Analysis of Spatial Heterogeneity of Responses in Metastatic Sites in Renal Cell Carcinoma Patients Treated with Nivolumab
Abstract
:1. Introduction
2. Materials
2.1. Patient Population
2.2. Imaging Examination
2.3. Analytical Statistics
3. Results
3.1. Patient Characteristics and Clinical Outcomes
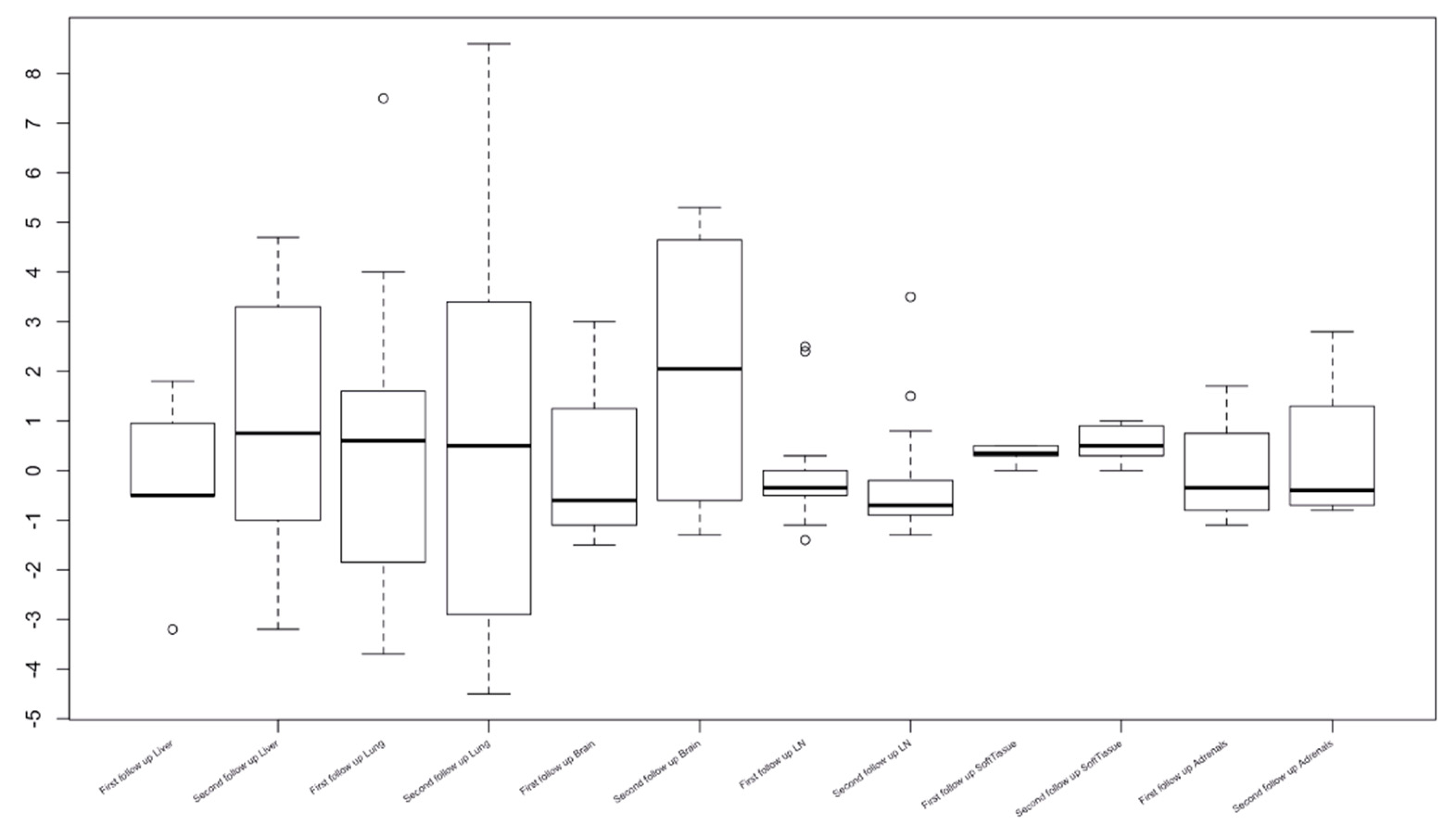
3.2. Organ-Specific Responses
3.3. Comparison of the RECIST 1.1 and iRECIST Criteria at the First Occurrence of Progression
3.4. Response Assessment by iRECIST and RECIST1.1
3.5. New Lesions during Nivolumab Therapy
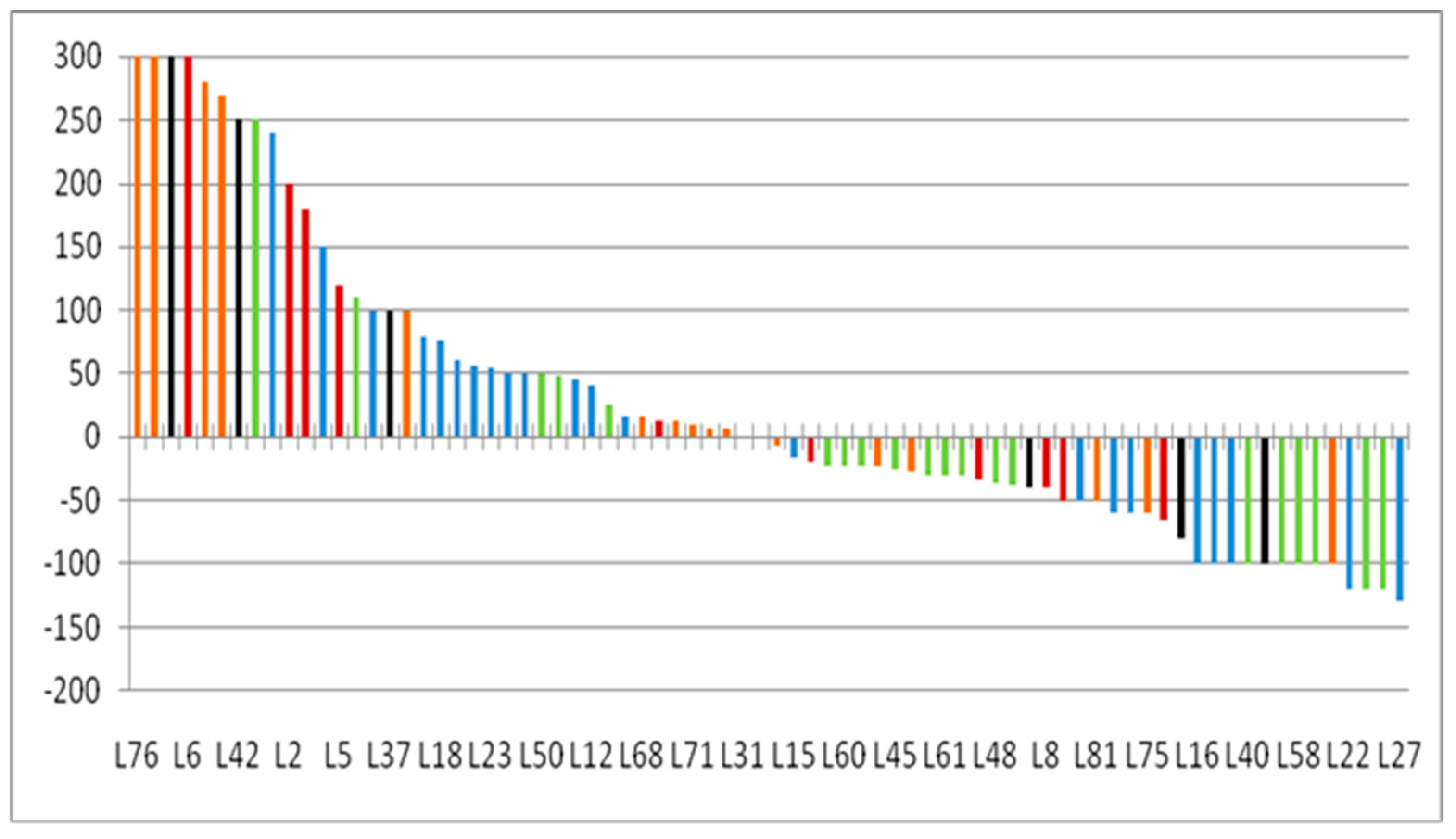
3.6. Lesion-Based Response Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CR | Complete remission |
| CT | Computed Tomography |
| RCC | Renal cell carcinoma |
| ORR | Objective response rate |
| OSRR | Organ-specific response rate |
| PD | Progressive disease |
| PD-L1 | Programmed death-ligand 1 |
| CPI | Check point inhibitors |
| LVNI | Lymphovascular neural invasion |
| ICPD | Immune-confirmed Progression |
| IUPD | Immune-unconfirmed Progression |
| LFU | Last follow up |
References
- Chow, W.-H.; Devesa, S.S.; Warren, J.L.; Fraumeni, J.J.F. Rising Incidence of Renal Cell Cancer in the United States. JAMA J. Am. Med. Assoc. 1999, 281, 1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, K.; Miller, J.D.; Li, J.Z.; Russell, M.W.; Charbonneau, C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): A literature review. Cancer Treat. Rev. 2008, 34, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Janzen, N.K.; Kim, H.L.; Figlin, R.A.; Belldegrun, A.S. Surveillance after Radical or Partial Nephrectomy for Localized Renal Cell Carcinoma and Management of Recurrent Disease. Urol. Clin. N. Am. 2003, 30, 843–852. [Google Scholar] [CrossRef]
- Fisher, R.; Gore, M.; Larkin, J. Current and Future Systemic Treatments for Renal Cell Carcinoma. Semin. Cancer Biol. 2013, 23, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Albiges, L.; Chamming’s, F.; Duclos, B.; Stern, M.; Motzer, R.J.; Ravaud, A.; Camus, P. Incidence and Management of MTOR Inhibitor-Associated Pneumonitis in Patients with Metastatic Renal Cell Carcinoma. Ann. Oncol. 2012, 23, 1943–1953. [Google Scholar] [CrossRef]
- Schmidinger, M. Understanding and Managing Toxicities of Vascular Endothelial Growth Factor (VEGF) Inhibitors. Eur. J. Cancer Suppl. 2013, 11, 172–191. [Google Scholar] [CrossRef] [Green Version]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef]
- Motzer, R.J.; Rini, B.I.; McDermott, D.F.; Redman, B.G.; Kuzel, T.M.; Harrison, M.R.; Vaishampayan, U.; Drabkin, H.A.; George, S.; Logan, T.F.; et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J. Clin. Oncol. 2015, 33, 1430–1437. [Google Scholar] [CrossRef]
- McDermott, D.F.; Drake, C.G.; Sznol, M.; Choueiri, T.K.; Powderly, J.D.; Smith, D.; Brahmer, J.R.; Carvajal, R.D.; Hammers, H.J.; Puzanov, I.; et al. Survival, Durable Response, and Long-Term Safety in Patients With Previously Treated Advanced Renal Cell Carcinoma Receiving Nivolumab. J. Clin. Oncol. 2015, 33, 2013–2020. [Google Scholar] [CrossRef]
- McDermott, D.F.; Rini, B.I.; Motzer, R.J.; Tannir, N.M.; Escudier, B.; Kollmannsberger, C.K.; Hammers, H.J.; Porta, C.; George, S.; Donskov, F.; et al. Treatment-free survival (TFS) after discontinuation of first-line nivolumab (NIVO) plus ipilimumab (IPI) or sunitinib (SUN) in intention-to-treat (ITT) and IMDC favorable-risk patients (pts) with advanced renal cell carcinoma (aRCC) from CheckMate 214. J. Clin. Oncol. 2019, 37, 564. [Google Scholar] [CrossRef]
- Carrasco, J.; Van Pel, A.; Neyns, B.; Lethé, B.; Brasseur, F.; Renkvist, N.; Van Der Bruggen, P.; Van Baren, N.; Paulus, R.; Thielemans, K.; et al. Vaccination of a Melanoma Patient with Mature Dendritic Cells Pulsed with MAGE-3 Peptides Triggers the Activity of Nonvaccine Anti-Tumor Cells. J. Immunol. 2008, 180, 3585–3593. [Google Scholar] [CrossRef] [PubMed]
- Bol, K.F.; Figdor, C.G.; Aarntzen, E.H.; Welzen, M.E.; van Rossum, M.M.; Blokx, W.A.; van de Rakt, M.W.; Scharenborg, N.M.; de Boer, A.J.; Pots, J.M.; et al. Intranodal Vaccination with MRNA-Optimized Dendritic Cells in Metastatic Melanoma Patients. Oncoimmunology 2015, 4, e1019197. [Google Scholar] [CrossRef] [PubMed]
- Koelzer, V.H.; Rothschild, S.I.; Zihler, D.; Wicki, A.; Willi, B.; Willi, N.; Voegeli, M.; Cathomas, G.; Zippelius, A.; Mertz, K.D. Systemic inflammation in a melanoma patient treated with immune checkpoint inhibitors—an autopsy study. J. Immunother. Cancer 2016, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Hodi, F.S.; Oble, D.A.; Drappatz, J.; Velazquez, E.F.; Ramaiya, N.; Ramakrishna, N.; Day, A.L.; Kruse, A.; Mac Rae, S.; Hoos, A.; et al. CTLA-4 blockade with ipilimumab induces significant clinical benefit in a female with melanoma metastases to the CNS. Nat. Clin. Pract. Oncol. 2008, 5, 557–561. [Google Scholar] [CrossRef]
- Hodi, F.S.; Butler, M.; Oble, D.A.; Seiden, M.V.; Haluska, F.G.; Kruse, A.; Macrae, S.; Nelson, M.; Canning, C.; Lowy, I.; et al. Immunologic and Clinical Effects of Antibody Blockade of Cytotoxic T Lymphocyte-Associated Antigen 4 in Previously Vaccinated Cancer Patients. Proc. Natl. Acad. Sci. USA 2008, 105, 3005–3010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishino, M.; Giobbie-Hurder, A.; Gargano, M.; Suda, M.; Ramaiya, N.H.; Hodi, F.S. Developing a Common Language for Tumor Response to Immunotherapy: Immune-Related Response Criteria Using Unidimensional Measurements. Clin. Cancer Res. 2013, 19, 3936–3943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolchok, J.D.; Hoos, A.; O’Day, S.; Weber, J.S.; Hamid, O.; Lebbé, C.; Maio, M.; Binder, M.; Bohnsack, O.; Nichol, G.; et al. Guidelines for the Evaluation of Immune Therapy Activity in Solid Tumors: Immune-Related Response Criteria. Clin. Cancer Res. 2009, 15, 7412–7420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; et al. IRECIST: Guidelines for Response Criteria for Use in Trials Testing Immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef] [Green Version]
- Persigehl, T.; Lennartz, S.; Schwartz, L.H. IRECIST: How to Do It. Cancer Imaging 2020, 20, 2. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Hellmann, M.D.; Hamid, O.; Tsai, K.K.; Loo, K.L.; Gubens, M.A.; Rosenblum, M.; Harview, C.L.; Taube, J.M.; Handley, N.; et al. Liver Metastasis and Treatment Outcome with Anti-PD-1 Monoclonal Antibody in Patients with Melanoma and NSCLC. Cancer Immunol. Res. 2017, 5, 417–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, S.; Diem, S.; Li, Q.; Krapf, M.; Flatz, L.; Leschka, S.; Desbiolles, L.; Klingbiel, D.; Jochum, W.; Früh, M. Organ-specific response to nivolumab in patients with non-small cell lung cancer (NSCLC). Cancer Immunol. Immunother. 2018, 67, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Ramaiya, N.H.; Chambers, E.S.; Adeni, A.E.; Hatabu, H.; Jänne, P.A.; Hodi, F.S.; Awad, M.M. Immune-related response assessment during PD-1 inhibitor therapy in advanced non-small-cell lung cancer patients. J. Immunother. Cancer 2016, 4, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkins, M.B.; Clark, J.I.; Quinn, D.I. Immune checkpoint inhibitors in advanced renal cell carcinoma: Experience to date and future directions. Ann. Oncol. 2017, 28, 1484–1494. [Google Scholar] [CrossRef]
- Diem, S.; Hasan Ali, O.; Ackermann, C.J.; Bomze, D.; Koelzer, V.H.; Jochum, W.; Speiser, D.E.; Mertz, K.D.; Flatz, L. Tumor Infiltrating Lymphocytes in Lymph Node Metastases of Stage III Melanoma Correspond to Response and Survival in Nine Patients Treated with Ipilimumab at the Time of Stage IV Disease. Cancer Immunol. Immunother. 2018, 67, 39–45. [Google Scholar] [CrossRef]
- Negishi, T.; Furubayashi, N.; Nakagawa, T.; Nishiyama, N.; Kitamura, H.; Hori, Y.; Kuroiwa, K.; Son, Y.; Seki, N.; Tomoda, T.; et al. Site-specific Response to Nivolumab in Renal Cell Carcinoma. Anticancer Res. 2021, 41, 1539–1545. [Google Scholar] [CrossRef]
- Vuong, L.; Kotecha, R.R.; Voss, M.H.; Hakimi, A.A. Tumor Microenvironment Dynamics in Clear-Cell Renal Cell Carcinoma. Cancer Discov. 2019, 9, 1349–1357. [Google Scholar] [CrossRef] [Green Version]
- Vano, Y.-A.; Elaidi, R.; Bennamoun, M.; Chevreau, C.; Borchiellini, D.; Pannier, D.; Maillet, D.; Gross-Goupil, M.; Tournigand, C.; Laguerre, B.; et al. Nivolumab, nivolumab–ipilimumab, and VEGFR-tyrosine kinase inhibitors as first-line treatment for metastatic clear-cell renal cell carcinoma (BIONIKK): A biomarker-driven, open-label, non-comparative, randomised, phase 2 trial. Lancet Oncol. 2022, 23, 612–624. [Google Scholar] [CrossRef]
- Lu, L.C.; Hsu, C.; Shao, Y.Y.; Chao, Y.; Yen, C.J.; Shih, I.L.; Hung, Y.P.; Chang, C.J.; Shen, Y.C.; Guo, J.C.; et al. Differential Organ-Specific Tumor Response to Immune Checkpoint Inhibitors in Hepatocellular Carcinoma. Liver Cancer 2019, 8, 480–490. [Google Scholar] [CrossRef]
- Pignon, J.-C.; Jegede, O.; Shukla, S.A.; Braun, D.A.; Horak, C.E.; Wind-Rotolo, M.; Ishii, Y.; Catalano, P.J.; Grosha, J.; Flaifel, A.; et al. irRECIST for the Evaluation of Candidate Biomarkers of Response to Nivolumab in Metastatic Clear Cell Renal Cell Carcinoma: Analysis of a Phase II Prospective Clinical Trial. Clin. Cancer Res. 2019, 25, 2174–2184. [Google Scholar] [CrossRef]


| Clinical Parameters | Total N = 21 (%) | Responders (N = 11) | Non-Responders (N = 10) |
|---|---|---|---|
| Age | 33–70 (Median = 58 yrs) | 33–70 (Median = 51 yrs) | 46–69 (Median = 58 yrs) |
| Sex (M/F) | 20/1 (95.2%/4.8%) | 10/1 (90.9%/9.1%) | 10/0 (100%/0%) |
| Smoking | 4 (19%) | 3 (27.2%) | 1 (10%) |
| Co-morbidity (Diabetes, Hypertension and COPD) | 12 (57.1%) | 7 (63.6%) | 5(50%) |
| Treatment | |||
| Partial/Radical nephrectomy | 2/19 (9.5%/90.5%) | 1/10 (9.1%/90.9%) | 1/9(10%/90%) |
| Nivolumab | 21 (100%) | 11 (100%) | 10 (100%) |
| Sunitinib/Adjuvant Radiation | 18/0 (85.8%/0%) | 18/0 (85.8%/0%) | 18/0 (85.8%/0%) |
| RCC (B/L //U/L) | 1/20 (4.8%/95.2%) | 1/10 (9.1%/90.9%) | 0/10 (0%/100%) |
| Clinical End point | |||
| Dead/Alive | 3/18 (14.2%/85.8%) | 1/10 (9.1%/90.9%) | 2/8 (20%/80%) |
| Clinical complications (including Dead)/Stable | 8/13 (38%/62%) | 4/7 (36.3%/63.6%) | 4/6 (40%/60%) |
| Histology | |||
| P T1 | 3 (14.2%) | 2 (18.2%) | 1 (10%) |
| P T2 | 1 (4.8%) | 0 (0%) | 1 (10%) |
| PT3 | 17 (80.9%) | 9 (81.8%) | 8 (80%) |
| Gross Tumor Volume | 305 cc (Median) | ||
| Histology | |||
| Clear cell | 18 (85.8%) | 8 (72.7%) | 10 (100%) |
| Papillary | 2 (9.5%) | 2 (18.1%) | 0 (0%) |
| MIT family | 1 (4.7%) | 1 (9.1%) | 0 (0%) |
| Sarcomatoid elements | |||
| Present | 6 (28.5%) | 2 (18.2%) | 4 (40%) |
| Absent | 15 (71.5%) | 9 (81.8%) | 6 (60%) |
| Fuhrman’s Grade | |||
| 2 | 5 (23.8%) | 3 (27.2%) | 2 (20%) |
| 3 | 14 (66.7%) | 8 (72.8%) | 6 (60%) |
| 4 | 2 (9.5%) | 0 (0%) | 2 (20%) |
| Renal pelvis | |||
| Involved | 7 (33.3%) | 4 (36.3%) | 3 (30%) |
| Not Involved | 14 (66.7%) | 7 (63.7%) | 7 (70%) |
| LVNI | |||
| Present | 12 (57.1%) | 8 (72.7%) | 4 (40%) |
| Absent | 9 (42.9%) | 3 (27.2%) | 6 (60%) |
| Lymph nodes | |||
| Present | 4 (19%) | 3 (27.2%) | 1 (10%) |
| Absent | 17 (81%) | 8 (72.8%) | 9 (90%) |
| Gerotas fascia, ureter, and renal vessels | |||
| Involved | 0 (0%) | 0 (0%) | 0 (0%) |
| Not involved | 21 (100%) | 11 (100%) | 10 (100%) |
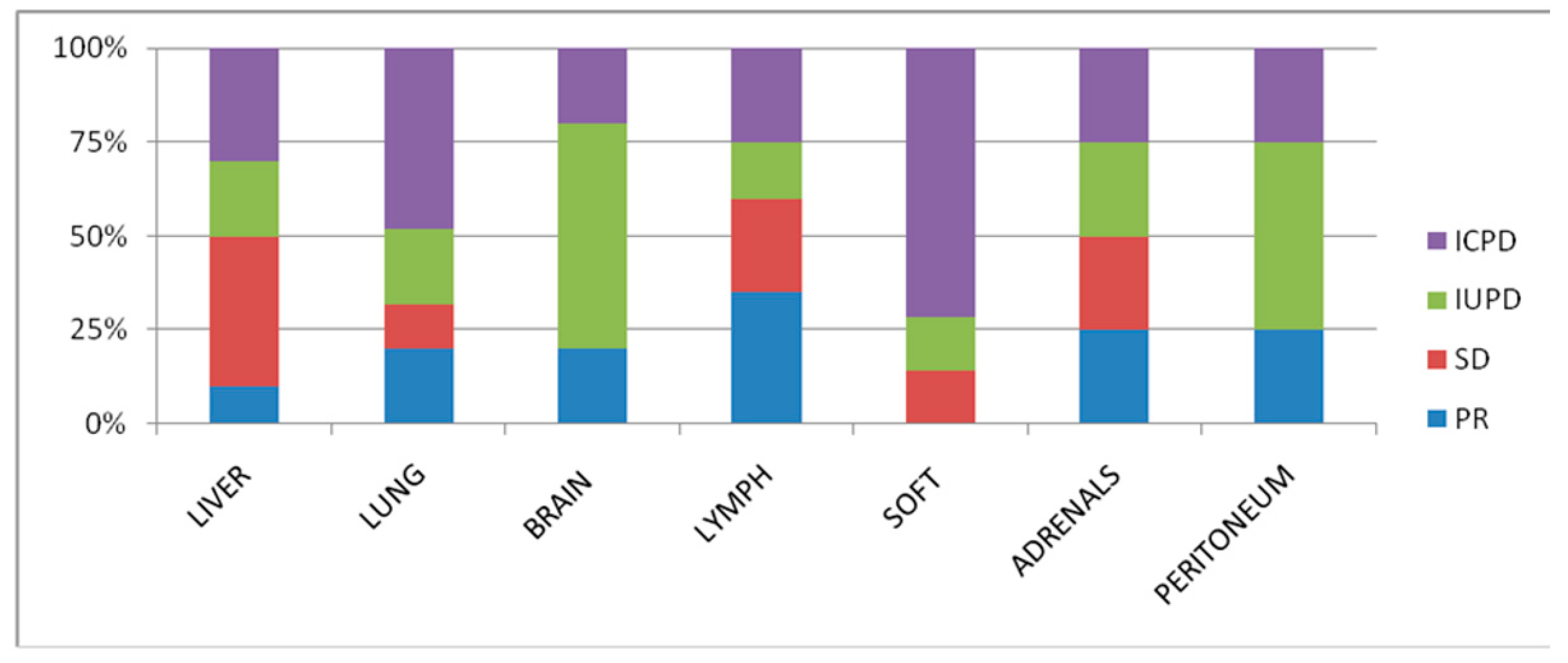
| Liver (n = 10) | Lung (n = 26) | Brain (n = 5) | Lymph Node (n = 20) | Soft-Tissue (n = 7) | Adrenals (n = 4) | Peritoneum (n = 4) | |
|---|---|---|---|---|---|---|---|
| Median Size Baseline (in mm) | 20 | 20 | 12 | 13 | 30 | 19 | 15.5 |
| Median Size LFU (in mm) | 19 | 20 | 25 | 12 | 40 | 25 | 20 |
| PR | 1 | 5 | 1 | 7 | 0 | 1 | 1 |
| SD | 4 | 3 | 0 | 5 | 1 | 1 | 0 |
| IUPD | 2 | 5 | 3 | 3 | 1 | 1 | 2 |
| ICPD | 3 | 12 | 1 | 5 | 5 | 1 | 1 |
| Objective Response (%) | 10 | 19 | 20 | 35 | 0 | 25 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jajodia, A.; Goel, V.; Patnaik, N.; Pasricha, S.; Gupta, G.; Batra, U.; Talwar, V. Analysis of Spatial Heterogeneity of Responses in Metastatic Sites in Renal Cell Carcinoma Patients Treated with Nivolumab. Tomography 2022, 8, 1363-1373. https://doi.org/10.3390/tomography8030110
Jajodia A, Goel V, Patnaik N, Pasricha S, Gupta G, Batra U, Talwar V. Analysis of Spatial Heterogeneity of Responses in Metastatic Sites in Renal Cell Carcinoma Patients Treated with Nivolumab. Tomography. 2022; 8(3):1363-1373. https://doi.org/10.3390/tomography8030110
Chicago/Turabian StyleJajodia, Ankush, Varun Goel, Nivedita Patnaik, Sunil Pasricha, Gurudutt Gupta, Ullas Batra, and Vineet Talwar. 2022. "Analysis of Spatial Heterogeneity of Responses in Metastatic Sites in Renal Cell Carcinoma Patients Treated with Nivolumab" Tomography 8, no. 3: 1363-1373. https://doi.org/10.3390/tomography8030110
APA StyleJajodia, A., Goel, V., Patnaik, N., Pasricha, S., Gupta, G., Batra, U., & Talwar, V. (2022). Analysis of Spatial Heterogeneity of Responses in Metastatic Sites in Renal Cell Carcinoma Patients Treated with Nivolumab. Tomography, 8(3), 1363-1373. https://doi.org/10.3390/tomography8030110






