Reclassification of Heart Failure with Preserved Ejection Fraction Following Cardiac Sympathetic Nervous System Activation: A New Cutoff Value of 58%
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Cardiac 123I-MIBG Scintigraphy
2.3. Statistical Analysis
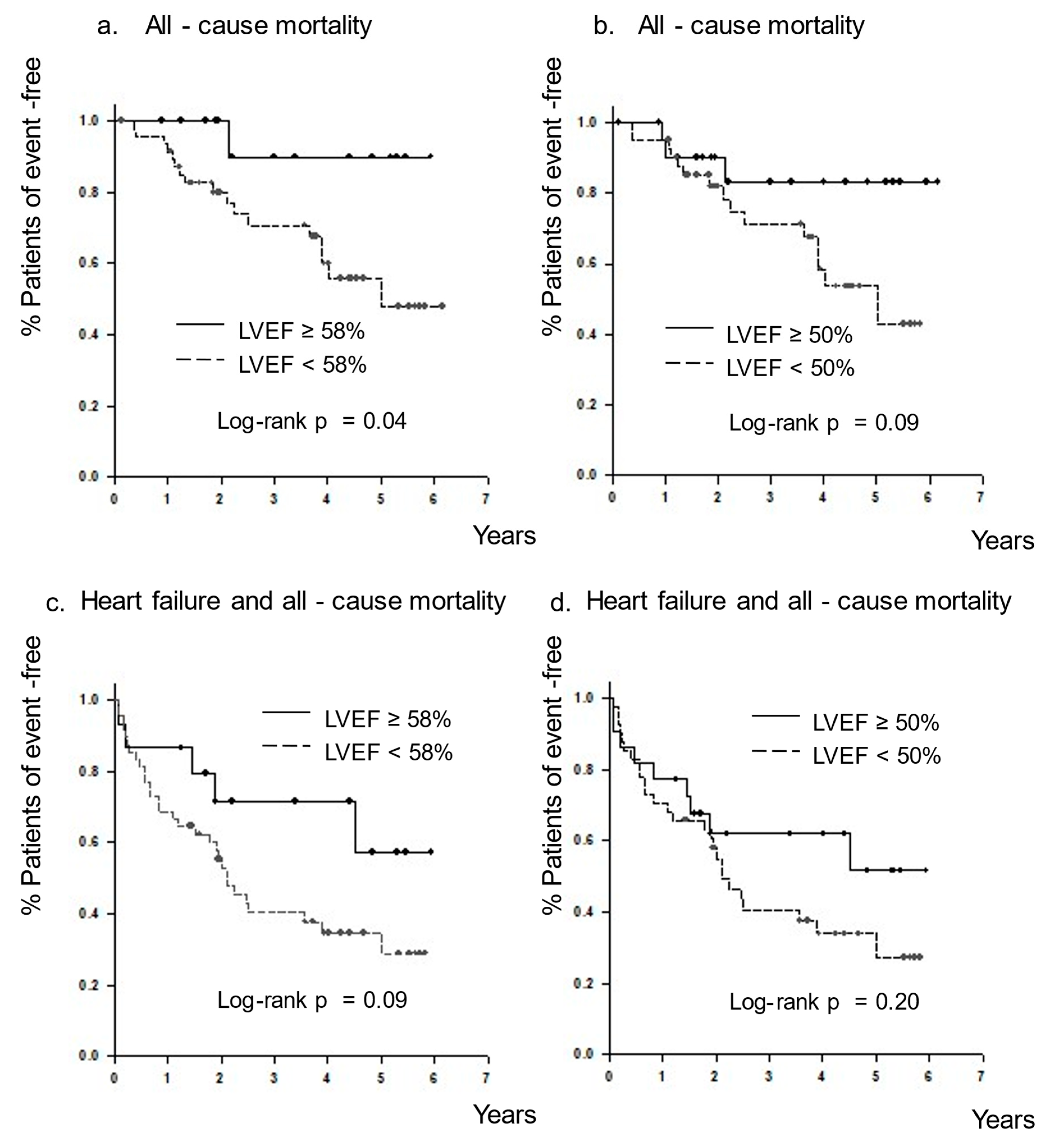
3. Results
3.1. Clinical Characteristics of Study Patients
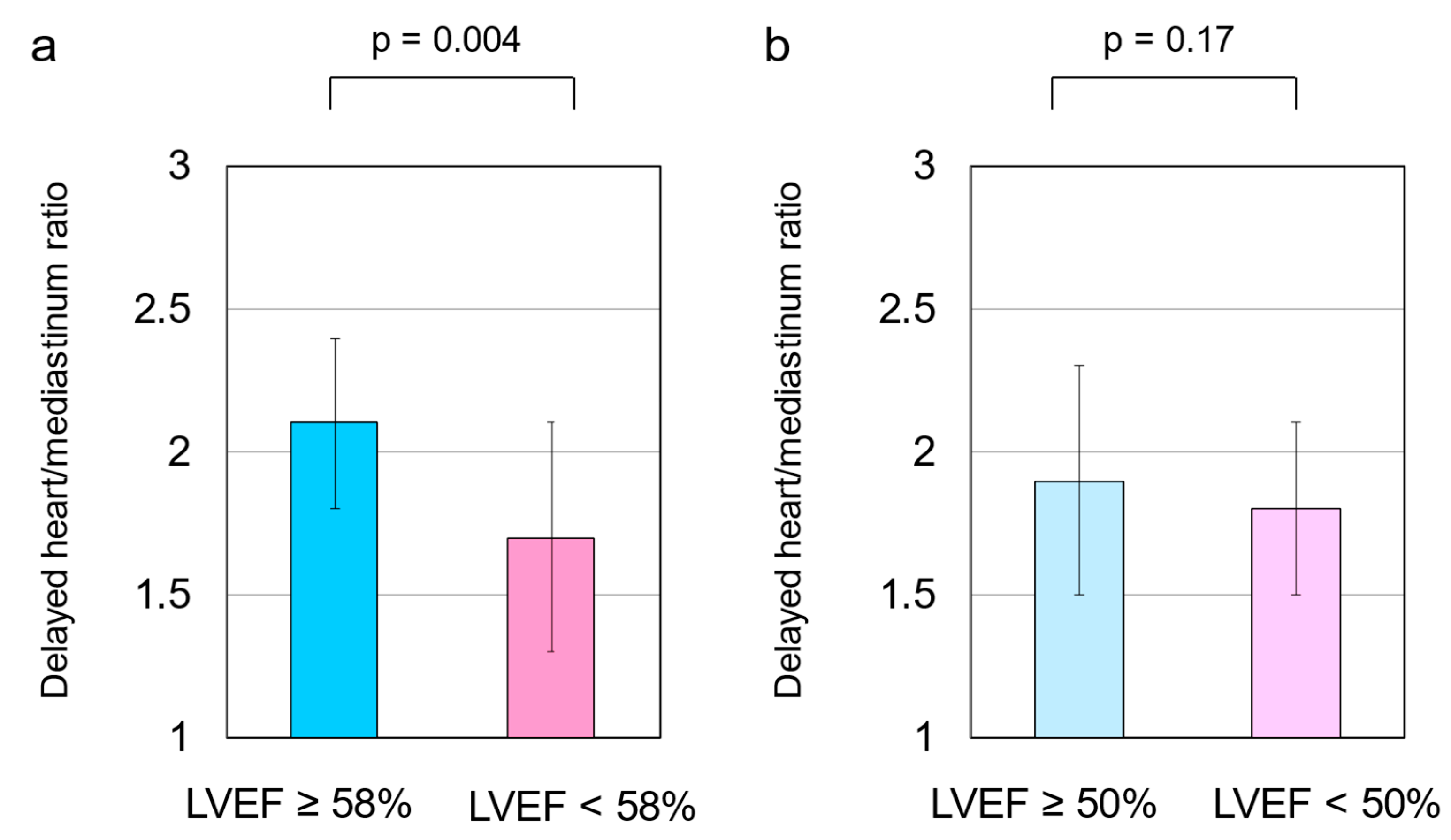
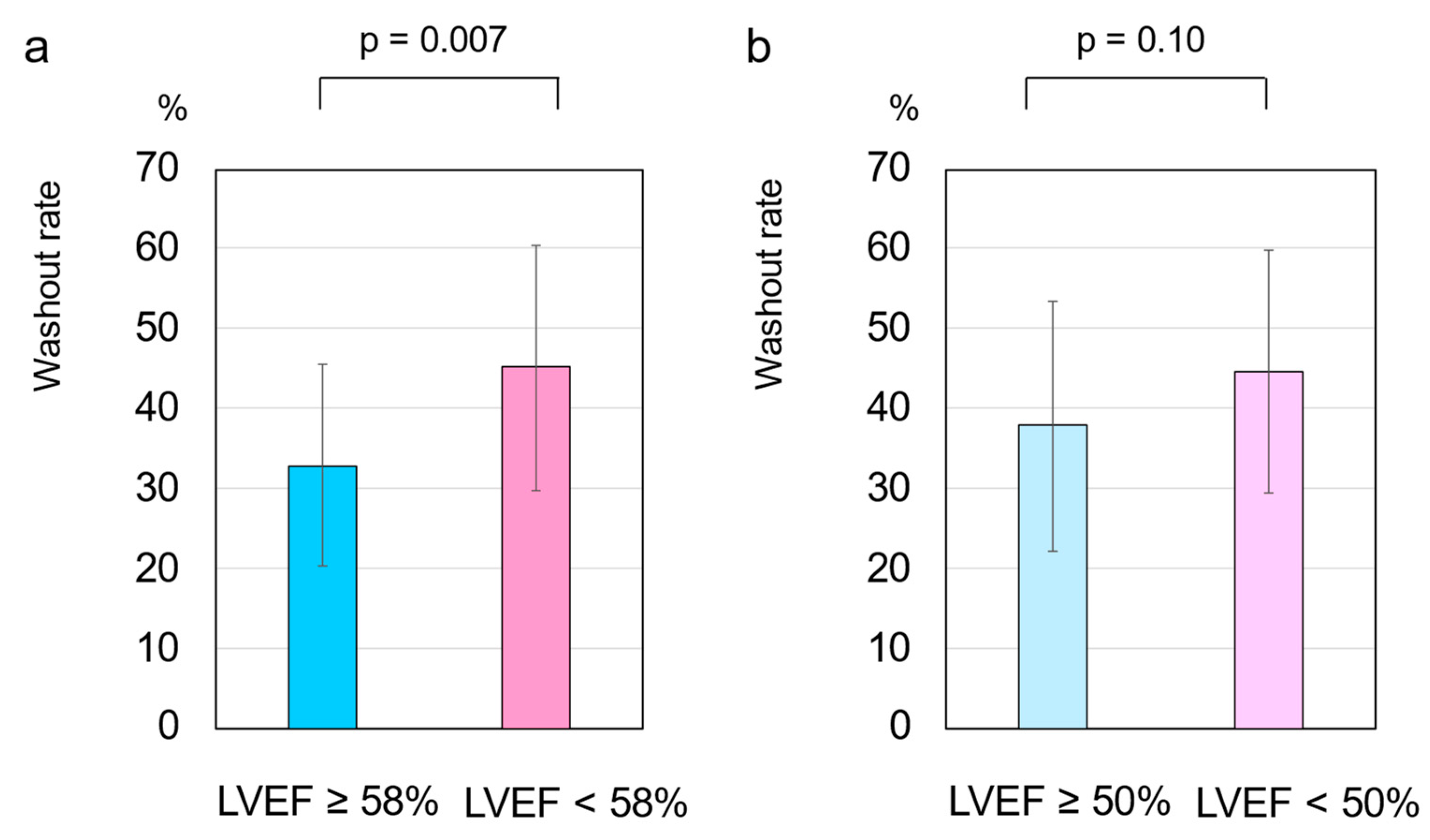
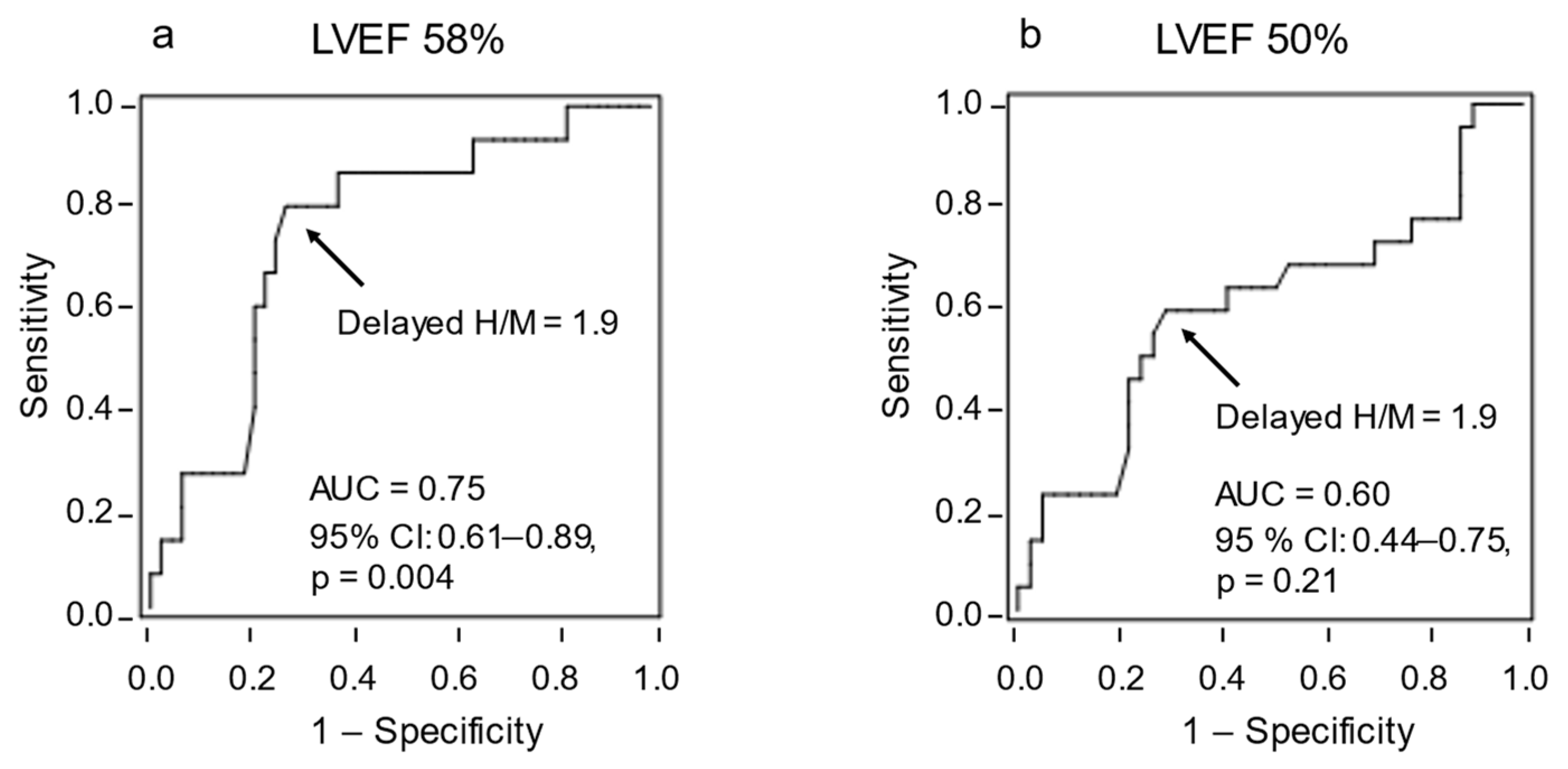
3.2. HF Cutoff Value of LVEF 58%
3.3. HF Cutoff Value of LVEF 50%
3.4. Medication
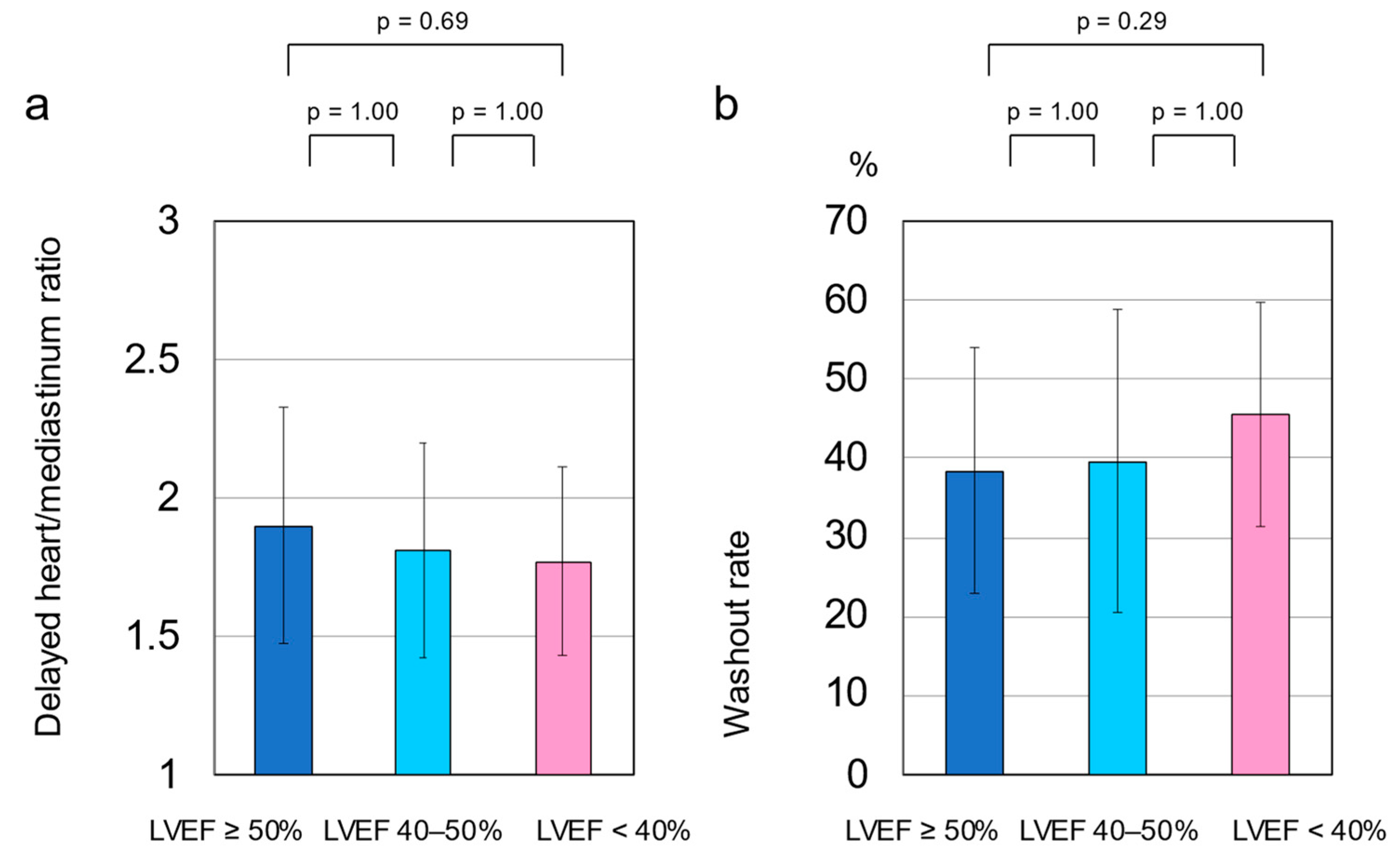
3.5. Cardiac SNS Activity
3.6. Differences in LVEF and Cardiac SNS Activity by Gender
3.7. All-Cause Mortality and the Combined Endpoint
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Schrier, R.W.; Abraham, W.T. Hormones and hemodynamics in heart failure. N. Engl. J. Med. 1999, 341, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Carrio, I. Cardiac neurotransmission imaging. J. Nucl. Med. 2001, 42, 1062–1076. [Google Scholar] [PubMed]
- Carrio, I.; Cowie, M.R.; Yamazaki, J.; Udelson, J.; Camici, P.G. Cardiac sympathetic imaging with mIBG in heart failure. JACC Cardiovasc. Imaging 2010, 3, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Manger, W.M.; Hoffman, B.B. Heart imaging in the diagnosis of pheochromocytoma and assessment of catecholamine uptake. J. Nucl. Med. 1983, 24, 1194–1196. [Google Scholar]
- Wieland, D.M.; Wu, J.; Brown, L.E.; Mangner, T.J.; Swanson, D.P.; Beierwaltes, W.H. Radiolabeled adrenergi neuron-blocking agents: Adrenomedullary imaging with [131I]iodobenzylguanidine. J. Nucl. Med. 1980, 21, 349–353. [Google Scholar]
- Jacobson, A.F.; Senior, R.; Cerqueira, M.D.; Wong, N.D.; Thomas, G.S.; Lopez, V.A.; Agostini, D.; Weiland, F.; Chandna, H.; Narula, J. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J. Am. Coll. Cardiol. 2010, 55, 2212–2221. [Google Scholar] [CrossRef]
- Nakajima, K.; Nakata, T.; Yamada, T.; Yamashina, S.; Momose, M.; Kasama, S.; Matsui, T.; Matsuo, S.; Travin, M.I.; Jacobson, A.F. A prediction model for 5-year cardiac mortality in patients with chronic heart failure using ¹²³I-metaiodobenzylguanidine imaging. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1673–1682. [Google Scholar] [CrossRef]
- Nakata, T.; Nakajima, K.; Yamashina, S.; Yamada, T.; Momose, M.; Kasama, S.; Matsui, T.; Matsuo, S.; Travin, M.I.; Jacobson, A.F. A pooled analysis of multicenter cohort studies of (123)I-mIBG imaging of sympathetic innervation for assessment of long-term prognosis in heart failure. JACC Cardiovasc. Imaging 2013, 6, 772–784. [Google Scholar] [CrossRef]
- Seo, M.; Yamada, T.; Tamaki, S.; Watanabe, T.; Morita, T.; Furukawa, Y.; Kawasaki, M.; Kikuchi, A.; Kawai, T.; Abe, M.; et al. Prognostic significance of cardiac I-123-metaiodobenzylguanidine imaging in patients with reduced, mid-range, and preserved left ventricular ejection fraction admitted for acute decompensated heart failure: A prospective study in Osaka Prefectural Acute Heart Failure Registry (OPAR). Eur. Heart J. Cardiovasc. Imaging 2020, 22, 58–66. [Google Scholar] [CrossRef]
- Goto, T.; Wakami, K.; Fukuta, H.; Fujita, H.; Tani, T.; Ohte, N. Patients with left ventricular ejection fraction greater than 58% have fewer incidences of future acute decompensated heart failure admission and all-cause mortality. Heart Vessels 2016, 31, 734–743. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoshida, T.; Ohte, N.; Narita, H.; Sakata, S.; Wakami, K.; Asada, K.; Miyabe, H.; Saeki, T.; Kimura, G. Lack of inertia force of late systolic aortic flow is a cause of left ventricular isolated diastolic dysfunction in patients with coronary artery disease. J. Am. Coll. Cardiol. 2006, 48, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, M.; Uchida, K.; Kondoh, Y.; Magosaki, N.; Niki, K.; Jones, C.J.; Sugimachi, M.; Sunagawa, K. Aortic blood momentum--the more the better for the ejecting heart in vivo? Cardiovasc. Res. 1997, 33, 433–446. [Google Scholar] [CrossRef]
- McKee, P.A.; Castelli, W.P.; McNamara, P.M.; Kannel, W.B. The natural history of congestive heart failure: The Framingham study. N. Engl. J. Med. 1971, 285, 1441–1446. [Google Scholar] [CrossRef]
- Senni, M.; Paulus, W.J.; Gavazzi, A.; Fraser, A.G.; Díez, J.; Solomon, S.D.; Smiseth, O.A.; Guazzi, M.; Lam, C.S.; Maggioni, A.P.; et al. New strategies for heart failure with preserved ejection fraction: The importance of targeted therapies for heart failure phenotypes. Eur. Heart J. 2014, 35, 2797–2815. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Hay, I.; Fetics, B.; Kass, D.A. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: Implications for systolic and diastolic reserve limitations. Circulation 2003, 107, 714–720. [Google Scholar] [CrossRef]
- Bavishi, C.; Chatterjee, S.; Ather, S.; Patel, D.; Messerli, F.H. Beta-blockers in heart failure with preserved ejection fraction: A meta-analysis. Heart Fail. Rev. 2015, 20, 193–201. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Y.; Feng, X.; Teng, Z.; Yuan, Y.; Bin, J. Effects of beta-blockers on heart failure with preserved ejection fraction: A meta-analysis. PLoS ONE 2014, 9, e90555. [Google Scholar] [CrossRef]
- Silverman, D.N.; Plante, T.B.; Infeld, M.; Callas, P.W.; Juraschek, S.P.; Dougherty, G.B.; Meyer, M. Association of β-Blocker Use with Heart Failure Hospitalizations and Cardiovascular Disease Mortality Among Patients with Heart Failure with a Preserved Ejection Fraction: A Secondary Analysis of the TOPCAT Trial. JAMA Netw. Open 2019, 2, e1916598. [Google Scholar] [CrossRef]
- Yamamoto, K.; Origasa, H.; Hori, M. Effects of carvedilol on heart failure with preserved ejection fraction: The Japanese Diastolic Heart Failure Study (J-DHF). Eur. J. Heart Fail. 2013, 15, 110–118. [Google Scholar] [CrossRef]
- Brubaker, P.H.; Kitzman, D.W. Chronotropic incompetence: Causes, consequences, and management. Circulation 2011, 123, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Ellestad, M.H.; Wan, M.K. Predictive implications of stress testing. Follow-up of 2700 subjects after maximum treadmill stress testing. Circulation 1975, 51, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.P.; Santema, B.T.; Voors, A.A. Atrial Fibrillation in Heart Failure: A Common and Deadly Combination. JACC Heart Fail. 2017, 5, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Goto, T.; Yamamoto, J.; Muto, K.; Kikuchi, S.; Wakami, K.; Fukuta, H.; Ohte, N. Compensatory Increase in Heart Rate Is Responsible for Exercise Tolerance among Male Patients with Permanent Atrial Fibrillation. Tohoku J. Exp. Med. 2018, 246, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, I.C.; Groenveld, H.F.; Crijns, H.J.; Tuininga, Y.S.; Tijssen, J.G.; Alings, A.M.; Hillege, H.L.; Bergsma-Kadijk, J.A.; Cornel, J.H.; Kamp, O.; et al. Lenient versus strict rate control in patients with atrial fibrillation. N. Engl. J. Med. 2010, 362, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Fukuta, H.; Goto, T.; Wakami, K.; Kamiya, T.; Ohte, N. Effect of beta-blockers on heart failure severity in patients with heart failure with preserved ejection fraction: A meta-analysis of randomized controlled trials. Heart Fail. Rev. 2020, 26, 165–171. [Google Scholar] [CrossRef]
| Characteristic | All Patients | LVEF ≥ 58% | LVEF < 58% | p-Value | LVEF ≥ 50% | LVEF < 50% | p-Value |
|---|---|---|---|---|---|---|---|
| Number (male %) | 63 (49.2) | 15 (46.7) | 48 (50) | 0.82 | 22 (45.5) | 41 (51.2) | 0.66 |
| Age (years) | 78.4 ± 9.6 | 82.5 ± 9.8 | 77.1 ± 9.3 | 0.06 | 79.8 ± 10.5 | 77.7 ± 9.2 | 0.40 |
| Height (cm) | 155.3 ± 9.1 | 155.9 ± 11.7 | 155.0 ± 8.2 | 0.75 | 155.2 ± 11.1 | 155.3 ± 7.9 | 0.96 |
| Weight (kg) | 52.0 ± 11.0 | 53.5 ± 11.3 | 51.6 ± 10.9 | 0.55 | 52.0 ± 11.4 | 52.0 ± 10.9 | 1.00 |
| Body mass index (kg/m2) | 21.5 ± 3.8 | 22.0 ± 4.1 | 21.3 ± 3.7 | 0.56 | 21.5 ± 3.8 | 21.5 ± 3.8 | 0.99 |
| NYHA class | 2.6 ± 0.7 | 2.4 ± 0.7 | 2.6 ± 0.7 | 0.35 | 2.5 ± 0.8 | 2.6 ± 0.7 | 0.66 |
| Systolic BP (mm Hg) | 114.0 ± 16.7 | 121.3 ± 13.1 | 111.7 ± 117.1 | 0.051 | 118.4 ± 14.0 | 111.6 ± 17.6 | 0.12 |
| Diastolic BP (mm Hg) | 65.8 ± 11.2 | 65.1 ± 11.5 | 66.0 ± 11.3 | 0.79 | 64.7 ± 10.7 | 66.3 ± 11.6 | 0.58 |
| Heart rate (beats/min) | 68.7 ± 11.0 | 63.9 ± 13.1 | 70.3 ± 10.0 | 0.050 | 66.1 ± 11.9 | 70.2 ± 10.4 | 0.17 |
| LVEF (%) | 44.6 ± 17.9 | 70.4 ± 8.3 | 36.6 ± 11.1 | <0.001 | 65.4 ± 10.2 | 33.5 ± 8.9 | <0.001 |
| BNP (mg/dL) | 310.9 [185.2–560.0] | 204.2 [144.6–313.0] | 326.2 [185.9–618.6] | 0.10 | 196.4 [105.5–318.0] | 342.6 [191.8–654.8] | 0.01 |
| HbA1c (%) | 6.3 ± 0.8 | 6.0 ± 0.6 | 6.4 ± 0.8 | 0.20 | 6.2 ± 0.9 | 6.3 ± 0.8 | 0.48 |
| Serum creatinine (mg/dL) | 1.1 ± 0.8 | 1.1 ± 0.5 | 1.1 ± 0.8 | 0.76 | 1.0 ± 0.5 | 1.2 ± 0.9 | 0.34 |
| eGFR (mL/min/1.73 m2) | 54.3 ± 24.6 | 51.1 ± 17.2 | 55.3 ± 26.6 | 0.57 | 55.7 ± 20.0 | 53.5 ± 26.9 | 0.74 |
| Hemoglobin (g/dL) | 12.2 ± 2.0 | 11.7 ± 1.9 | 12.3 ± 2.1 | 0.32 | 11.8 ± 2.0 | 12.4 ± 2.1 | 0.22 |
| Sodium (mEq/L) | 140.0 ± 2.6 | 140.8 ± 1.5 | 139.7 ± 2.9 | 0.17 | 140.5 ± 2.2 | 139.7 ± 2.9 | 0.31 |
| Characteristic | All Patients | LVEF ≥ 58% | LVEF < 58% | p-Value | LVEF ≥ 50% | LVEF < 50% | p-Value |
|---|---|---|---|---|---|---|---|
| Etiology | |||||||
| Ischemic CM (n, %) | 12 (19.0) | 0 (0) | 12 (25) | 0.03 | 2 (9.1) | 10 (24.4) | 0.14 |
| Non-ischemic CM (n, %) | 31 (49.2) | 4 (26.7) | 27 (56.3) | 0.045 | 6 (27.3) | 25 (61.0) | 0.01 |
| Hypertensive (n, %) | 3 (4.8) | 2 (13.3) | 1 (2.1) | 0.07 | 3 (13.6) | 0 (0) | 0.02 |
| Valvular (n, %) | 5 (7.9) | 2 (13.3) | 3 (6.3) | 0.38 | 2 (9.1) | 3 (7.3) | 0.80 |
| Abnormal heart rhythms (n, %) | 12 (19.0) | 7 (46.7) | 5 (10.4) | 0.002 | 9 (40.9) | 3 (7.3) | 0.001 |
| Co-morbidity | |||||||
| Hypertension (n, %) | 27 (42.9) | 8 (53.3) | 19 (39.6) | 0.35 | 13(59.1) | 14 (34.1) | 0.06 |
| Diabetes mellitus (n, %) | 17 (27.0) | 3 (20) | 14 (29.2) | 0.49 | 5 (22.7) | 12 (29.3) | 0.58 |
| Prior heart failure (n, %) | 24 (38.1) | 2 (13.3) | 22 (45.8) | 0.02 | 5 (22.7) | 19 (46.3) | 0.07 |
| Atrial fibrillation (n, %) | 38 (76.2) | 13 (86.7) | 25 (52.1) | 0.02 | 18 (81.8) | 20 (48.8) | 0.01 |
| Characteristic | All Patients | LVEF ≥ 58% | LVEF < 58% | p-Value | LVEF ≥ 50% | LVEF < 50% | p-Value |
|---|---|---|---|---|---|---|---|
| Anti-platelet (%) | 28.6 | 20.0 | 31.2 | 0.40 | 18.2 | 34.1 | 0.18 |
| Anti-coagulants (%) | 57.1 | 80 | 50 | 0.04 | 77.2 | 46.3 | 0.02 |
| Diuretics (%) | 69.8 | 53.3 | 75.0 | 0.11 | 59.1 | 75.6 | 0.17 |
| Statins (%) | 25.4 | 26.7 | 25.0 | 0.90 | 18.2 | 29.3 | 0.34 |
| ACEIs (%) | 39.7 | 13.3 | 47.9 | 0.02 | 22.7 | 48.8 | 0.04 |
| ARBs (%) | 22.2 | 20.0 | 22.9 | 0.81 | 22.7 | 22.0 | 0.94 |
| AAs (%) | 39.7 | 33.3 | 41.7 | 0.57 | 27.3 | 46.3 | 0.14 |
| β-blockers (%) | 66.6 | 53.3 | 70.8 | 0.21 | 50.0 | 75.6 | 0.04 |
| Bisoprolol (%, mg) | 30.2 | 26.7 (0.7 ± 1.4) | 31.3 (0.6 ± 1.2) | 0.83 | 31.8 (0.7 ± 1.3) | 29.3 (0.6 ± 1.2) | 0.82 |
| Carvedilol (%, mg) | 38.1 | 33.3 (1.5 ± 2.8) | 39.6 (2.3 ± 4.0) | 0.46 | 22.7 (1.2 ± 1.4) | 46.3 (2.7 ± 4.2) | 0.09 |
| CCBs (%) | 23.8 | 40.0 | 18.8 | 0.09 | 40.9 | 14.6 | 0.02 |
| LVEF ≥ 58% | LVEF < 58% | |||||
| Male (n = 7) | Female (n = 8) | p-Value | Male (n = 24) | Female (n = 24) | p-Value | |
| LVEF (%) | 69.9 ± 9.5 | 70.8 ± 7.7 | 0.85 | 34.9 ± 11.4 | 38.3 ± 10.8 | 0.29 |
| Delayed H/M | 2.1 ± 0.2 | 2.1 ± 0.5 | 0.90 | 1.8 ± 0.4 | 1.7 ± 0.3 | 0.41 |
| WR (%) | 29.3 ± 4.4 | 36.1 ± 16.6 | 0.32 | 41.6 ± 15.0 | 48.6 ± 15.3 | 0.11 |
| LVEF ≥ 50% | LVEF < 50% | |||||
| Male (n = 10) | Female (n = 12) | p-value | Male (n = 21) | Female (n = 20) | p-value | |
| LVEF (%) | 65.6 ± 10.4 | 65.1 ± 10.5 | 0.91 | 31.9 ± 8.6 | 35.2 ± 9.0 | 0.24 |
| Delayed H/M | 2.0 ± 0.4 | 1.9 ± 0.5 | 0.57 | 1.8 ± 0.3 | 1.7 ± 0.4 | 0.64 |
| WR (%) | 30.8 ± 8.9 | 43.6 ± 18.1 | 0.06 | 42.6 ± 14.9 | 46.6 ± 15.6 | 0.40 |
| Total Patients (n = 63) | LVEF ≥ 58% (n = 15) | LVEF < 58% (n = 48) | LVEF ≥ 50% (n = 22) | LVEF < 50% (n = 41) | |
|---|---|---|---|---|---|
| Hospitalization due to heart failure | 28 | 5 | 23 | 9 | 19 |
| All-cause mortality | 18 | 1 | 17 | 3 | 15 |
| Cardiac death | 10 | 0 | 10 | 1 | 9 |
| Non-cardiac death | 8 | 1 | 7 | 2 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goto, T.; Nakayama, T.; Yamamoto, J.; Mori, K.; Shintani, Y.; Kikuchi, S.; Fujita, H.; Fukuta, H.; Seo, Y. Reclassification of Heart Failure with Preserved Ejection Fraction Following Cardiac Sympathetic Nervous System Activation: A New Cutoff Value of 58%. Tomography 2022, 8, 1595-1607. https://doi.org/10.3390/tomography8030132
Goto T, Nakayama T, Yamamoto J, Mori K, Shintani Y, Kikuchi S, Fujita H, Fukuta H, Seo Y. Reclassification of Heart Failure with Preserved Ejection Fraction Following Cardiac Sympathetic Nervous System Activation: A New Cutoff Value of 58%. Tomography. 2022; 8(3):1595-1607. https://doi.org/10.3390/tomography8030132
Chicago/Turabian StyleGoto, Toshihiko, Takafumi Nakayama, Junki Yamamoto, Kento Mori, Yasuhiro Shintani, Shohei Kikuchi, Hiroshi Fujita, Hidekatsu Fukuta, and Yoshihiro Seo. 2022. "Reclassification of Heart Failure with Preserved Ejection Fraction Following Cardiac Sympathetic Nervous System Activation: A New Cutoff Value of 58%" Tomography 8, no. 3: 1595-1607. https://doi.org/10.3390/tomography8030132
APA StyleGoto, T., Nakayama, T., Yamamoto, J., Mori, K., Shintani, Y., Kikuchi, S., Fujita, H., Fukuta, H., & Seo, Y. (2022). Reclassification of Heart Failure with Preserved Ejection Fraction Following Cardiac Sympathetic Nervous System Activation: A New Cutoff Value of 58%. Tomography, 8(3), 1595-1607. https://doi.org/10.3390/tomography8030132






