Rate of Change of Liver Iron Content by MR Imaging Methods: A Comparison Study
Abstract
1. Introduction
2. Methods
2.1. MR Imaging Protocol
2.1.1. Spin-Echo Imaging (R2 Relaxometry)
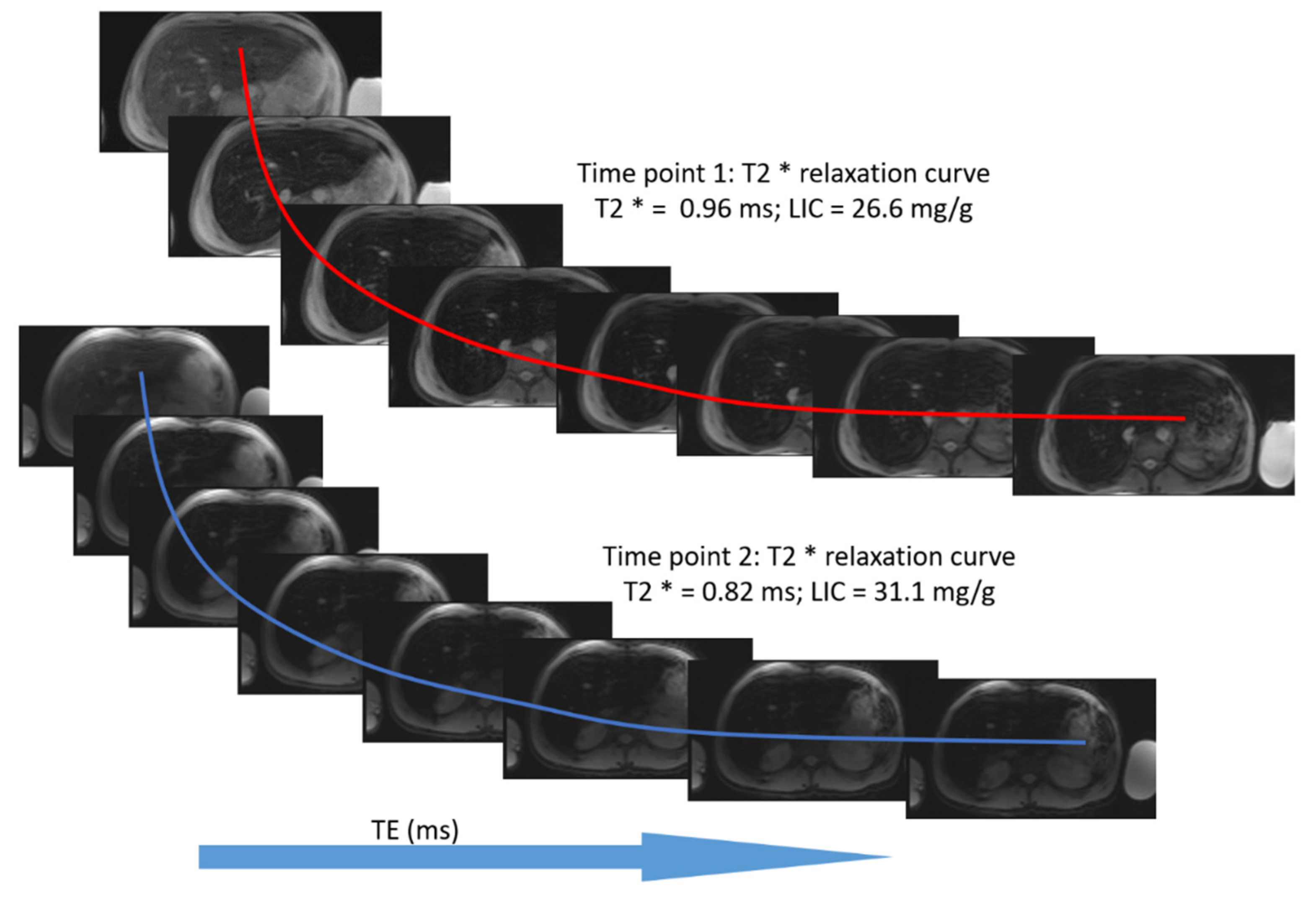
2.1.2. GRE Imaging (R2* Relaxometry)
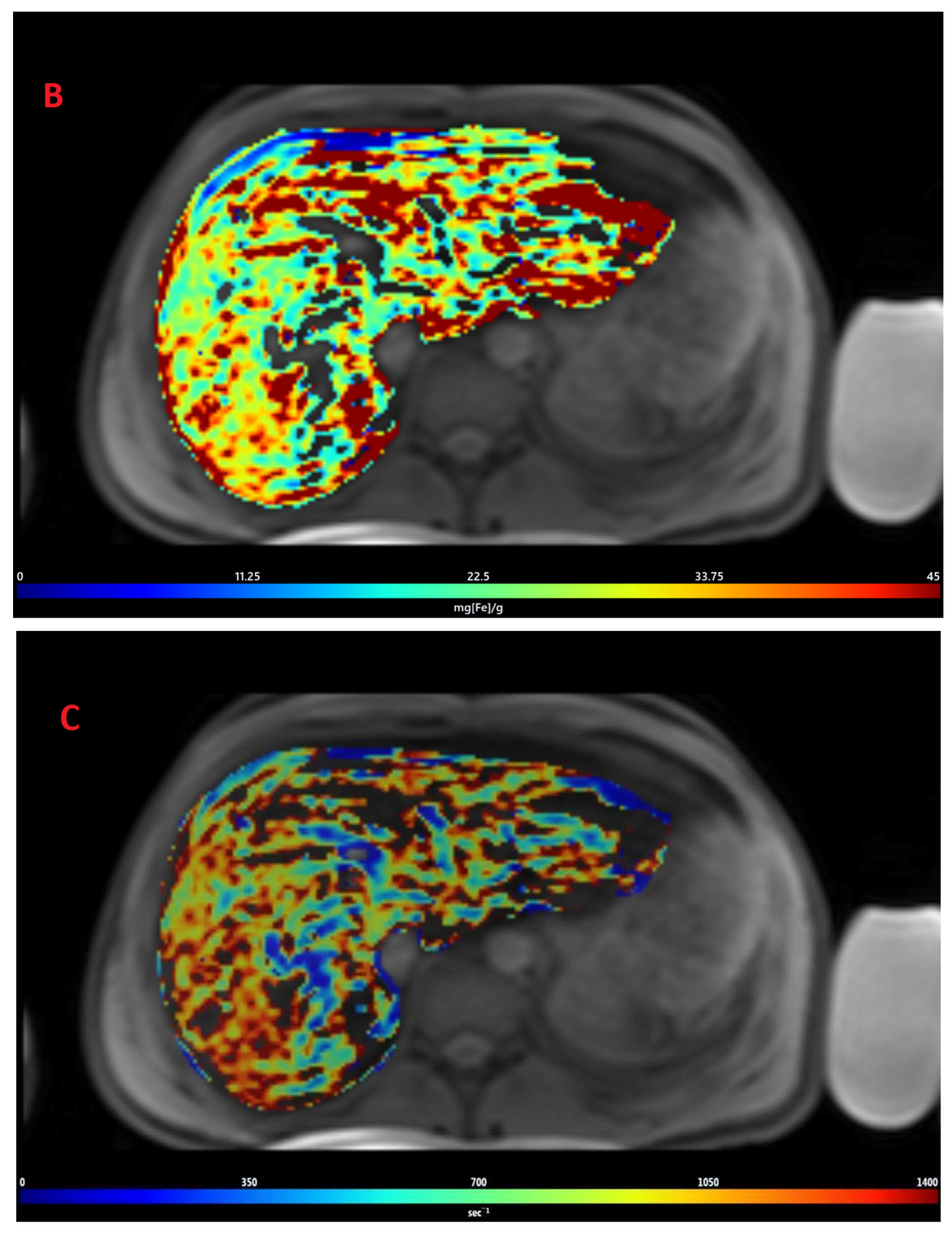
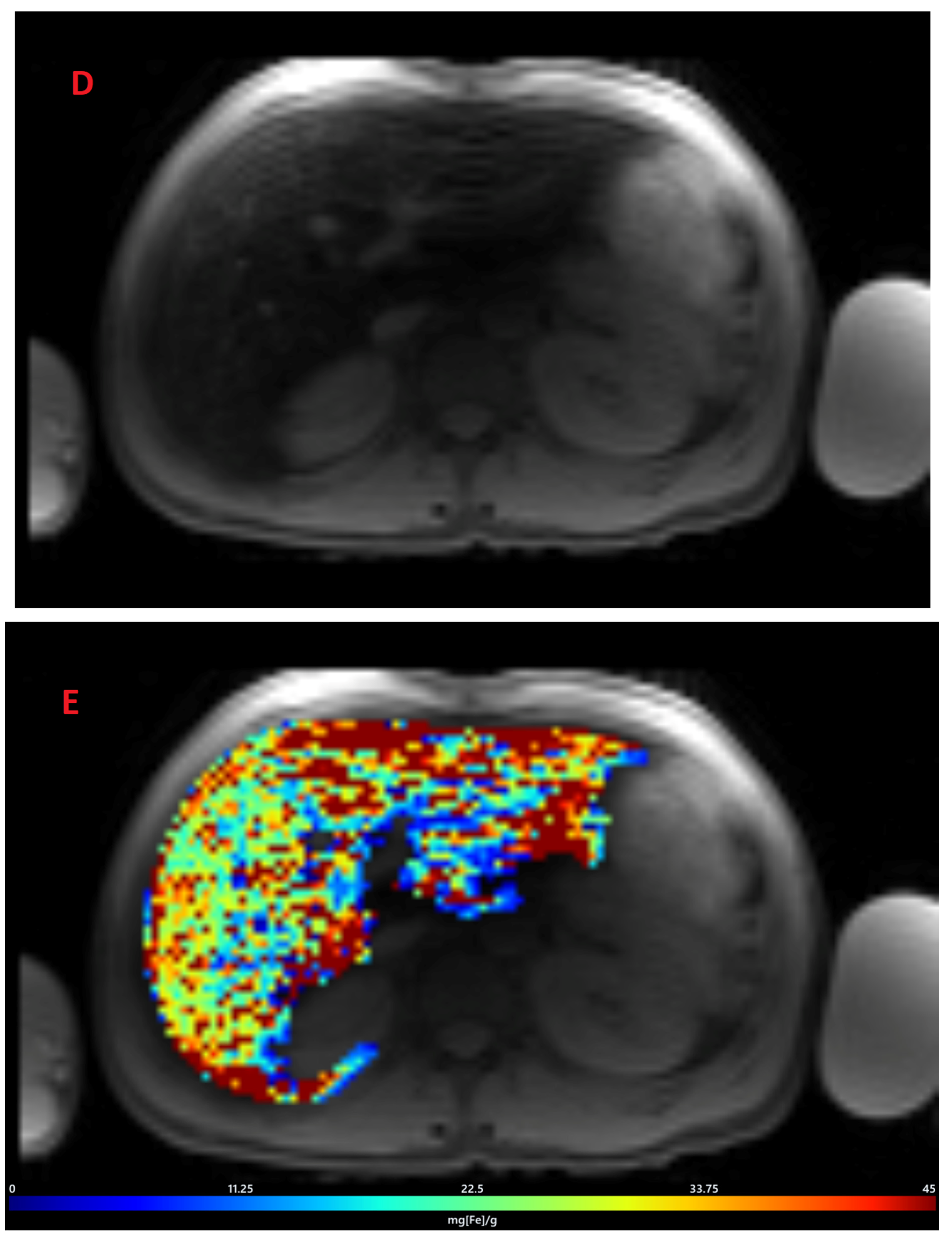
2.2. Post-Processing of Images
2.3. Statistical Analysis
3. Results
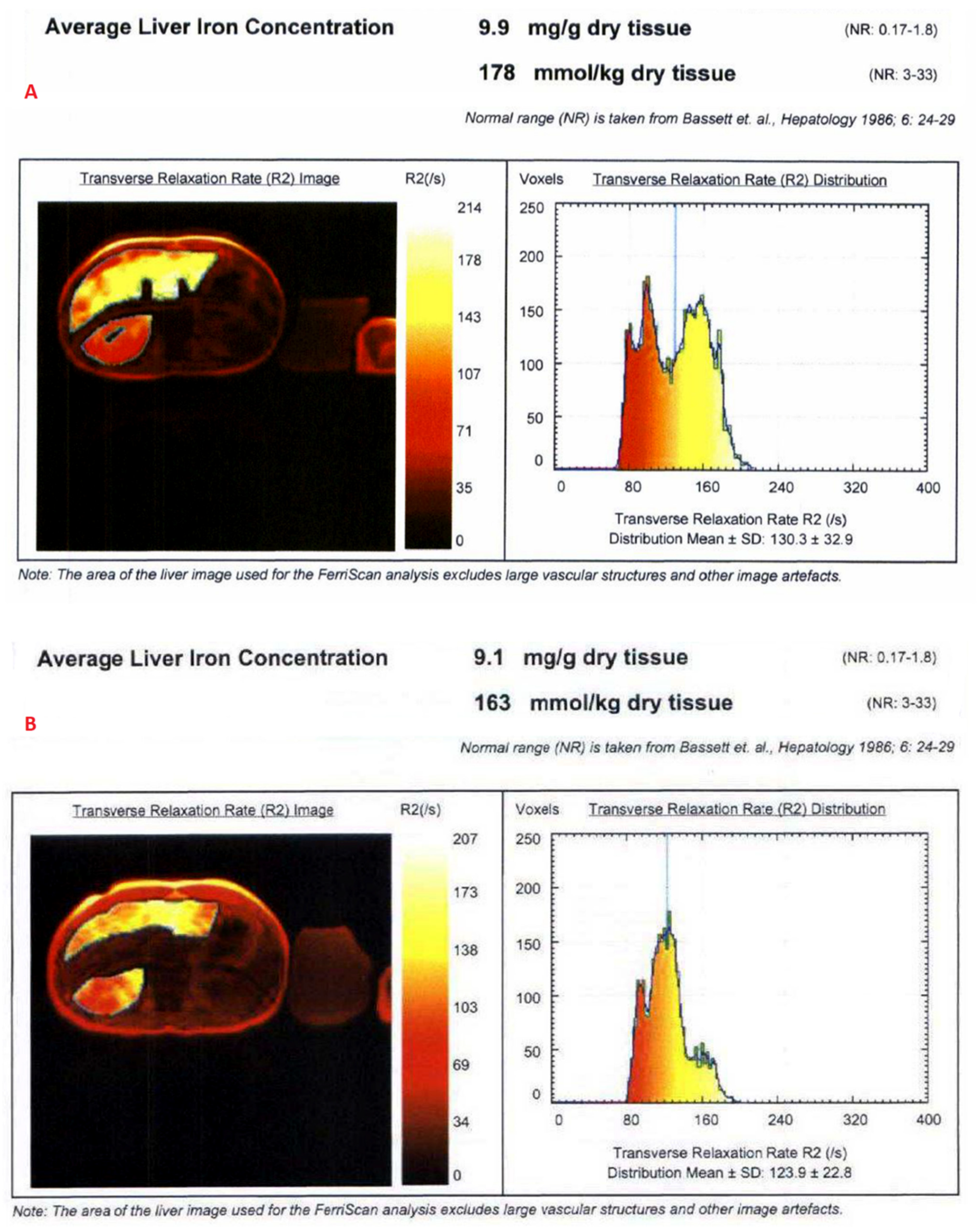
3.1. Estimation of LIC by R2 Relaxometry (LIC R2)
3.2. Estimation of LIC by R2* Relaxometry (LIC R2*)
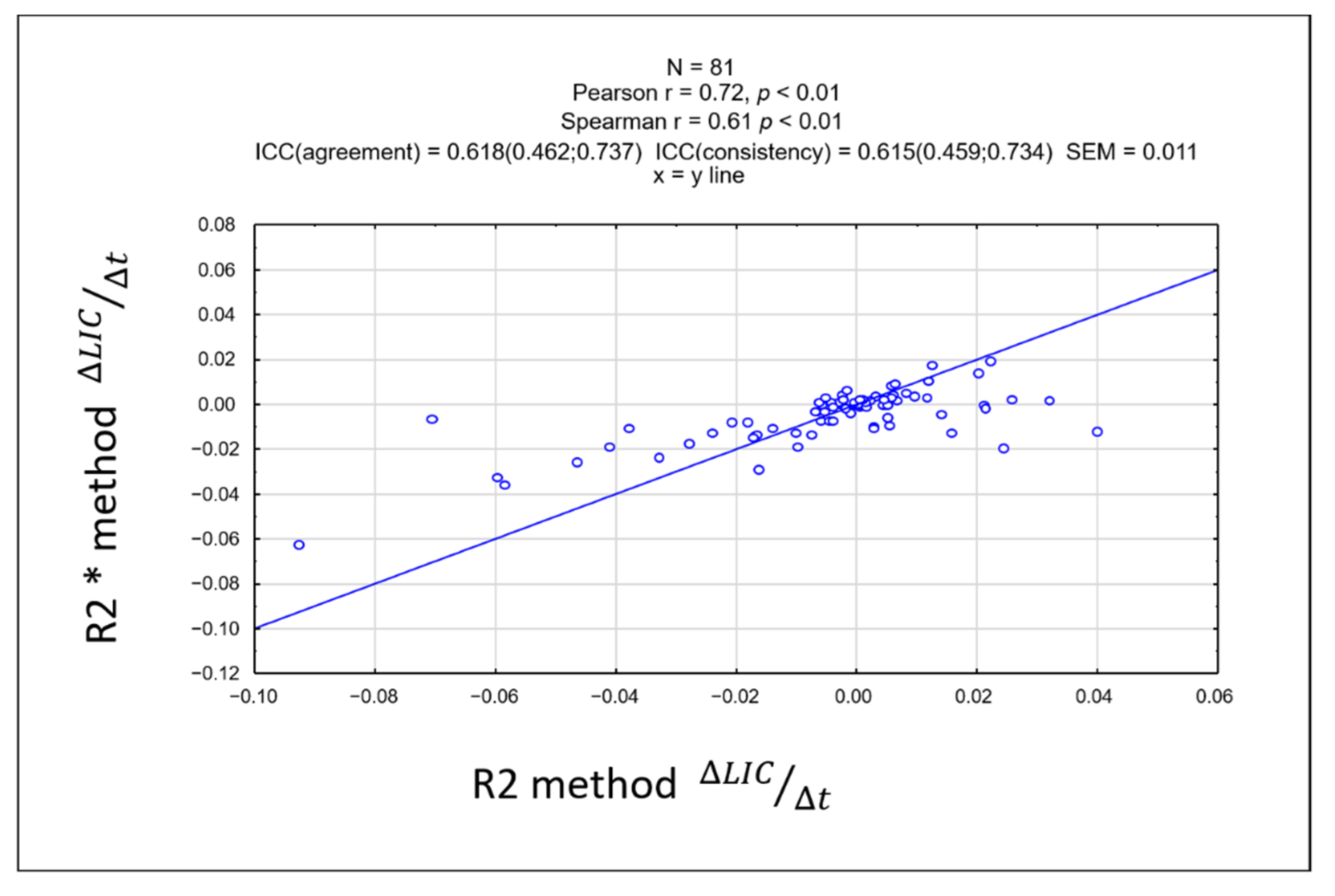
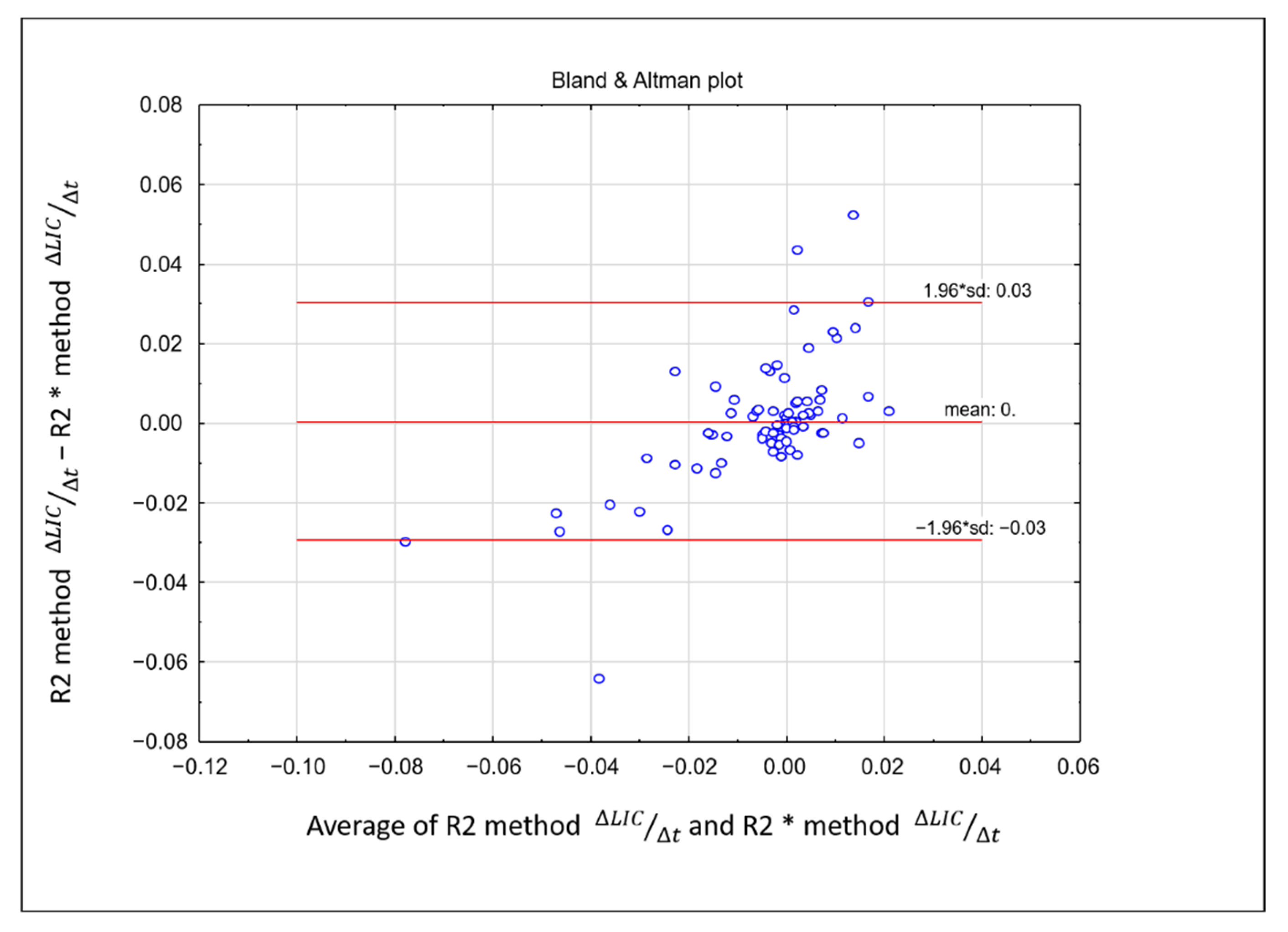
3.3. Comparison between LIC R2 and LIC R2*
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lieu, P.T.; Heiskala, M.; Peterson, P.A.; Yang, Y. The roles of iron in health and disease. Mol. Asp. Med. 2001, 22, 1–87. [Google Scholar] [CrossRef]
- Calle-Toro, J.S.; Barrera, C.A.; Khrichenko, D.; Otero, H.J.; Serai, S.D. R2 relaxometry based MR imaging for estimation of liver iron content: A comparison between two methods. Abdom. Radiol. 2019, 44, 3058–3068. [Google Scholar] [CrossRef]
- Ware, H.M.; Kwiatkowski, J.L. Evaluation and treatment of transfusional iron overload in children. Pediatr. Clin. N. Am. 2013, 60, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Crownover, B.K.; Covey, C.J. Hereditary hemochromatosis. Am. Fam. Physician 2013, 87, 183–190. [Google Scholar]
- Powell, L.W. Diagnosis of hemochromatosis. Semin. Gastrointest. Dis. 2002, 13, 80–88. [Google Scholar] [PubMed]
- Serai, S.D.; Fleck, R.J.; Quinn, C.T.; Zhang, B.; Podberesky, D.J. Retrospective comparison of gradient recalled echo R2* and spin-echo R2 magnetic resonance analysis methods for estimating liver iron content in children and adolescents. Pediatr. Radiol. 2015, 45, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Labranche, R.; Gilbert, G.; Cerny, M.; Vu, K.-N.; Soulières, D.; Olivié, D.; Billiard, J.-S.; Yokoo, T.; Tang, A. Liver Iron Quantification with MR Imaging: A Primer for Radiologists. Radiographics 2018, 38, 392–412. [Google Scholar] [CrossRef] [PubMed]
- Castera, L.; Pinzani, M. Biopsy and non-invasive methods for the diagnosis of liver fibrosis: Does it take two to tango? Gut 2010, 59, 861–866. [Google Scholar] [CrossRef]
- Regev, A.; Berho, M.; Jeffers, L.J.; Milikowski, C.; Molina, E.G.; Pyrsopoulos, N.T.; Feng, Z.Z.; Reddy, K.R.; Schiff, E.R. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am. J. Gastroenterol. 2002, 97, 2614–2618. [Google Scholar] [CrossRef]
- Siegel, C.A.; Silas, A.M.; Suriawinata, A.A.; van Leeuwen, D.J. Liver biopsy 2005: When and how? Cleve. Clin. J. Med. 2005, 72, 199–201, 206, 208. [Google Scholar] [CrossRef]
- Sirlin, C.B.; Reeder, S.B. Magnetic resonance imaging quantification of liver iron. Magn. Reson. Imaging Clin. N. Am. 2010, 18, 359–381. [Google Scholar] [CrossRef] [PubMed]
- Tanner, M.A.; He, T.; Westwood, M.A.; Firmin, D.N.; Pennell, D.J. Thalassemia International Federation Heart T2* Investigators. Multi-center validation of the transferability of the magnetic resonance T2* technique for the quantification of tissue iron. Haematologica 2006, 91, 1388–1391. [Google Scholar] [PubMed]
- Wood, J.C.; Enriquez, C.; Ghugre, N.; Tyzka, J.M.; Carson, S.; Nelson, M.D.; Coates, T.D. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood 2005, 106, 1460–1465. [Google Scholar] [CrossRef]
- Chan, W.C.; Tejani, Z.; Budhani, F.; Massey, C.; Haider, M.A. R2* as a surrogate measure of ferriscan iron quantification in thalassemia. J. Magn. Reson. Imaging 2014, 39, 1007–1011. [Google Scholar] [CrossRef]
- St Pierre, T.G.; Clark, P.R.; Chua-anusorn, W.; Fleming, A.J.; Jeffrey, G.P.; Olynyk, J.K.; Pootrakul, P.; Robins, E.; Lindeman, R. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood 2005, 105, 855–861. [Google Scholar] [CrossRef]
- Voskaridou, E.; Douskou, M.; Terpos, E.; Papassotiriou, I.; Stamoulakatou, A.; Ourailidis, A.; Loutradi, A.; Loukopoulos, D. Magnetic resonance imaging in the evaluation of iron overload in patients with beta thalassaemia and sickle cell disease. Br. J. Haematol. 2004, 126, 736–742. [Google Scholar] [CrossRef]
- Wang, Z.J.; Haselgrove, J.C.; Martin, M.B.; Hubbard, A.M.; Li, S.; Loomes, K.; Moore, J.R.; Zhao, H.; Cohen, A.R. Evaluation of iron overload by single voxel MRS measurement of liver T2. J. Magn. Reson. Imaging 2002, 15, 395–400. [Google Scholar] [CrossRef]
- Towbin, A.J.; Serai, S.D.; Podberesky, D.J. Magnetic resonance imaging of the pediatric liver: Imaging of steatosis, iron deposition, and fibrosis. Magn. Reson. Imaging Clin. N. Am. 2013, 21, 669–680. [Google Scholar] [CrossRef]
- Barrera, C.A.; Otero, H.J.; Hartung, H.D.; Biko, D.M.; Serai, S.D. Protocol optimization for cardiac and liver iron content assessment using MRI: What sequence should I use? Clin. Imaging 2019, 56, 52–57. [Google Scholar] [CrossRef]
- St Pierre, T.G.; El-Beshlawy, A.; Elalfy, M.; Al Jefri, A.; Al Zir, K.; Daar, S.; Habr, D.; Kriemler-Krahn, U.; Taher, A. Multicenter validation of spin-density projection-assisted R2-MRI for the noninvasive measurement of liver iron concentration. Magn. Reson. Med. 2014, 71, 2215–2223. [Google Scholar] [CrossRef]
- St Pierre, T.G.; Clark, P.R.; Chua-Anusorn, W. Measurement and mapping of liver iron concentrations using magnetic resonance imaging. Ann. N. Y. Acad. Sci. 2005, 1054, 379–385. [Google Scholar] [CrossRef]
- Clark, P.R.; Chua-Anusorn, W.; St Pierre, T.G. Proton transverse relaxation rate (R2) images of liver tissue; mapping local tissue iron concentrations with MRI [corrected]. Magn. Reson. Med. 2003, 49, 572–575. [Google Scholar] [CrossRef]
- Serai, S.D.; Smith, E.A.; Trout, A.T.; Dillman, J.R. Agreement between manual relaxometry and semi-automated scanner-based multi-echo Dixon technique for measuring liver T2* in a pediatric and young adult population. Pediatr. Radiol. 2018, 48, 94–100. [Google Scholar] [CrossRef]
- Barrera, C.A.; Khrichenko, D.; Serai, S.D.; Hartung, H.D.; Biko, D.M.; Otero, H.J. Biexponential R2* relaxometry for estimation of liver iron concentration in children: A better fit for high liver iron states. J. Magn. Reson. Imaging 2019, 50, 1191–1198. [Google Scholar] [CrossRef]
- Evans, J.D. Straightforward Statistics for the Behavioral Sciences; Brooks/Cole Publishing Company: Pacific Grove, CA, USA, 1996. [Google Scholar]
- Bassett, M.L.; Halliday, J.W.; Powell, L.W. Value of hepatic iron measurements in early hemochromatosis and determination of the critical iron level associated with fibrosis. Hepatology 1986, 6, 24–29. [Google Scholar] [CrossRef]
- Lambing, A.; Kachalsky, E.; Mueller, M.L. The dangers of iron overload: Bring in the iron police. J. Am. Acad. Nurse Pract. 2012, 24, 175–183. [Google Scholar] [CrossRef]
- Shander, A.; Sazama, K. Clinical consequences of iron overload from chronic red blood cell transfusions, its diagnosis, and its management by chelation therapy. Transfusion 2010, 50, 1144–1155. [Google Scholar] [CrossRef]
- Wood, J.C. Use of magnetic resonance imaging to monitor iron overload. Hematol. Oncol. Clin. N. Am. 2014, 28, 747–764. [Google Scholar] [CrossRef]
- Serai, S.D.; Trout, A.T.; Fleck, R.J.; Quinn, C.T.; Dillman, J.R. Measuring liver T2* and cardiac T2* in a single acquisition. Abdom. Radiol. 2018, 43, 2303–2308. [Google Scholar] [CrossRef]
- Healy, G.M.; Kannengiesser, S.A.R.; Espin-Garcia, O.; Ward, R.; Kuo, K.H.M.; Jhaveri, K.S. Comparison of Inline R2* MRI versus FerriScan for liver iron quantification in patients on chelation therapy for iron overload: Preliminary results. Eur. Radiol. 2021, 31, 9296–9305. [Google Scholar] [CrossRef]



| R2 Method | R2* Method | |
|---|---|---|
| No. of echoes | 5 | 8 |
| Minimum TE (ms) | 6 | 1 |
| Δ TE (ms) | 3 | 1.4 |
| TR (ms) | 1000 | 200 |
| No. of slices | 11 | 4 |
| Slice thickness (mm) | 5 | 10 |
| Matrix size | 256 × 256 | 128 × 128 |
| Flip angle (degree) | 90 | 20 |
| Approx. scan time (minutes/seconds) | 10:00 | 0:13 |
| Diseases | Number (%) Total = 81 |
|---|---|
| Beta thalassemia | 44 (54%) |
| Sickle cell disease | 16 (20%) |
| Hemoglobin H-constant Spring variant | 4 (5%) |
| Diamond–Blackfan anemia | 3 (4%) |
| Idiopathic aplastic anemia | 3 (4%) |
| Pyruvate kinase deficiency | 2 (2%) |
| Aplastic anemia | 2 (2%) |
| Hemoglobin E-beta thalassemia | 3 (4%) |
| Sideroblastic anemia | 2 (2%) |
| Non-autoimmune hemolytic anemia | 1 (1%) |
| Hereditary hemolytic anemia | 1 (1%) |
| LIC Methods | |||
|---|---|---|---|
| Mean ± SD | Confidence | Confidence | |
| −95% | 95% | ||
| Δ LIC R2 (mg/g per month) | −0.0043 ± 0.0214 | −0.009037 | 0.000409 |
| Δ LIC R2* (mg/g per month) | −0.0047 ± 0.012 | −0.007384 | −0.002108 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatakrishna, S.S.B.; Otero, H.J.; Ghosh, A.; Khrichenko, D.; Serai, S.D. Rate of Change of Liver Iron Content by MR Imaging Methods: A Comparison Study. Tomography 2022, 8, 2508-2521. https://doi.org/10.3390/tomography8050209
Venkatakrishna SSB, Otero HJ, Ghosh A, Khrichenko D, Serai SD. Rate of Change of Liver Iron Content by MR Imaging Methods: A Comparison Study. Tomography. 2022; 8(5):2508-2521. https://doi.org/10.3390/tomography8050209
Chicago/Turabian StyleVenkatakrishna, Shyam Sunder B., Hansel J. Otero, Adarsh Ghosh, Dmitry Khrichenko, and Suraj D. Serai. 2022. "Rate of Change of Liver Iron Content by MR Imaging Methods: A Comparison Study" Tomography 8, no. 5: 2508-2521. https://doi.org/10.3390/tomography8050209
APA StyleVenkatakrishna, S. S. B., Otero, H. J., Ghosh, A., Khrichenko, D., & Serai, S. D. (2022). Rate of Change of Liver Iron Content by MR Imaging Methods: A Comparison Study. Tomography, 8(5), 2508-2521. https://doi.org/10.3390/tomography8050209






