Alert Germ Infections: Chest X-ray and CT Findings in Hospitalized Patients Affected by Multidrug-Resistant Acinetobacter baumannii Pneumonia
Abstract
1. Introduction
2. Methods and Materials
2.1. Patients
2.2. Chest Radiographs
2.3. CT Scans
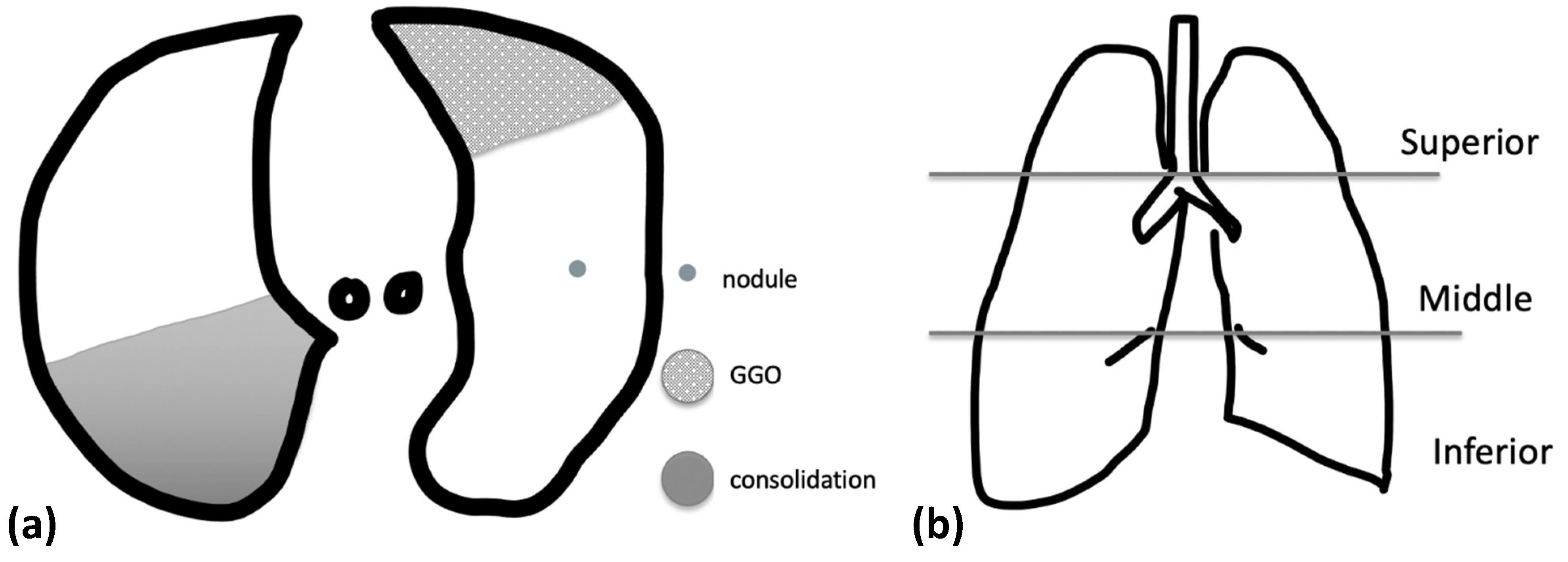
2.4. Imaging Evaluation
2.5. Statistical Analysis
3. Results
3.1. Clinical Features
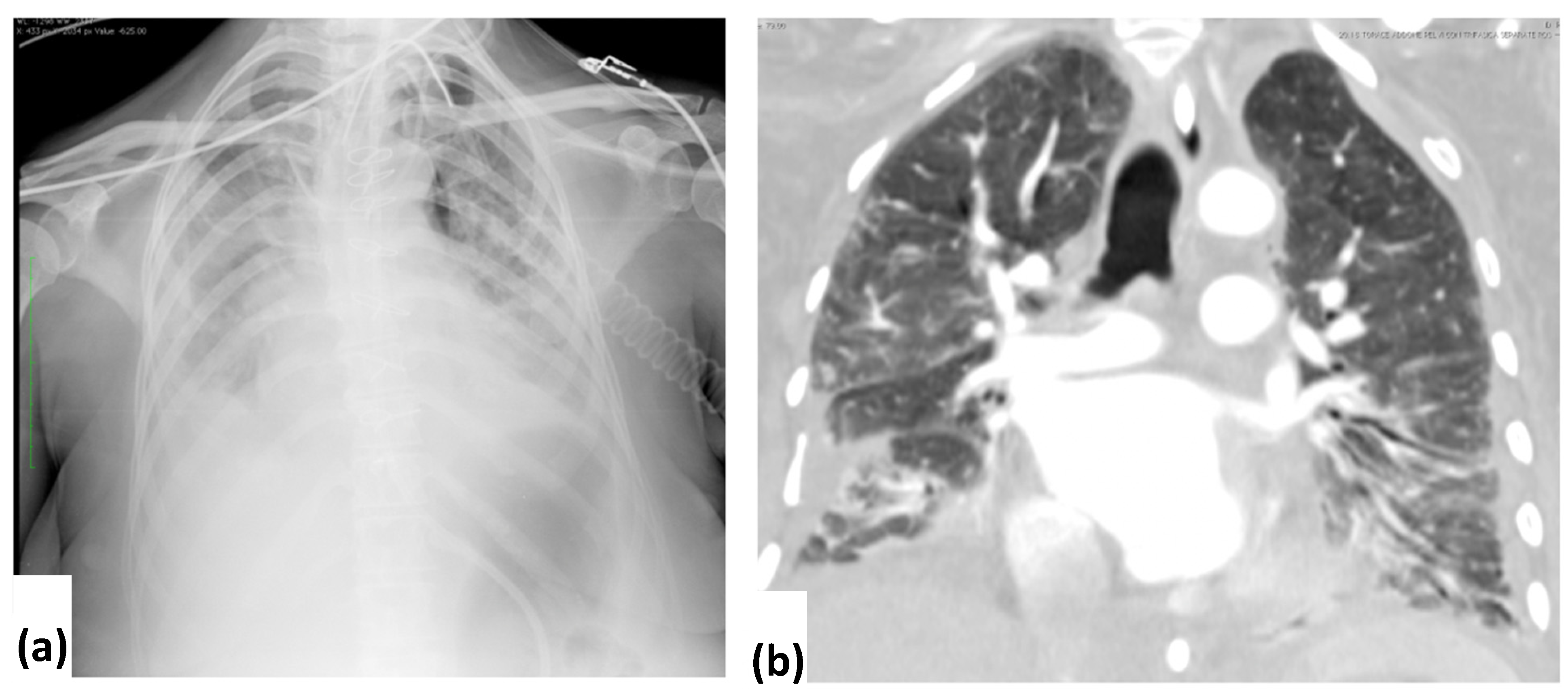
3.2. CXR Findings and Disease Distribution
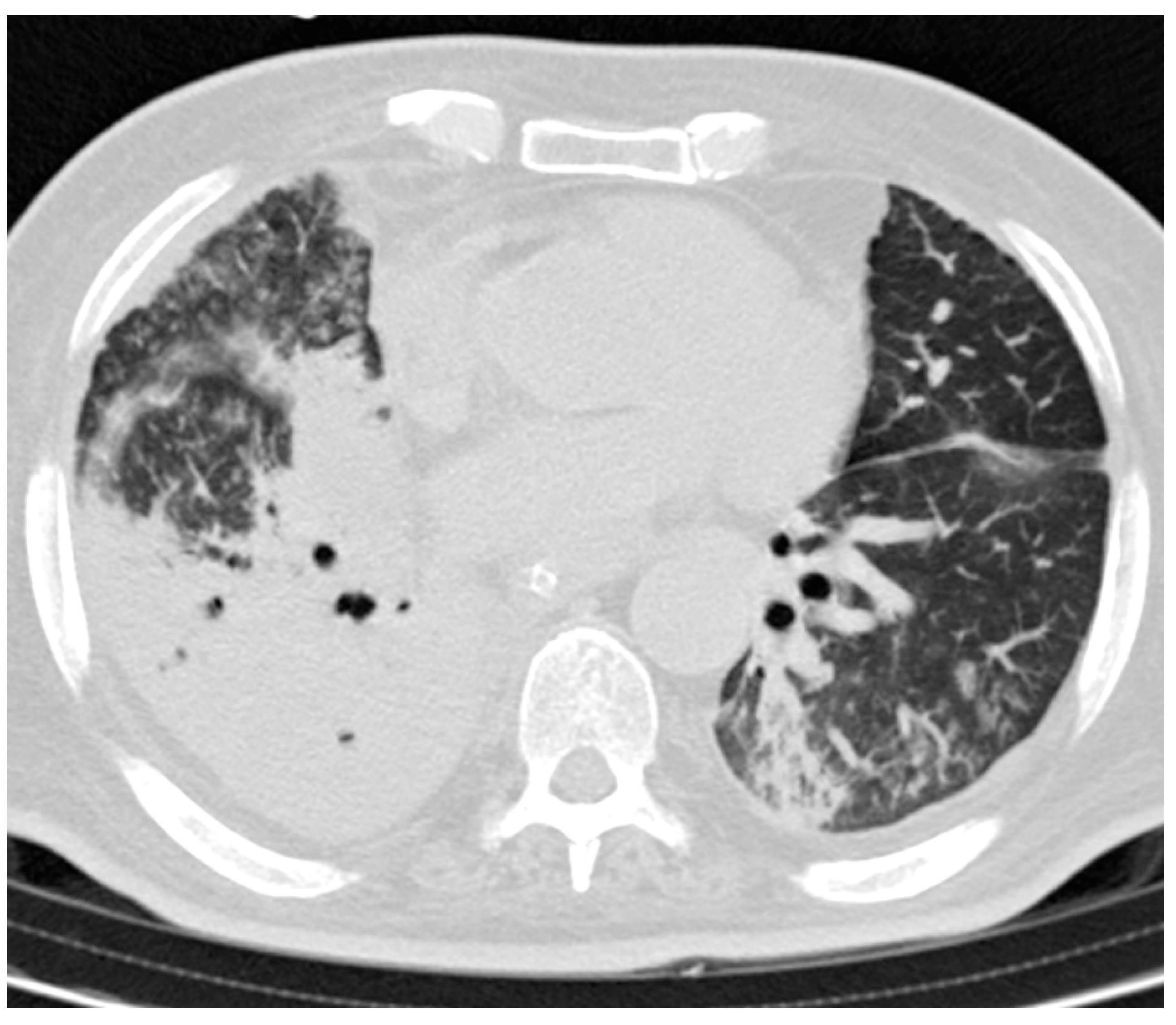
3.3. CT Findings and Disease Distribution
3.4. Statistical Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paluchowska, P.; Skałkowska, M.; Spelak, A.; Budak, A. Wystepowanie patogenów alarmowych w środowisku szpitalnym. Cześć II. Wielolekooporne pałeczki niefermentujace [Occurrence of alert pathogens in hospital environment Part II. Multidrug-resistant non-fermenting bacilli]. Med. Dosw. Mikrobiol. 2012, 64, 45–53. (In Polish) [Google Scholar] [PubMed]
- Al-Anazi, K.A.; Al-Jasser, A.M. Infections Caused by Acinetobacter baumannii in Recipients of Hematopoietic Stem Cell Transplantation. Front. Oncol. 2014, 4, 186. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Zhu, H.; Gao, B.; Weng, H.; Ding, Z.; Li, M.; Weng, X.; He, G. Diagnosis of severe community-acquired pneumonia caused by Acinetobacter baumannii through next-generation sequencing: A case report. BMC Infect. Dis. 2020, 20, 45. [Google Scholar] [CrossRef] [PubMed]
- Hartzell, J.D.; Kim, A.S.; Kortepeter, M.G.; Moran, A.K. Acinetobacter pneumonia: A review. MedGenMed 2007, 9, 4. [Google Scholar]
- Howard, A.; O’Donoghue, M.; Feeney, A.; Sleator, R.D. Acinetobacter baumannii. Virulence 2012, 3, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.E. Emerging gram-negative antibiotic resistance: Daunting challenges, declining sensitivities, and dire consequences. Respir. Care 2008, 53, 471–479. [Google Scholar]
- Koenig, S.M.; Truwit, J.D. Ventilator-Associated Pneumonia: Diagnosis, Treatment, and Prevention. Clin. Microbiol. Rev. 2006, 19, 637–657. [Google Scholar] [CrossRef]
- Yang, Y.-S.; Lee, Y.-T.; Huang, T.-W.; Sun, J.-R.; Kuo, S.-C.; Yang, C.-H.; Chen, T.-L.; Lin, J.-C.; Fung, C.-P.; Chang, F.-Y. Acinetobacter baumannii nosocomial pneumonia: Is the outcome more favorable in non-ventilated than ventilated patients? BMC Infect. Dis. 2013, 13, 142. [Google Scholar] [CrossRef]
- Rungruanghiranya, S.; Somboonwit, C.; Kanchanapoom, T. Acinetobacter Infection in the Intensive Care Unit. J. Infect. Dis. Antimicrob. Agents 2005, 22, 77–92. [Google Scholar]
- Chowdhury, T.; Mainali, A.; Bellamkonda, A.; Gousy, N. Acinetobacter: A Rare Cause of Rapid Development of Cavitary Lung Lesion Following COVID-19 Infection. Cureus 2022, 14, e24366. [Google Scholar] [CrossRef] [PubMed]
- Rangel, K.; Chagas, T.; De-Simone, S. Acinetobacter baumannii Infections in Times of COVID-19 Pandemic. Pathogens 2021, 10, 1006. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, C.; Krishna, S.; Shenoy, A.; Prakashini, K. Clinical, radiological and microbiological corroboration to assess the role of endotracheal aspirate in diagnosing ventilator-associated pneumonia in an intensive care unit of a tertiary care hospital, India. Int. J. Infect. Control. 2009, 6. [Google Scholar] [CrossRef]
- Franquet, T. Imaging of pneumonia: Trends and algorithms. Eur. Respir. J. 2001, 18, 196–208. [Google Scholar] [CrossRef]
- Sharma, S.; Maycher, B.; Eschun, G. Radiological imaging in pneumonia: Recent innovations. Curr. Opin. Pulm. Med. 2007, 13, 159–169. [Google Scholar] [CrossRef]
- Werarak, P.; Waiwarawut, J.; Tharavichitkul, P.; Pothirat, C.; Rungruanghiranya, S.; Geater, S.L.; Chongthaleong, A.; Sittipunt, C.; Horsin, P.; Chalermskulrat, W.; et al. Acinetobacter baumannii nosocomial pneumonia in tertiary care hospitals in Thailand. J. Med. Assoc. Thai. 2012, 95 (Suppl. 2), S23–S33. [Google Scholar]
- Haroon, A.; Higa, F.; Fujita, J.; Watanabe, A.; Aoki, N.; Niki, Y.; Kadota, J.-I.; Yanagihara, K.; Kaku, M.; Hori, S.; et al. Pulmonary Computed Tomography Findings in 39 Cases of Streptococcus pneumoniae Pneumonia. Intern. Med. 2012, 51, 3343–3349. [Google Scholar] [CrossRef][Green Version]
- Bouhemad, B.; Dransart-Rayé, O.; Mojoli, F.; Mongodi, S. Lung ultrasound for diagnosis and monitoring of ventilator-associated pneumonia. Ann. Transl. Med. 2018, 6, 418. [Google Scholar] [CrossRef]
- Tonnii, S.; Chua, H.H. Case series: Fulminant community-acquired Acinetobacter pneumonia. Med. J. Malaysia 2020, 75, 186–188. [Google Scholar]
- Lin, S.-Y.; Huang, Z.-H.; Chen, H.-C.; Chang, D.-M.; Lu, C.-C. Multidrug-resistance Acinetobacter baumannii pneumonia in a rheumatoid arthritis patient receiving tumor necrosis factor inhibitor. Medicine 2018, 97, e11730. [Google Scholar] [CrossRef]
- Chen, Y.M.; Fang, W.F.; Kao, H.C.; Chen, H.C.; Tsai, Y.C.; Shen, L.S.; Li, C.L.; Chang, H.C.; Huang, K.T.; Lin, M.C.; et al. Influencing factors of successful eradication of multidrug-resistant Acinetobacter baumannii in the respiratory tract with aerosolized colistin. Biomed. J. 2014, 37, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, A.; Kasiakou, S.K.; Mastora, Z.; Rellos, K.; Kapaskelis, A.M.; Falagas, M.E. Aerosolized colistin for the treatment of nosocomial pneumonia due to multidrug-resistant Gram-negative bacteria in patients without cystic fibrosis. Crit. Care 2005, 9, R53–R59. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, A.; Fotakis, D.; Virtzili, S.; Vletsas, C.; Raftopoulou, S.; Mastora, Z.; Falagas, M.E. Aerosolized colistin as adjunctive treatment of ventilator-associated pneumonia due to multidrug-resistant Gram-negative bacteria: A prospective study. Respir. Med. 2008, 102, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Ginocchio, C.C. Role of NCCLS in antimicrobial susceptibility testing and monitoring. Am. J. Health Syst. Pharm. 2002, 59 (Suppl. 3), S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.A.; Lin, Y.C.; Lu, P.L.; Chen, H.C.; Chang, H.L.; Sheu, C.C. Antibiotic strategies and clinical outcomes in critically ill patients with pneumonia caused by carbapenem-resistant Acinetobacter baumannii. Clin. Microbiol. Infect. 2018, 24, 908.e1–908.e7. [Google Scholar] [CrossRef] [PubMed]
- Larcher, R.; Pantel, A.; Arnaud, E.; Sotto, A.; Lavigne, J.-P. First report of cavitary pneumonia due to community-acquired Acinetobacter pittii, study of virulence and overview of pathogenesis and treatment. BMC Infect. Dis. 2017, 17, 477. [Google Scholar] [CrossRef]
- Chittawatanarat, K.; Jaipakdee, W.; Chotirosniramit, N.; Chandacham, K.; Jirapongcharoenlap, T. Microbiology, resistance patterns, and risk factors of mortality in ventilator-associated bacterial pneumonia in a Northern Thai tertiary-care university based general surgical intensive care unit. Infect. Drug Resist. 2014, 7, 203–210. [Google Scholar] [CrossRef]
| Number of Patients | |
|---|---|
| Patients | 94 |
| Male | 53 |
| Female | 41 |
| Mean age (years) | 64.2 |
| CXR | 94 |
| GGO | 27 |
| Consolidation | 17 |
| GGO and consolidation | 20 |
| Anatomic sides involved | |
| Monolateral | 31 |
| Bilateral | 33 |
| Pleural effusion | 45 |
| Bilateral | 24 |
| Involved zone | |
| Superior | 27 |
| Middle | 44 |
| Inferior | 49 |
| Score (mean ± SD) | 15.06 ± 18.29 |
| CT | 68 |
| GGO | 5 |
| Consolidation | 26 |
| GGO and consolidation | 32 |
| Anatomic sides involved | |
| Monolateral | 9 |
| Bilateral | 54 |
| Pleural effusion | 43 |
| Bilateral | 34 |
| Involved zone | |
| Superior zone | 47 |
| Middle zone | 56 |
| Inferior zone | 61 |
| Predominant distribution | |
| Peripheral | 40 |
| Central | 4 |
| Central and peripheral | 19 |
| Other findings | |
| Nodular opacities | 10 |
| Cavitation | 4 |
| Bronchial wall thickening | 5 |
| Air bronchogram | 40 |
| Interlobar septal thickening | 17 |
| Bronchiesctasis | 10 |
| Lymphadenopathies | 18 |
| Pneumotorax | 2 |
| Pneumomediastinum | 1 |
| Score (mean ± SD) | 21.88 ± 15.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capasso, R.; Pinto, A.; Serra, N.; Atripaldi, U.; Corcione, A.; Bocchini, G.; Guarino, S.; Lieto, R.; Rea, G.; Sica, G.; et al. Alert Germ Infections: Chest X-ray and CT Findings in Hospitalized Patients Affected by Multidrug-Resistant Acinetobacter baumannii Pneumonia. Tomography 2022, 8, 1534-1543. https://doi.org/10.3390/tomography8030126
Capasso R, Pinto A, Serra N, Atripaldi U, Corcione A, Bocchini G, Guarino S, Lieto R, Rea G, Sica G, et al. Alert Germ Infections: Chest X-ray and CT Findings in Hospitalized Patients Affected by Multidrug-Resistant Acinetobacter baumannii Pneumonia. Tomography. 2022; 8(3):1534-1543. https://doi.org/10.3390/tomography8030126
Chicago/Turabian StyleCapasso, Raffaella, Antonio Pinto, Nicola Serra, Umberto Atripaldi, Adele Corcione, Giorgio Bocchini, Salvatore Guarino, Roberta Lieto, Gaetano Rea, Giacomo Sica, and et al. 2022. "Alert Germ Infections: Chest X-ray and CT Findings in Hospitalized Patients Affected by Multidrug-Resistant Acinetobacter baumannii Pneumonia" Tomography 8, no. 3: 1534-1543. https://doi.org/10.3390/tomography8030126
APA StyleCapasso, R., Pinto, A., Serra, N., Atripaldi, U., Corcione, A., Bocchini, G., Guarino, S., Lieto, R., Rea, G., Sica, G., & Valente, T. (2022). Alert Germ Infections: Chest X-ray and CT Findings in Hospitalized Patients Affected by Multidrug-Resistant Acinetobacter baumannii Pneumonia. Tomography, 8(3), 1534-1543. https://doi.org/10.3390/tomography8030126







