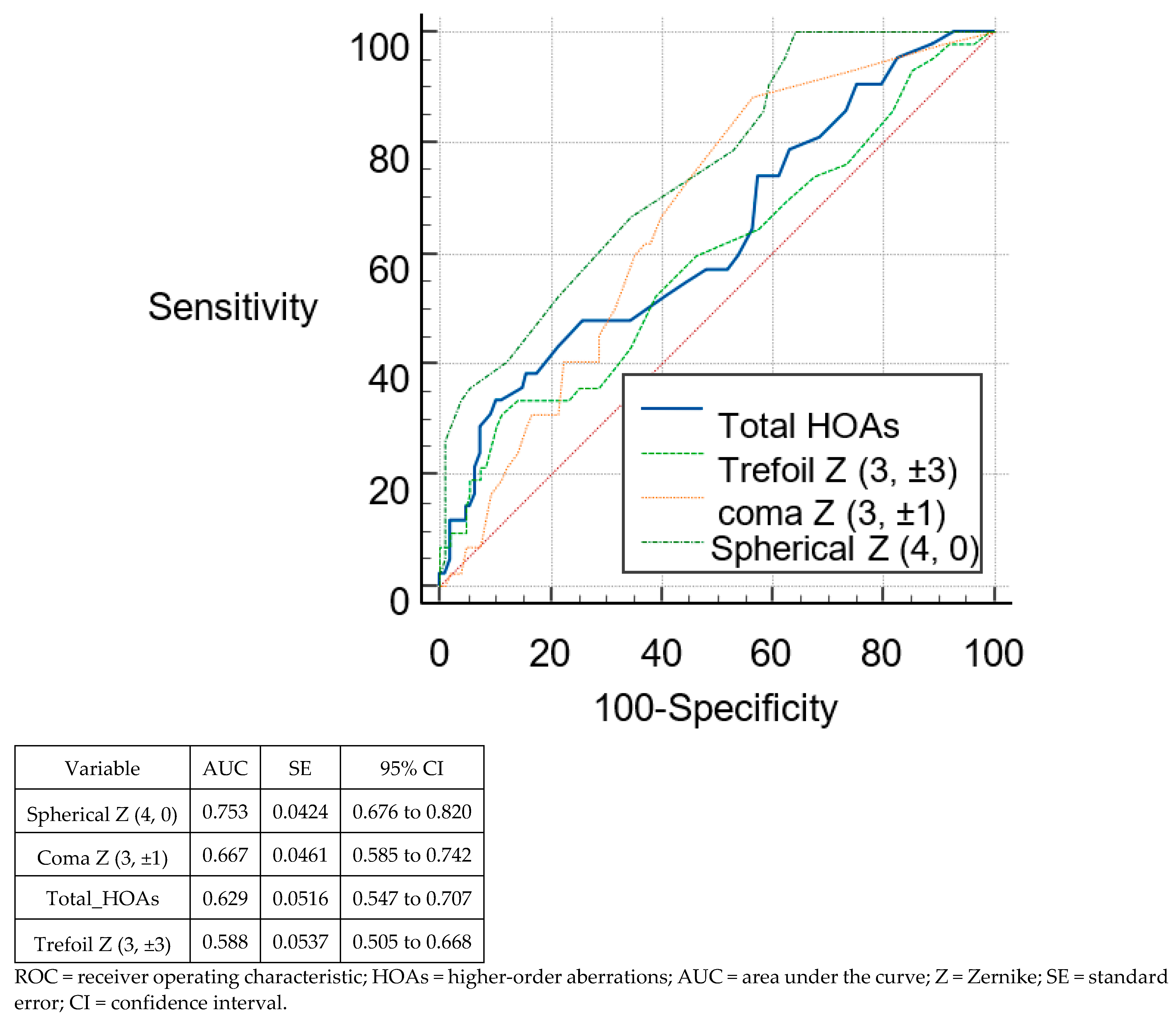
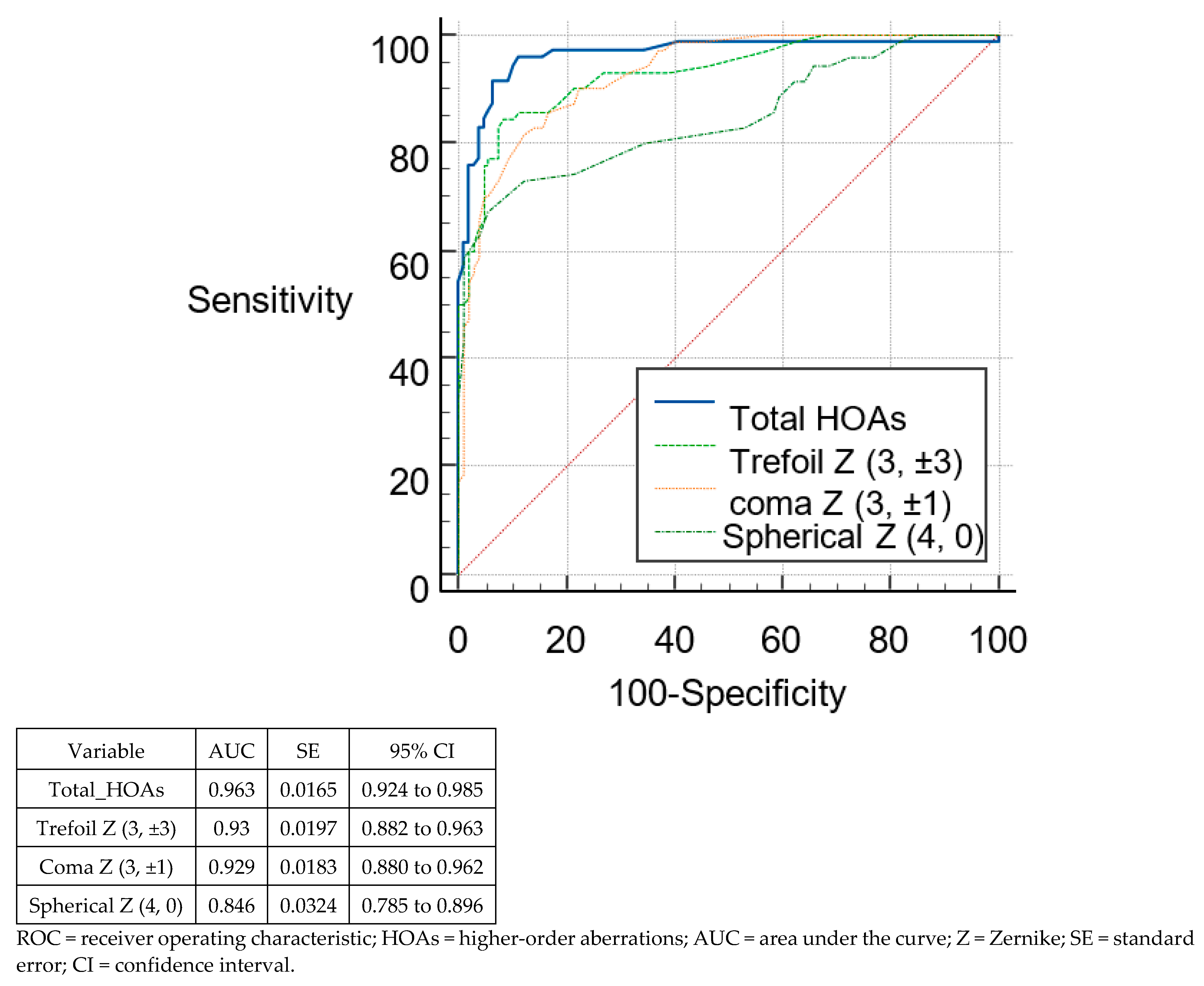
3.3. Discriminant Analysis and ROC Curves
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rabinowitz, Y.S. Keratoconus. Surv. Ophthalmol. 1998, 42, 297–319. [Google Scholar] [CrossRef] [PubMed]
- Burns, D.M.; Johnston, F.M.; Frazer, D.G.; Patterson, C.; Jackson, A.J. Keratoconus: An analysis of corneal asymmetry. Br. J. Ophthalmol. 2004, 88, 1252–1255. [Google Scholar] [CrossRef] [PubMed]
- Naderan, M.; Jahanrad, A.; Farjadnia, M. Clinical biomicroscopy and retinoscopy findings of keratoconus in a Middle Eastern population. Clin. Exp. Optom. 2018, 101, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Salman, A.; Darwish, T.; Ali, A.; Ghabra, M.; Shaaban, R. Sensitivity and specificity of Sirius indices in diagnosis of keratoconus and suspect keratoconus. Eur. J. Ophthalmol. 2021, 23, 11206721211060139. [Google Scholar] [CrossRef] [PubMed]
- Bühren, J.; Kook, D.; Yoon, G.; Kohnen, T. Detection of subclinical keratoconus by using corneal anterior and posterior surface aberrations and thickness spatial profiles. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3424–3432. [Google Scholar] [CrossRef] [PubMed]
- Bühren, J.; Kühne, C.; Kohnen, T. Defining subclinical keratoconus using corneal first-surface higher-order aberrations. Am. J. Ophthalmol. 2007, 143, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Jafri, B.; Li, X.; Yang, H.; Rabinowitz, Y.S. Higher order wavefront aberrations and topography in early and suspected keratoconus. J. Refract. Surg. 2007, 23, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y. Strategies for improving the early diagnosis of keratoconus. Clin. Optom. 2016, 8, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Arbelaez, M.C.; Versaci, F.; Vestri, G.; Barboni, P.; Savini, G. Use of a support vector machine for keratoconus and subclinical keratoconus detection by topographic and tomographic data. Ophthalmology 2012, 119, 2231–2238. [Google Scholar] [CrossRef] [PubMed]
- Zadnik, K.; Barr, J.T.; Edrington, T.B.; Everett, D.F.; Jameson, M.; McMahon, T.T.; Shin, J.A.; Sterling, J.L.; Wagner, H.; Gordon, M.O. Baseline findings in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2537–2546. [Google Scholar]
- Li, X.; Yang, H.; Rabinowitz, Y.S. Keratoconus: Classification scheme based on videokeratography and clinical signs. J. Cataract. Refract. Surg. 2009, 35, 1597–1603. [Google Scholar] [CrossRef] [PubMed]
- Zadnik, K.; Barr, J.T.; Gordon, M.O.; Edrington, T.B. Biomicroscopic signs and disease severity in keratoconus. Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study Group. Cornea 1996, 15, 139–146. [Google Scholar] [CrossRef] [PubMed]
- ISO 24157:2008; Ophthalmic Optics and Instruments-Reporting Aberrations of the Human Eye. ISO: Geneva, Switzerland, 2008.
- Randleman, J.B. Evaluating risk factors for ectasia: What is the goal of assessing risk? J. Refract. Surg. 2010, 26, 236–237. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.; Gatinel, D. Evaluation of total and corneal wavefront high order aberrations for the detection of forme fruste keratoconus. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2978–2992. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Fujikado, T.; Kuroda, T.; Mihashi, T.; Hirohara, Y.; Nishida, K.; Watanabe, H.; Tano, Y. Wavefront aberrations measured with Hartmann-Shack sensor in patients with keratoconus. Ophthalmology 2002, 109, 1996–2003. [Google Scholar] [CrossRef] [PubMed]
- Alió, J.L.; Shabayek, M.H. Corneal higher order aberrations: A method to grade keratoconus. J. Refract. Surg. 2006, 22, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Gobbe, M.; Guillon, M. Corneal wavefront aberration measurements to detect keratoconus patients. Cont. Lens Anterior Eye 2005, 28, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Piñero, D.P.; Alió, J.L.; Alesón, A.; Escaf, M.; Miranda, M. Pentacam posterior and anterior corneal aberrations in normal and keratoconic eyes. Clin. Exp. Optom. 2009, 92, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Naderan, M.; Jahanrad, A.; Farjadnia, M. Ocular, corneal, and internal aberrations in eyes with keratoconus, forme fruste keratoconus, and healthy eyes. Int. Ophthalmol. 2018, 38, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, W.; Jiang, J.; Zhuang, X.; Chen, W.; Peng, M.; Wang, J.; Lu, F.; Shen, M.; Wang, Y. Characteristic of entire corneal topography and tomography for the detection of sub-clinical keratoconus with Zernike polynomials using Pentacam. Sci. Rep. 2017, 7, 16486. [Google Scholar] [CrossRef] [PubMed]

| Normal | SKC | KC | |
|---|---|---|---|
| Patients (n) | 108 | 34 | 46 |
| Eyes (n) | 108 | 42 | 70 |
| Sex | |||
| F | 77 | 17 | 24 |
| M | 31 | 17 | 22 |
| Age, (mean ± SD) | 24.17 ± 5.58 | 26.62 ± 6.2 | 24.87 ± 5.98 |
| Sphere (D), (mean ± SD) | −1.33 ± 2.83 | −1.04 ± 1.81 | −1.25 ± 2.02 |
| Cylinder (D), (mean ± SD) | −1.14 ± 0.95 | −1.07 ± 0.71 | −2.67 ± 1.61 |
| Anterior HOAs | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Indices | Normal (n = 108) | SKC (n = 42) | KC (n = 70) | p-Value | p * | |||||
| Mean | SD | Mean | SD | Mean | SD | Normal vs. SKC | Normal vs. KC | SKC vs. KC | ||
| Total HOAs | 0.28 | 0.14 | 0.73 | 0.34 | 2.11 | 0.90 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Trefoil Z (3, ±3) | 0.12 | 0.07 | 0.30 | 0.23 | 0.56 | 0.27 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Coma Z (3, ±1) | 0.15 | 0.13 | 0.55 | 0.29 | 1.90 | 0.88 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Spherical Z (4, 0) | 0.02 | 0.15 | −0.15 | 0.18 | 0.16 | 0.35 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Posterior HOAs | ||||||||||
| Total HOAs | 0.21 | 0.12 | 0.27 | 0.16 | 0.85 | 0.48 | 0.609 | <0.0001 | <0.0001 | <0.0001 |
| Trefoil Z (3, ±3) | 0.13 | 0.10 | 0.18 | 0.14 | 0.60 | 0.38 | 0.82 | <0.0001 | <0.0001 | <0.0001 |
| Coma Z (3, ±1) | 0.08 | 0.09 | 0.11 | 0.08 | 0.38 | 0.26 | 0.883 | <0.0001 | <0.0001 | <0.0001 |
| Spherical Z (4, 0) | 0.04 | 0.06 | −0.02 | 0.04 | −0.08 | 0.11 | <0.0001 | |||
| Indices | Cut-Off Value | ROC AUC | Sensitivity (%) | Specificity (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Normal vs. SKC | Normal vs. KC | Normal vs. SKC | Normal vs. KC | Normal vs. SKC | Normal vs. KC | Normal vs. SKC | Normal vs. KC | |
| Total HOAs | >0.4 | >0.65 | 0.918 | 1 | 90.48 | 100 | 81.48 | 99.07 |
| Trefoil Z (3, ±3) | >0.2 | >0.34 | 0.756 | 0.959 | 59.52 | 82.86 | 87.96 | 100 |
| Coma Z (3, ±1) | >0.2 | >0.57 | 0.922 | 1 | 95.24 | 100 | 75 | 100 |
| Spherical Z (4, 0) | ≤−0.01 | >0.16 | 0.767 | 0.594 | 83.33 | 40 | 73.15 | 90.74 |
| Indices | Cut-Off Value | ROC AUC | Sensitivity (%) | Specificity (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Normal vs. SKC | Normal vs. KC | Normal vs. SKC | Normal vs. KC | Normal vs. SKC | Normal vs. KC | Normal vs. SKC | Normal vs. KC | |
| Total HOAs | >0.33 | >0.37 | 0.629 | 0.963 | 33.33 | 91.43 | 89.81 | 93.52 |
| Trefoil Z (3, ±3) | >0.23 | >0.25 | 0.588 | 0.93 | 30.95 | 84.29 | 88.89 | 91.67 |
| Coma Z (3, ±1) | >0.03 | >0.18 | 0.667 | 0.929 | 88.1 | 81.43 | 43.52 | 87.96 |
| Spherical Z (4, 0) | ≤0.05 | ≤−0.03 | 0.753 | 0.846 | 100 | 67.14 | 36.11 | 94.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salman, A.; Kailani, O.; Marshall, J.; Ghabra, M.; Balamoun, A.A.; Darwish, T.R.; Badla, A.A.; Alhaji, H. Evaluation of Anterior and Posterior Corneal Higher Order Aberrations for the Detection of Keratoconus and Suspect Keratoconus. Tomography 2022, 8, 2864-2873. https://doi.org/10.3390/tomography8060240
Salman A, Kailani O, Marshall J, Ghabra M, Balamoun AA, Darwish TR, Badla AA, Alhaji H. Evaluation of Anterior and Posterior Corneal Higher Order Aberrations for the Detection of Keratoconus and Suspect Keratoconus. Tomography. 2022; 8(6):2864-2873. https://doi.org/10.3390/tomography8060240
Chicago/Turabian StyleSalman, Abdelrahman, Obeda Kailani, John Marshall, Marwan Ghabra, Ashraf Armia Balamoun, Taym R. Darwish, Abdul Aziz Badla, and Hala Alhaji. 2022. "Evaluation of Anterior and Posterior Corneal Higher Order Aberrations for the Detection of Keratoconus and Suspect Keratoconus" Tomography 8, no. 6: 2864-2873. https://doi.org/10.3390/tomography8060240
APA StyleSalman, A., Kailani, O., Marshall, J., Ghabra, M., Balamoun, A. A., Darwish, T. R., Badla, A. A., & Alhaji, H. (2022). Evaluation of Anterior and Posterior Corneal Higher Order Aberrations for the Detection of Keratoconus and Suspect Keratoconus. Tomography, 8(6), 2864-2873. https://doi.org/10.3390/tomography8060240







