Contrast Enhanced Ultrasound Compared with MRI and CT in the Evaluation of Post-Renal Transplant Complications
Abstract
1. Introduction
2. Imaging Modalities
2.1. Unenhanced US
2.2. CT
2.3. Magnetic Resonance Imaging
2.4. CEUS
2.5. CEUS Technique
- -
- A cortico-medullary phase is an arterial phase in which the renal cortex is mostly enhanced.
- -
- A nephrographic phase provides a more homogeneous enhancement of the cortex and medulla, at 30–70 s after injection.
- -
- The delayed imaging achieved after >70 s from contrast injection does not represent a urographic phase because such contrast agents in US imaging are not actually excreted by the kidney [15].
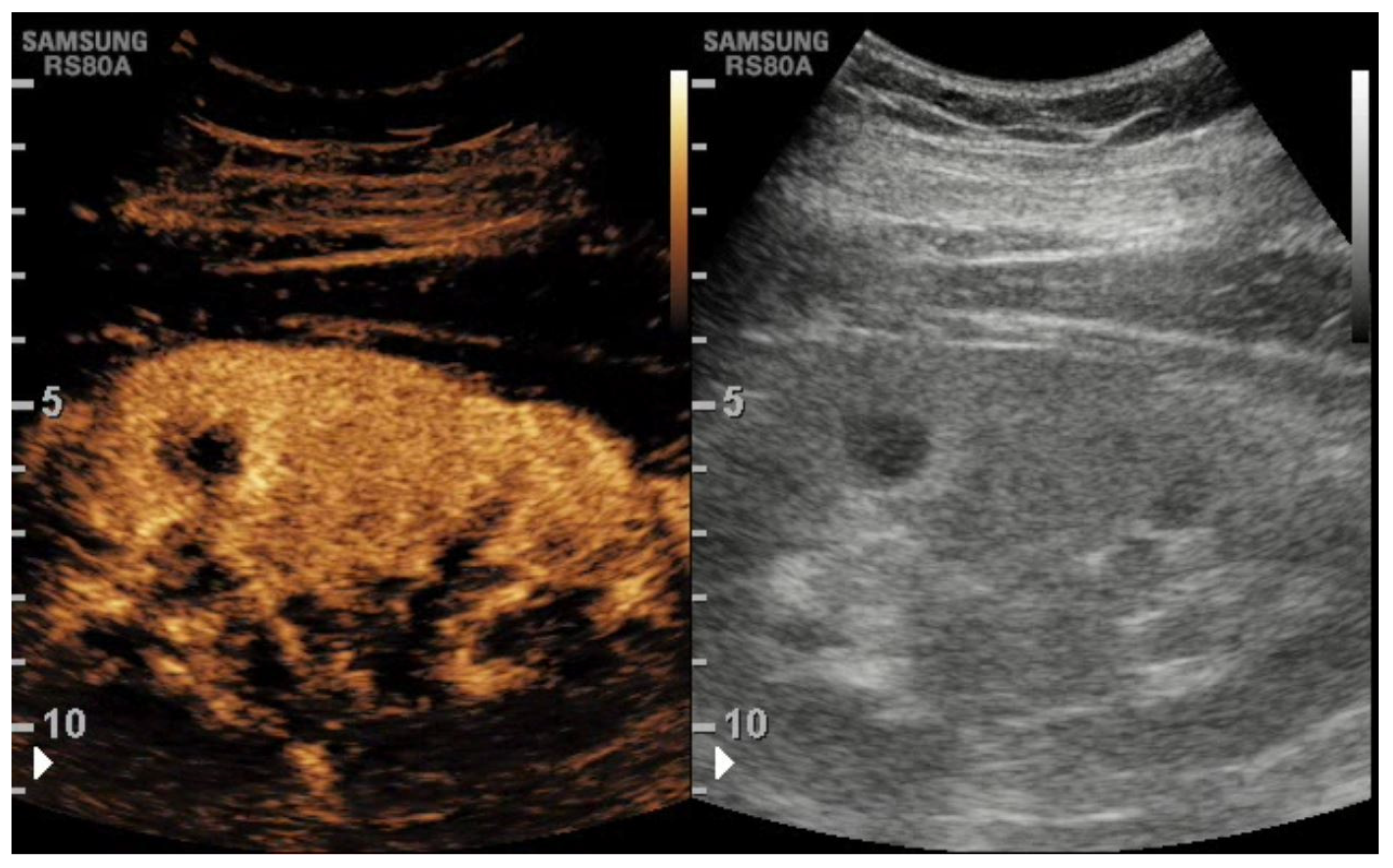
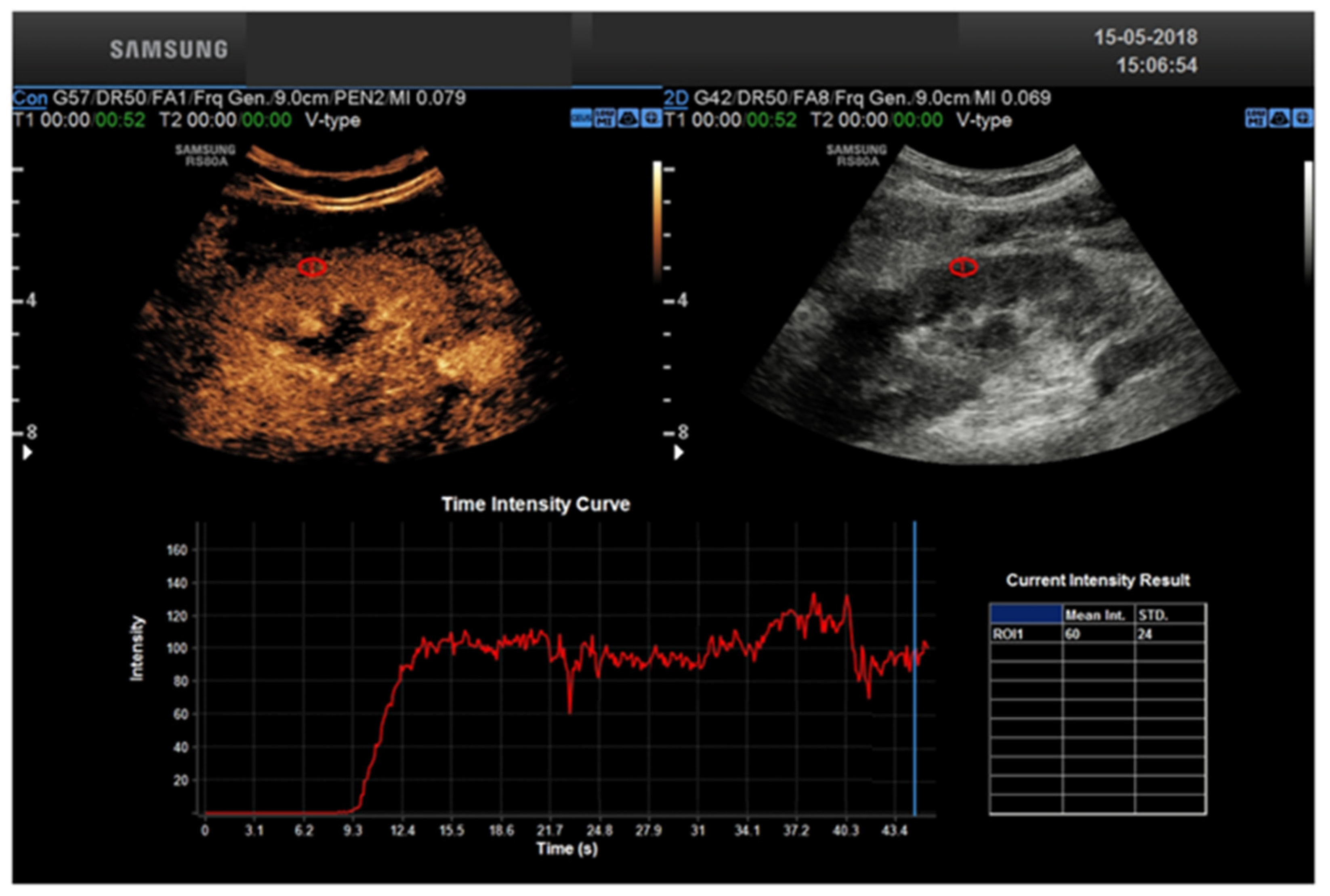
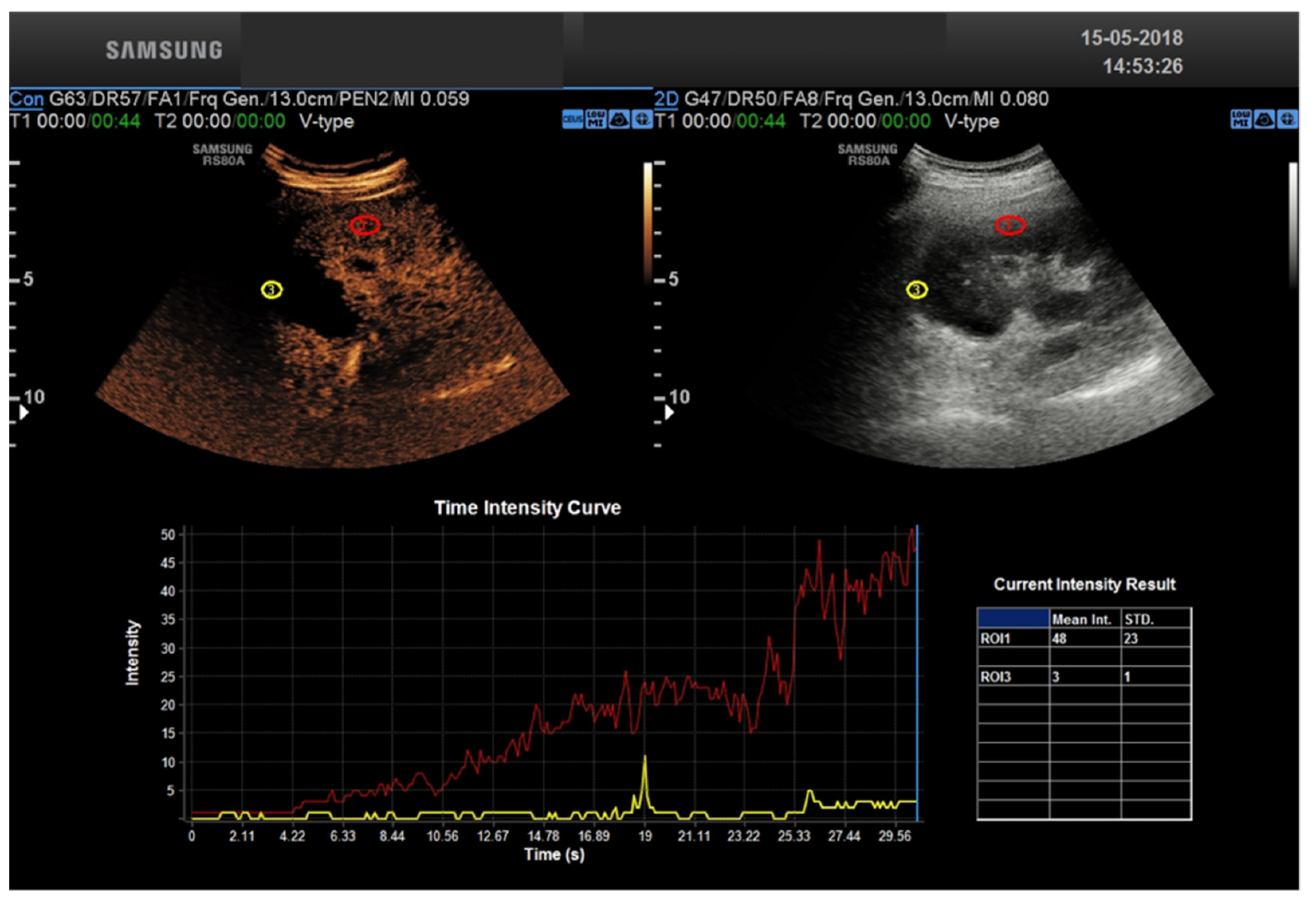
3. CEUS Role for Transplant Kidney Complications
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hart, A.; Lentine, K.L.; Smith, J.M.; Miller, J.M.; Skeans, M.A.; Prentice, M.; Robinson, A.; Foutz, J.; Booker, S.E.; Israni, A.K.; et al. OPTN/SRTR 2019 Annual Data Report: Kidney. Am. J. Transplant. 2021, 21, 21–137. [Google Scholar] [CrossRef] [PubMed]
- Cecka, J.M.; Terasaki, P.I. The UNOS Scientific Renal Transplant Registry. Clin. Transpl. 1992, 1–16. [Google Scholar]
- Bellini, M.I.; Courtney, A.E.; McCaughan, J.A. Living Donor Kidney Transplantation Improves Graft and Recipient Survival in Patients with Multiple Kidney Transplants. J. Clin. Med. 2020, 9, 2118. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Wiebe, N.; Knoll, G.; Bello, A.; Browne, S.; Jadhav, D.; Klarenbach, S.; Gill, J. Systematic Review: Kidney Transplantation Compared With Dialysis in Clinically Relevant Outcomes. Am. J. Transplant. 2011, 11, 2093–2109. [Google Scholar] [CrossRef] [PubMed]
- Haberal, M.; Boyvat, F.; Akdur, A.; Kırnap, M.; Özçelik, Ü.; Yarbuğ Karakayalı, F. Surgical Complications After Kidney Transplantation. Exp. Clin. Transplant. 2016, 14, 587–595. [Google Scholar]
- Rajiah, P.; Lim, Y.Y.; Taylor, P. Renal transplant imaging and complications. Gastrointest. Radiol. 2006, 31, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Berger, P.M.; Diamond, J.R. Ureteral obstruction as a complication of renal transplantation: A review. J. Nephrol. 1998, 11, 20–23. [Google Scholar] [PubMed]
- Hellemans, R.; Pengel, L.H.; Choquet, S.; Maggiore, U.; for ESOT Workstream 3 of the TLJ (Transplant Learning Journey) Project. Managing immunosuppressive therapy in potentially cured post-kidney transplant cancer (excluding non-melanoma skin cancer): An overview of the available evidence and guidance for shared decision-making. Transpl. Int. 2021, 34, 1789–1800. [Google Scholar] [CrossRef]
- Taffel, M.T.; Nikolaidis, P.; Beland, M.D.; Blaufox, M.D.; Dogra, V.S.; Goldfarb, S.; Gore, J.L.; Harvin, H.J.; Heilbrun, M.E.; Heller, M.T.; et al. ACR Appropriateness Criteria® Renal Transplant Dysfunction. J. Am. Coll. Radiol. 2017, 14, S272–S281. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Granata, A.; Clementi, S.; Londrino, F.; Romano, G.; Veroux, M.; Fiorini, F.; Fatuzzo, P.M. Renal transplant vascular complications: The role of Doppler ultrasound. J. Ultrasound 2014, 18, 101–107. [Google Scholar] [CrossRef]
- Sebastià, C.; Quiroga, S.; Boyé, R.; Cantarell, C.; Fernandez-Planas, M.; Alvarez, A. Helical CT in Renal Transplantation: Normal Findings and Early and Late Complications. RadioGraphics 2001, 21, 1103–1117. [Google Scholar] [CrossRef] [PubMed]
- Naesens, M.; Heylen, L.; Lerut, E.; Claes, K.; de Wever, L.; Claus, F.; Oyen, R.; Kuypers, D.; Evenepoel, P.; Bammens, B.; et al. Intrarenal Resistive Index after Renal Transplantation. N. Engl. J. Med. 2013, 369, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.I.; Charalampidis, S.; Herbert, P.E.; Bonatsos, V.; Crane, J.; Muthusamy, A.; Dor, F.J.M.F.; Papalois, V. Cold Pulsatile Machine Perfusion versus Static Cold Storage in Kidney Transplantation: A Single Centre Experience. BioMed Res. Int. 2019, 2019, 7435248. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Tortorici, F.; Amabile, M.; D’Andrea, V. Assessing Kidney Graft Viability and Its Cells Metabolism during Machine Perfusion. Int. J. Mol. Sci. 2021, 22, 1121. [Google Scholar] [CrossRef] [PubMed]
- Como, G.; Da Re, J.; Adani, G.L.; Zuiani, C.; Girometti, R. Role for contrast-enhanced ultrasound in assessing complications after kidney transplant. World J. Radiol. 2020, 12, 156–171. [Google Scholar] [CrossRef]
- Fananapazir, G.; McGahan, J.P.; Corwin, M.T.; Stewart, S.L.; Vu, C.T.; Wright, L.; Troppmann, C. Screening for Transplant Renal Artery Stenosis: Ultrasound-Based Stenosis Probability Stratification. Am. J. Roentgenol. 2017, 209, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfar, A.; Tavakoli, A.; Augustine, T.; Pararajasingam, R.; Riad, H.; Chalmers, N. Management of transplant renal artery stenosis and its impact on long-term allograft survival: A single-centre experience. Nephrol. Dial. Transplant. 2010, 26, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.-S.; Liu, M.; Luo, J.; Tian, W.-S.; Liang, J.-Y.; Xu, M.; Zheng, Y.-L.; Xie, X.-Y. Transplant renal artery stenosis: Evaluation with contrast-enhanced ultrasound. Eur. J. Radiol. 2017, 90, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Peltzer, K.; de Figueiredo, G.N.; Fischereder, M.; Habicht, A.; Rübenthaler, J.; Clevert, D.-A. Vascular rejection in renal transplant: Diagnostic value of contrast-enhanced ultrasound (CEUS) compared to biopsy. Clin. Hemorheol. Microcirc. 2018, 69, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Schutter, R.; Lantinga, V.A.; Borra, R.J.H.; Moers, C. MRI for diagnosis of post-renal transplant complications: Current state-of-the-art and future perspectives. Magn. Reson. Mater. Physics Biol. Med. 2019, 33, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Sugi, M.D.; Joshi, G.; Maddu, K.K.; Dahiya, N.; Menias, C.O. Imaging of Renal Transplant Complications throughout the Life of the Allograft: Comprehensive Multimodality Review. RadioGraphics 2019, 39, 1327–1355. [Google Scholar] [CrossRef] [PubMed]
- McArthur, C.; Baxter, G. Current and potential renal applications of contrast-enhanced ultrasound. Clin. Radiol. 2012, 67, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Averkiou, M.; Nielsen, M.B.; Barr, R.G.; Burns, P.N.; Calliada, F.; Cantisani, V.; Choi, B.; Chammas, M.C.; Clevert, D.-A.; et al. How to perform Contrast-Enhanced Ultrasound (CEUS). Ultrasound Int. Open 2018, 4, E2–E15. [Google Scholar] [CrossRef]
- Sidhu, P.S.; Cantisani, V.; Dietrich, C.F.; Gilja, O.H.; Saftoiu, A.; Bartels, E.; Bertolotto, M.; Calliada, F.; Clevert, D.-A.; Cosgrove, D.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall der Med. Eur. J. Ultrasound 2018, 39, e2–e44. [Google Scholar] [CrossRef] [PubMed]
- Rafailidis, V.; Huang, D.Y.; Yusuf, G.T.; Sidhu, P.S. General principles and overview of vascular contrast-enhanced ultrasonography. Ultrasonography 2020, 39, 22–42. [Google Scholar] [CrossRef]
- Barr, R.G.; Wilson, S.R.; Lyshchik, A.; McCarville, B.; Darge, K.; Grant, E.; Robbin, M.; Wilmann, J.K.; Chong, W.K.; Fleischer, A.; et al. Contrast -Enhanced Ultrasound. Ultrasound Q. 2020, 36, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.J.; Sidhu, P.S.; Nielsen, M.B. Contrast-Enhanced Ultrasound in Renal Transplants: Applications and Future Directions. Ultraschall der Med. Eur. J. Ultrasound 2013, 34, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Akbar, S.A.; Jafri, S.Z.H.; Amendola, M.A.; Madrazo, B.L.; Salem, R.; Bis, K.G. Complications of Renal Transplantation. RadioGraphics 2005, 25, 1335–1356. [Google Scholar] [CrossRef] [PubMed]
- Ben Hamida, F.; Barbouch, S.; Bardi, R.; Helal, I.; Kaaroud, H.; Ben Fatma, L.; Hedri, H.; Abderrahim, E.; Ben Abdallah, T.; Ayed, K.; et al. Acute rejection episodes after kidney transplantation. Saudi J. Kidney Dis. Transplant. 2009, 20, 370. [Google Scholar] [PubMed]
- Yang, C.; Wu, S.; Yang, P.; Shang, G.; Qi, R.; Xu, M.; Rong, R.; Zhu, T.; He, W. Prediction of renal allograft chronic rejection using a model based on contrast-enhanced ultrasonography. Microcirculation 2019, 26, e12544. [Google Scholar] [CrossRef]
- Benozzi, L.; Cappelli, G.; Granito, M.; Davoli, D.; Favali, D.; Montecchi, M.; Grossi, A.; Torricelli, P.; Albertazzi, A. Contrast-Enhanced Sonography in Early Kidney Graft Dysfunction. Transplant. Proc. 2009, 41, 1214–1215. [Google Scholar] [CrossRef] [PubMed]
- Baxter, G.; Ireland, H.; Moss, J.; Harden, P.; Junor, B.; Rodger, R.; Briggs, J. Colour Doppler ultrasound in renal transplant artery stenosis: Which Doppler index? Clin. Radiol. 1995, 50, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Rennert, J.; Farkas, S.; Georgieva, M.; Loss, M.; Dornia, C.; Jung, W.; Stroszczynski, C.; Jung, E.-M. Identification of early complications following pancreas and renal transplantation using contrast enhanced ultrasound (CEUS)—first results. Clin. Hemorheol. Microcirc. 2014, 58, 343–352. [Google Scholar] [CrossRef] [PubMed]
- El Zorkany, K.; Bridson, J.M.; Sharma, A.; Halawa, A. Transplant Renal Vein Thrombosis. Exp Clin Transplant. 2017, 15, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Middleton, W.D.; Kurtz, A.B.; Hertzberg, B.S. Ultrasound: The requisites, 2nd ed.; Mosby: St. Louis, MO, USA, 2004. [Google Scholar]
- Pini, A.; Faggioli, G.; Pini, R.; Mauro, R.; Gallitto, E.; Mascoli, C.; Grandinetti, V.; Donati, G.; Odaldi, F.; Ravaioli, M.; et al. Assessment and Management of Transplant Renal Artery Stenosis. A Literature Review. Ann. Vasc. Surg. 2022, 82, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Dimitroulis, D.; Bokos, J.; Zavos, G.; Nikiteas, N.; Karidis, N.; Katsaronis, P.; Kostakis, A. Vascular Complications in Renal Transplantation: A Single-Center Experience in 1367 Renal Transplantations and Review of the Literature. Transplant. Proc. 2009, 41, 1609–1614. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.I.; Charalampidis, S.; Stratigos, I.; Dor, F.J.; Papalois, V. The Effect of Donors’ Demographic Characteristics in Renal Function Post-Living Kidney Donation. Analysis of a UK Single Centre Cohort. J. Clin. Med. 2019, 8, 883. [Google Scholar] [CrossRef]
- Dobrijevic, E.L.K.; Au, E.H.K.; Rogers, N.M.; Clayton, P.A.; Wong, G.; Allen, R.D.M. Association Between Side of Living Kidney Donation and Post-Transplant Outcomes. Transpl. Int. 2022, 35, 10117. [Google Scholar] [CrossRef]
- Rodríguez, S.; Palacios, V.H.; Mayayo, E.S.; Dos Santos, V.G.; Nicolás, V.D.; Gallego, M.D.S.; Álvaro, J.L.; Revilla, F.J.B. The Usefulness of Contrast-Enhanced Ultrasound in the Assessment of Early Kidney Transplant Function and Complications. Diagnostics 2017, 7, 53. [Google Scholar] [CrossRef]
- Moreno, C.C.; Mittal, P.K.; Ghonge, N.P.; Bhargava, P.; Heller, M.T. Imaging Complications of Renal Transplantation. Radiol. Clin. N. Am. 2015, 54, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Stacul, F.; on behalf of the Contrast Media Safety Committee of European Society of Urogenital Radiology (ESUR); van der Molen, A.J.; Reimer, P.; Webb, J.A.W.; Thomsen, H.S.; Morcos, S.K.; Almén, T.; Aspelin, P.; Bellin, M.-F.; et al. Contrast induced nephropathy: Updated ESUR Contrast Media Safety Committee guidelines. Eur. Radiol. 2011, 21, 2527–2541. [Google Scholar] [CrossRef] [PubMed]
- Ulusan, S.; Koc, Z.; Tokmak, N. Accuracy of sonography for detecting renal stone: Comparison with CT. J. Clin. Ultrasound. 2007, 35, 256–261. [Google Scholar] [CrossRef] [PubMed]
- ACR Manual on Contrast Media Version 9. ACR Committee on Drugs and Contrast Media. Available online: http://www.acr.org/w/media/ACR/Documents/PDF/QualitySafety/Resources/Contrast%20Manual/2013_Contrast_Media.pdf (accessed on 20 May 2015).
- Serhal, A.; Aouad, P.; Serhal, M.; Pathrose, A.; Lombardi, P.; Carr, J.; Avery, R.; Edelman, R.R. Evaluation of Renal Allograft Vasculature Using Non-contrast 3D Inversion Recovery Balanced Steady-state Free Precession MRA and 2D Quiescent-interval Slice-selective MRA. Explor. Res. Hypothesis Med. 2021, 6, 90–98. [Google Scholar] [CrossRef] [PubMed]
|
|
|
|
|
|
|
|
|
|
|
|
| PROS | CONS | |
|---|---|---|
| CEUS | Lack of ionizing radiation Inexpensive Repeatable Real time examination Fast Macro and micro vascularization assessment Safe in patients with renal impairment Can be performed at the bedside (no need to transport the patient) Can be used to guide procedures | Absence of wide view compared to CT and MRI. Requires experienced operators. |
| US with Doppler | Follows most of the advantages of CEUS; It doesn’t use contrast media | Requires an expert operator; Affected by artifacts |
| CT | Panoramic view High spatial resolution Volumetric rendering Fast | Nephrotoxic contrast medium Ionizing radiation Not feasible in patients with high creatinine blood values |
| MRI | Panoramic view Lack of ionizing radiation Tissue characterization | Nephrotoxic contrast medium Expensive Time consuming |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
David, E.; Del Gaudio, G.; Drudi, F.M.; Dolcetti, V.; Pacini, P.; Granata, A.; Pretagostini, R.; Garofalo, M.; Basile, A.; Bellini, M.I.; et al. Contrast Enhanced Ultrasound Compared with MRI and CT in the Evaluation of Post-Renal Transplant Complications. Tomography 2022, 8, 1704-1715. https://doi.org/10.3390/tomography8040143
David E, Del Gaudio G, Drudi FM, Dolcetti V, Pacini P, Granata A, Pretagostini R, Garofalo M, Basile A, Bellini MI, et al. Contrast Enhanced Ultrasound Compared with MRI and CT in the Evaluation of Post-Renal Transplant Complications. Tomography. 2022; 8(4):1704-1715. https://doi.org/10.3390/tomography8040143
Chicago/Turabian StyleDavid, Emanuele, Giovanni Del Gaudio, Francesco Maria Drudi, Vincenzo Dolcetti, Patrizia Pacini, Antonio Granata, Renzo Pretagostini, Manuela Garofalo, Antonio Basile, Maria Irene Bellini, and et al. 2022. "Contrast Enhanced Ultrasound Compared with MRI and CT in the Evaluation of Post-Renal Transplant Complications" Tomography 8, no. 4: 1704-1715. https://doi.org/10.3390/tomography8040143
APA StyleDavid, E., Del Gaudio, G., Drudi, F. M., Dolcetti, V., Pacini, P., Granata, A., Pretagostini, R., Garofalo, M., Basile, A., Bellini, M. I., D’Andrea, V., Scaglione, M., Barr, R., & Cantisani, V. (2022). Contrast Enhanced Ultrasound Compared with MRI and CT in the Evaluation of Post-Renal Transplant Complications. Tomography, 8(4), 1704-1715. https://doi.org/10.3390/tomography8040143









