Preliminary Analysis of the Effects of Ad26.COV2.S Vaccination on CT Findings and High Intensive Care Admission Rates of COVID-19 Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Chest HRCT Acquisition and Examination
2.3. Statistical Analysis
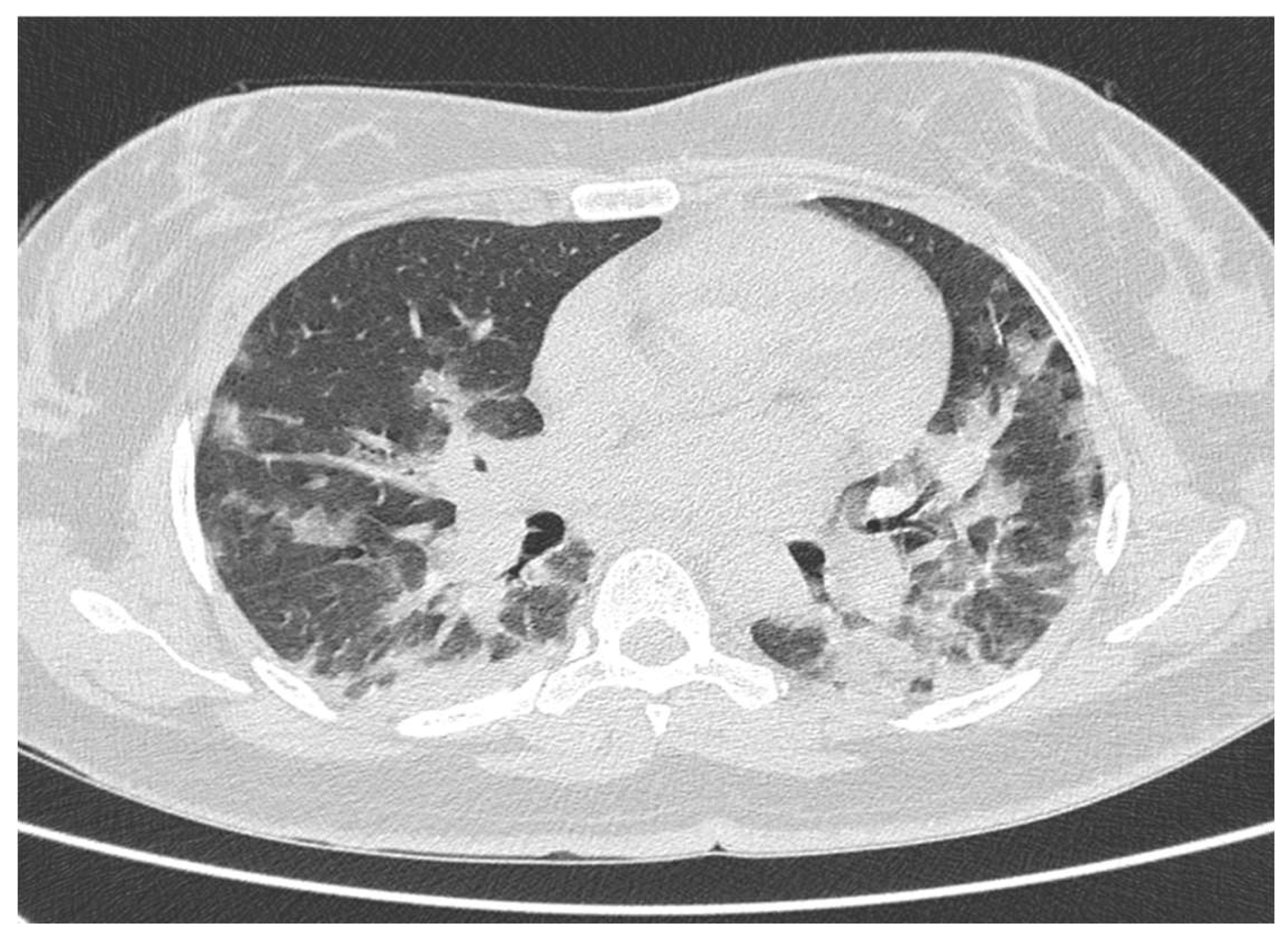
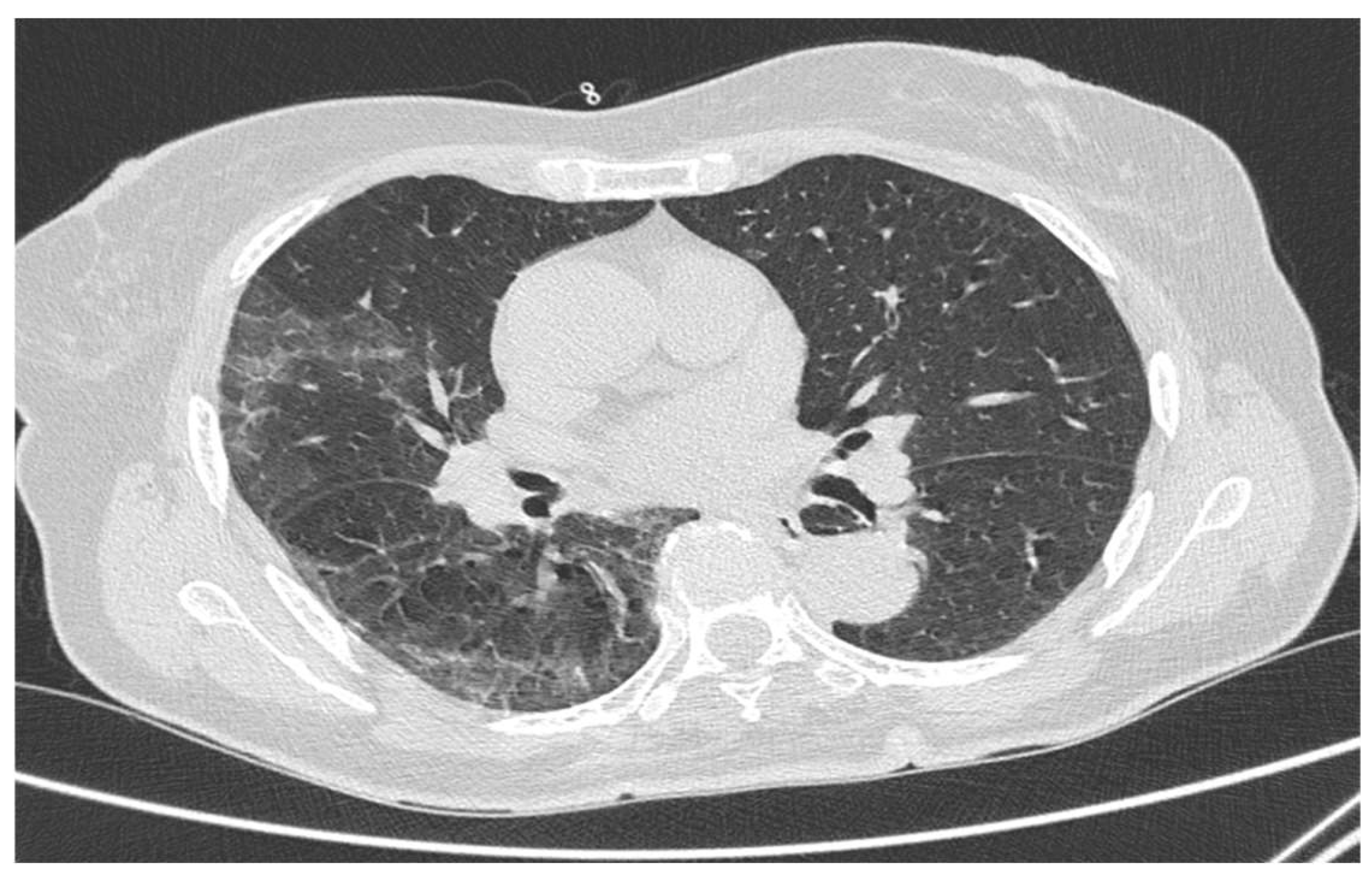
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Heymann, D.L.; Shindo, N. COVID-19: What is next for public health? Lancet 2020, 395, 542–545. [Google Scholar] [CrossRef]
- Vital Surveillances: The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19)—China. Available online: https://weekly.chinacdc.cn/en/article/id/e53946e2-c6c4-41e9-9a9b-fea8db1a8f51 (accessed on 17 February 2020).
- Fang, Y.; Zhang, H.; Xie, J.; Lin, M.; Ying, L.; Pang, P.; Ji, W. Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR. Radiology 2020, 296, E115–E117. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Lee, K.H.; Kim, J.Y.; Lee, Y.K.; Ko, H.; Kim, K.H.; Park, C.M.; Kim, Y.-H. Chest Radiographic and CT Findings of the 2019 Novel Coronavirus Disease (COVID-19): Analysis of Nine Patients Treated in Korea. Korean J. Radiol. 2020, 21, 494. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Ye, T.; Sun, P.; Gui, S.; Liang, B.; Li, L.; Zheng, D.; Wang, J.; Hesketh, R.L.; Yang, L.; et al. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology 2020, 295, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Ishfaq, A.; Yousaf Farooq, S.M.; Goraya, A.; Yousaf, M.; Gulani, S.A.; Kiran, A.; Ayoub, M.; Javed, A.; Bacha, R. Role of High Resolution Computed Tomography chest in the diagnosis and evaluation of COVID -19 patients -A systematic review and meta-analysis. Eur. J. Radiol. Open 2021, 8, 100350. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Li, X.; Liu, H.; Zhen, Y.; Zhang, X.; Xiong, Q.; Luo, Y.; Gao, C.; Zeng, W. Chest CT Severity Score: An Imaging Tool for Assessing Severe COVID-19. Radiol. Cardiothorac. Imaging 2020, 2, e200047. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Conway, C.M. Editorial: “Old lamps for new”. Br. J. Anaesth. 1975, 47, 811–812. [Google Scholar] [PubMed]
- Akamatsu, N.; Nakajima, H.; Ono, M.; Miura, Y. Increase in acetyl CoA synthetase activity after phenobarbital treatment. Biochem. Pharmacol. 1975, 24, 1725–1727. [Google Scholar] [CrossRef]
- Prokop, M.; van Everdingen, W.; van Rees Vellinga, T.; van Ufford, H.Q.; Stögeret, L.; Beenen, L.; Geurts, B.; Gietema, H.; Krdzalic, J.; Schaefer-Prokop, C.; et al. CO-RADS: A Categorical CT Assessment Scheme for Patients Suspected of Having COVID-19—Definition and Evaluation. Radiology 2020, 296, E97–E104. [Google Scholar] [CrossRef] [PubMed]
- Polinski, J.M.; Weckstein, A.R.; Batech, M.; Kabelac, C.; Kamath, T.; Harvey, R.; Jain, S.; Rassen, J.A.; Khan, N.; Schneeeiss, S. Durability of the Single-Dose Ad26.COV2.S Vaccine in the Prevention of COVID-19 Infections and Hospitalizations in the US Before and during the Delta Variant Surge. JAMA. Netw. Open 2022, 5, e222959. [Google Scholar] [CrossRef] [PubMed]
- Self, W.H.; Tenforde, M.W.; Rhoads, J.P.; Gaglani, M.; Ginde, A.A.; Douin, D.J.; Olson, S.M.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; et al. Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations Among Adults Without Immunocompromising Conditions - United States, March-August 2021. MMWR. Morb. Mortal Wkly. Rep. 2021, 70, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.-J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Teran-Tinedo, J.R.; Gonzalez-Rubio, J.; Najera, A.; Castany-Faro, A.; Contreras, M.L.N.; Garcia, I.M.; Mellado, L.L.; González, M.L.; Garvin, P.P.; Crespo, G.S.; et al. Clinical characteristics and respiratory care in hospitalized vaccinated SARS-CoV-2 patients. eClinicalMedicine 2022, 48, 101453. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Blish, C.A.; Sallusto, F.; Iwasaki, A. The immunology and immunopathology of COVID-19. Science 2022, 375, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- The anti-COVID-19 vaccine Janssen Ad26.COV2.S. Available online: https://www.epicentro.iss.it/vaccini/covid-19-vaccino-janssen (accessed on 10 August 2021).
- Callaway, E. Heavily mutated Omicron variant puts scientists on alert. Nature 2021, 600, 21. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, W.F.; St. Denis, K.J.; Hoelzemer, A.; Lam, E.C.; Nitido, A.D.; Sheehan, M.L.; Berrios, C.; Ofoman, O.; Chang, C.C.; Hauser, B.M.; et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 2022, 185, 457–466.e4. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, A. Omicron emerges. New Sci. 2021, 252, 7. [Google Scholar] [CrossRef]
- Emerging Variants of SARS-CoV-2 And Novel Therapeutics Against Coronavirus (COVID-19). Available online: http://www.ncbi.nlm.nih.gov/books/NBK570580/ (accessed on 27 July 2022).
- Cau, R.; Falaschi, Z.; Paschè, A.; Danna, P.; Arioli, R.; Arru, C.D.; Zagaria, D.; Tricca, S.; Suri, J.S.; Karla, M.K.; et al. CT Findings of Covid-19 Pneumonia in Icu-Patients. J. Public Health Res. 2021, 10, jphr.2021.2270. [Google Scholar] [CrossRef] [PubMed]
| Group 1 | Group 2 | ||
|---|---|---|---|
| Mean ± SD | Mean ± SD | p | |
| Age | 63.5 ± 4.2 | 64.7 ± 3.7 | 0.178 |
| RSNA SS | 9.7 ± 4.4 | 16.2 ± 10.5 | 0.030 |
| N% | N% | ||
| TOT | 10 (100%) | 61 (100%) | |
| Male | 4 (40%) | 32 (52.5%) | 0.465 |
| Female | 6 (60%) | 29 (47.5%) | |
| Admission to healthcare settings | |||
| Home Care | 8 (80%) | 33 (54.1%) | 0.124 |
| Ordinary Hospitalization | 1 (10%) | 16 (26.2%) | 0.265 |
| Subintensive Care | 1 (10%) | 8 (13.1%) | 0.784 |
| Intensive Care | 0 (0.0%) | 4 (6.6%) | 0.405 |
| High Intensivity Hospitalization | 1 (10%) | 12 (19.7%) | 0.464 |
| Admission to Healthcare Settings | TOT | Male | Female | Age ± SD | RSNA SS ± SD |
|---|---|---|---|---|---|
| Home Care | 41 (100%) | 18 (43.9%) | 23 (56.1%) | 63.8 ± 3.4 | 8.0 ± 3.6 |
| Ordinary Hospitalization | 17 (100%) | 11 (64.7%) | 6 (35.3%) | 65.2 ± 4.3 | 22.2 ± 6.7 |
| Subintensive Care | 9 (100%) | 6 (66.7%) | 3 (33.3%) | 65.3 ± 4.4 | 28.0 ± 6.6 |
| Intensive Care | 4 (100%) | 1 (25.0%) | 3 (75.0%) | 67.7 ± 2.6 | 31.3 ± 4.9 |
| High Intensivity Hospitalization | 13 (100%) | 7 (53.8%) | 6 (46.2%) | 66.1 ± 4.1 | 29.0 ± 6.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negroni, D.; Carriero, S.; Passarella, I.; Siani, A.; Biondetti, P.; Pizzolante, A.; Saba, L.; Guzzardi, G. Preliminary Analysis of the Effects of Ad26.COV2.S Vaccination on CT Findings and High Intensive Care Admission Rates of COVID-19 Patients. Tomography 2022, 8, 2403-2410. https://doi.org/10.3390/tomography8050199
Negroni D, Carriero S, Passarella I, Siani A, Biondetti P, Pizzolante A, Saba L, Guzzardi G. Preliminary Analysis of the Effects of Ad26.COV2.S Vaccination on CT Findings and High Intensive Care Admission Rates of COVID-19 Patients. Tomography. 2022; 8(5):2403-2410. https://doi.org/10.3390/tomography8050199
Chicago/Turabian StyleNegroni, Davide, Serena Carriero, Ilaria Passarella, Agnese Siani, Pierpaolo Biondetti, Antonio Pizzolante, Luca Saba, and Giuseppe Guzzardi. 2022. "Preliminary Analysis of the Effects of Ad26.COV2.S Vaccination on CT Findings and High Intensive Care Admission Rates of COVID-19 Patients" Tomography 8, no. 5: 2403-2410. https://doi.org/10.3390/tomography8050199
APA StyleNegroni, D., Carriero, S., Passarella, I., Siani, A., Biondetti, P., Pizzolante, A., Saba, L., & Guzzardi, G. (2022). Preliminary Analysis of the Effects of Ad26.COV2.S Vaccination on CT Findings and High Intensive Care Admission Rates of COVID-19 Patients. Tomography, 8(5), 2403-2410. https://doi.org/10.3390/tomography8050199







