MDCT Findings in Gastrointestinal Perforations and the Predictive Value according to the Site of Perforation
Abstract
:1. Introduction
2. Materials and Methods
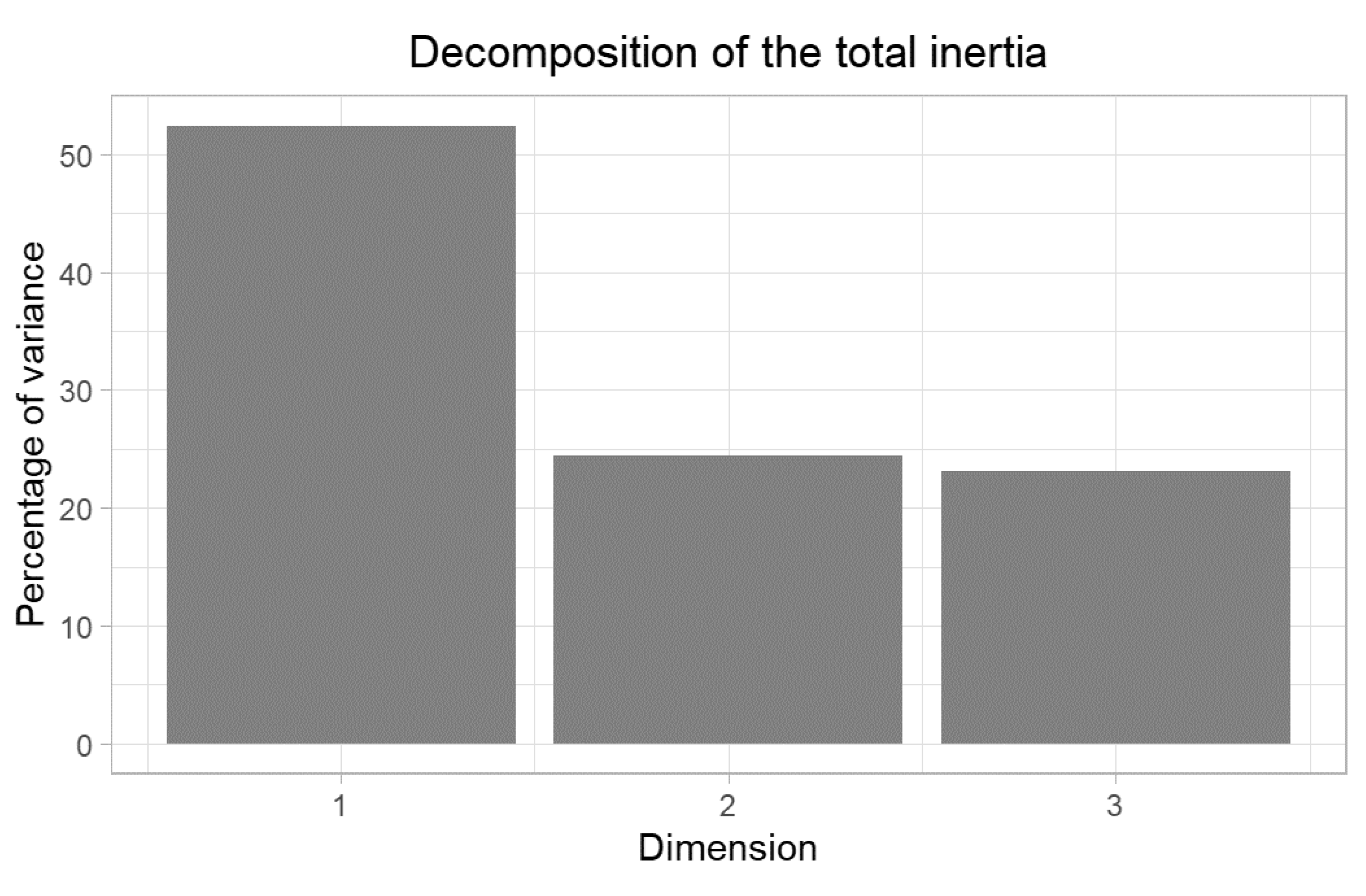
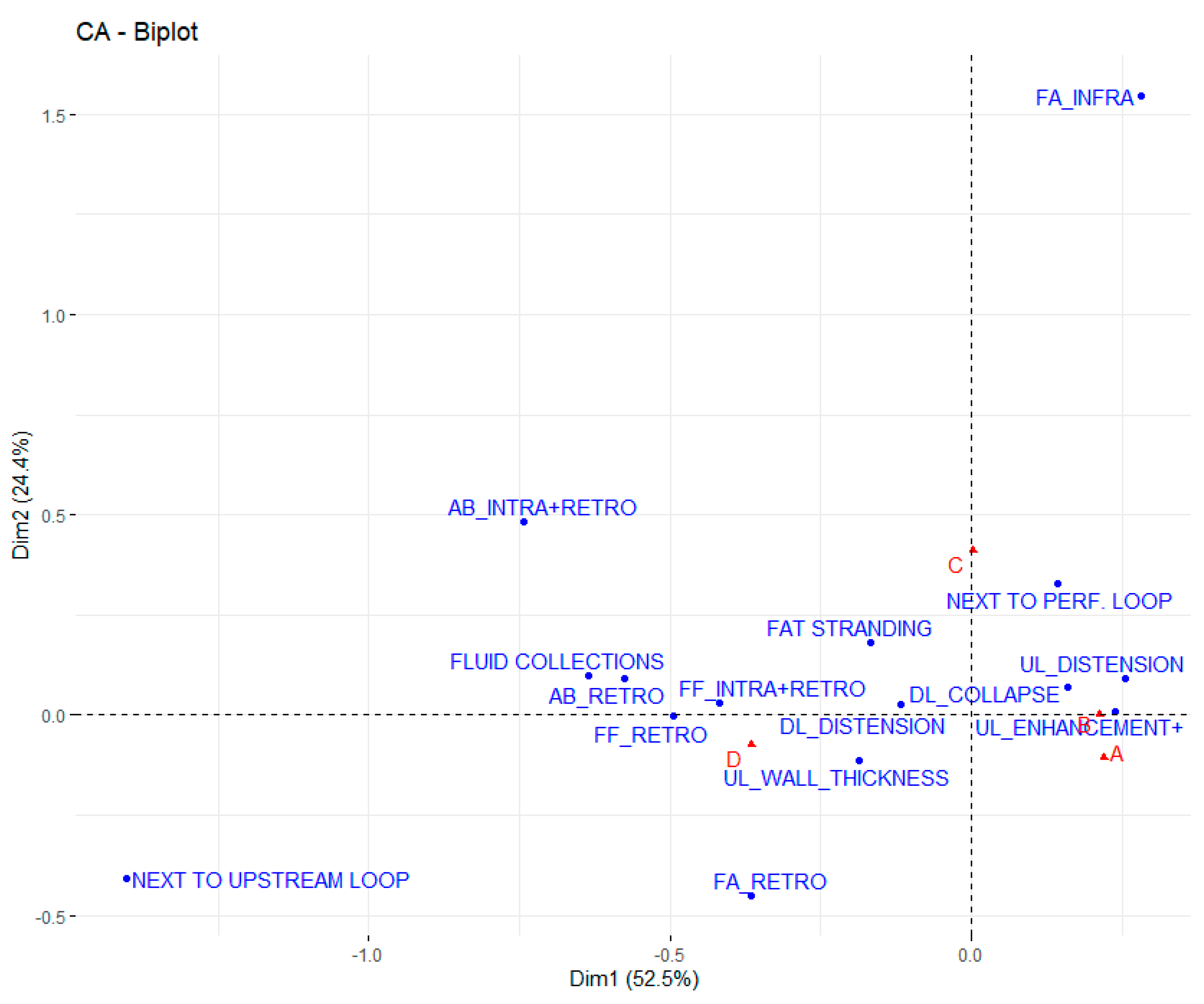
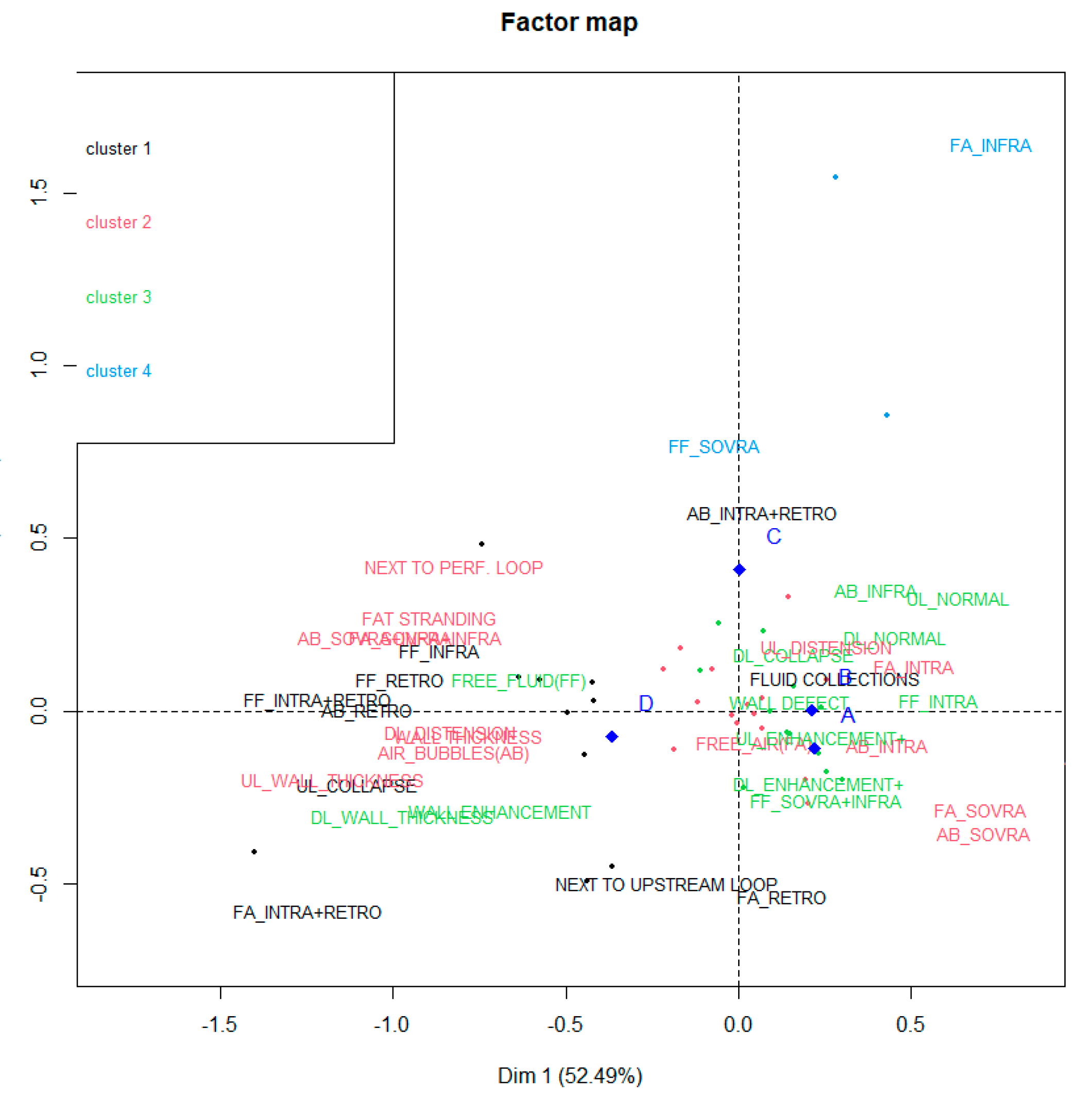
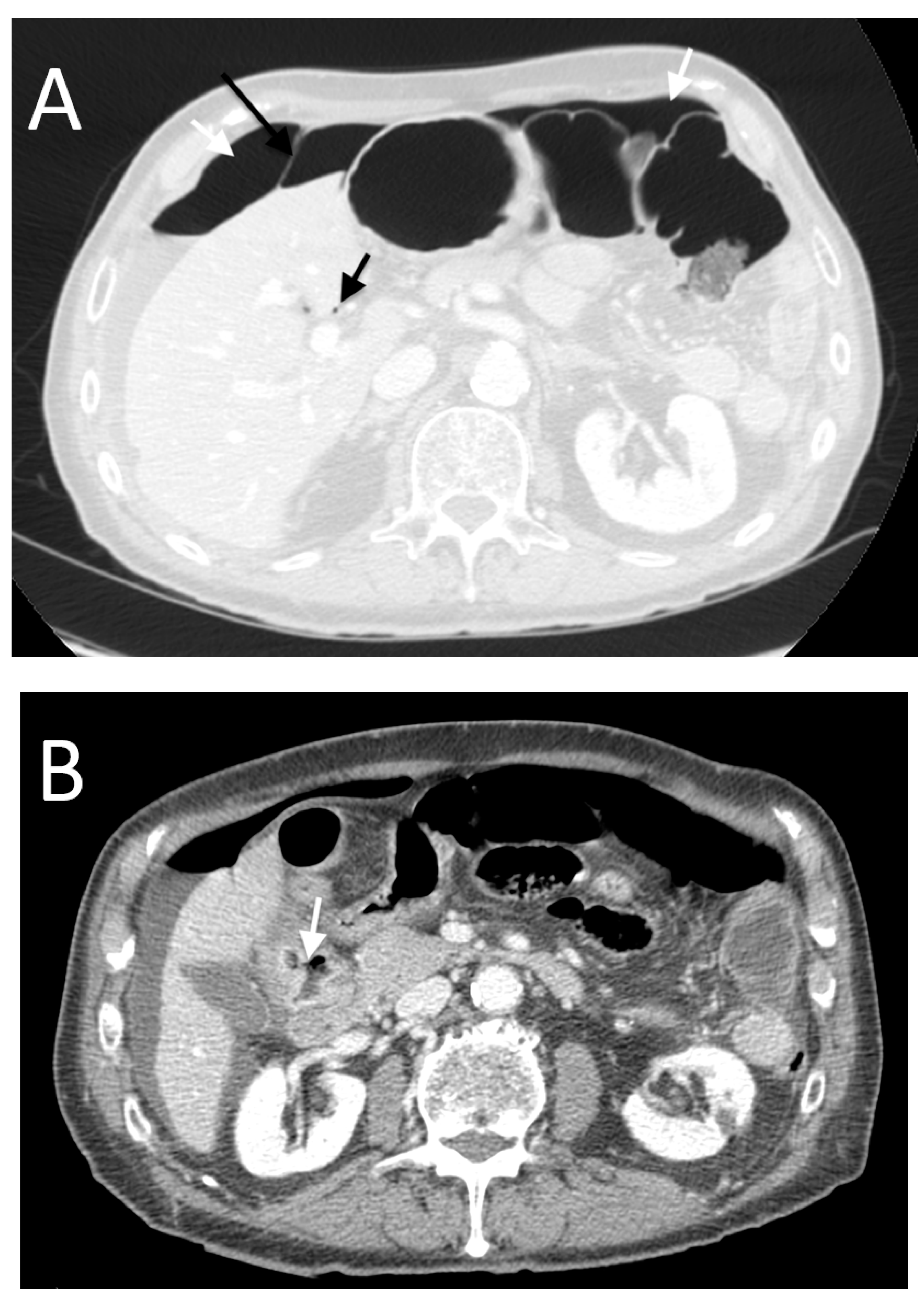
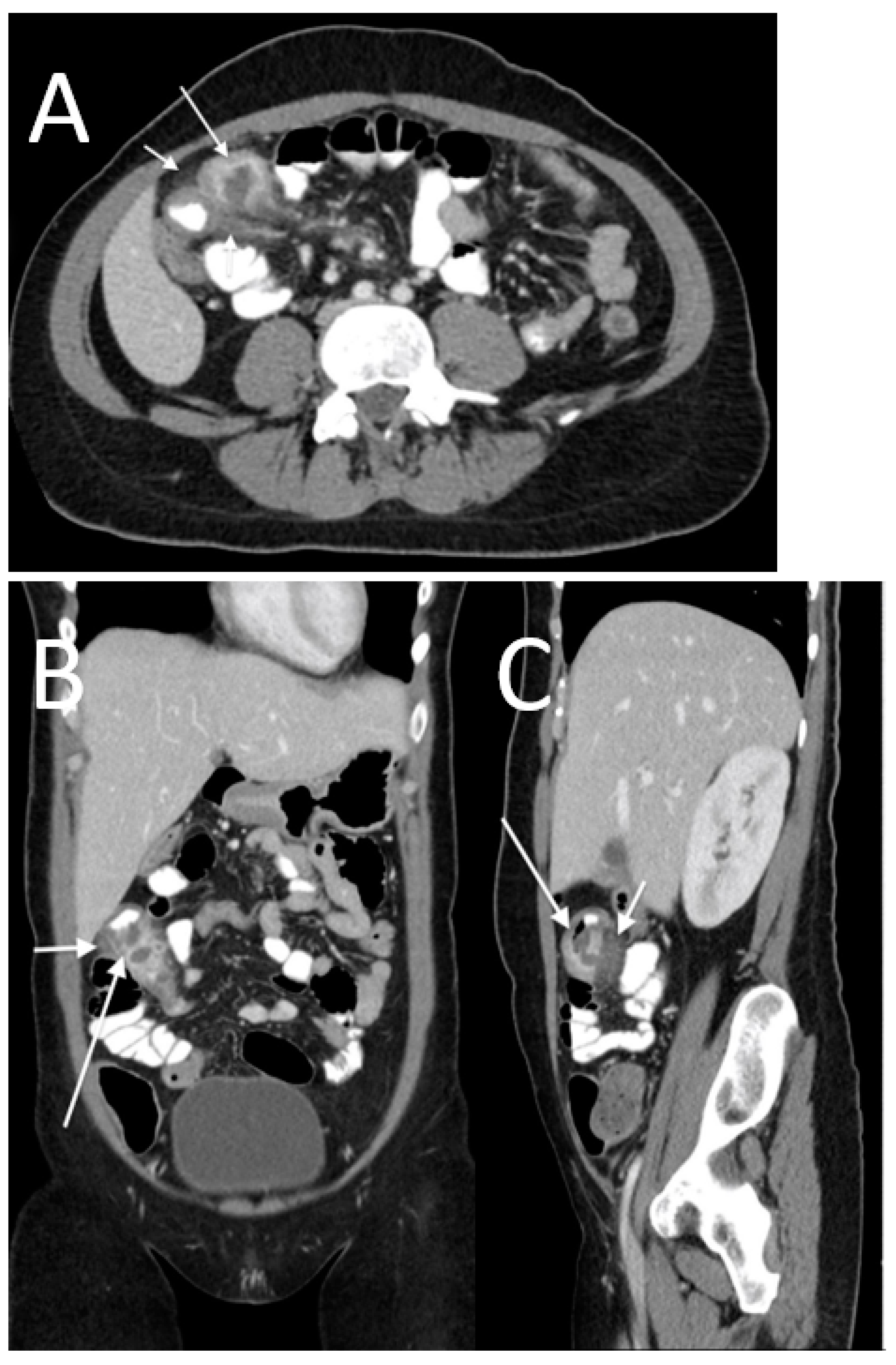
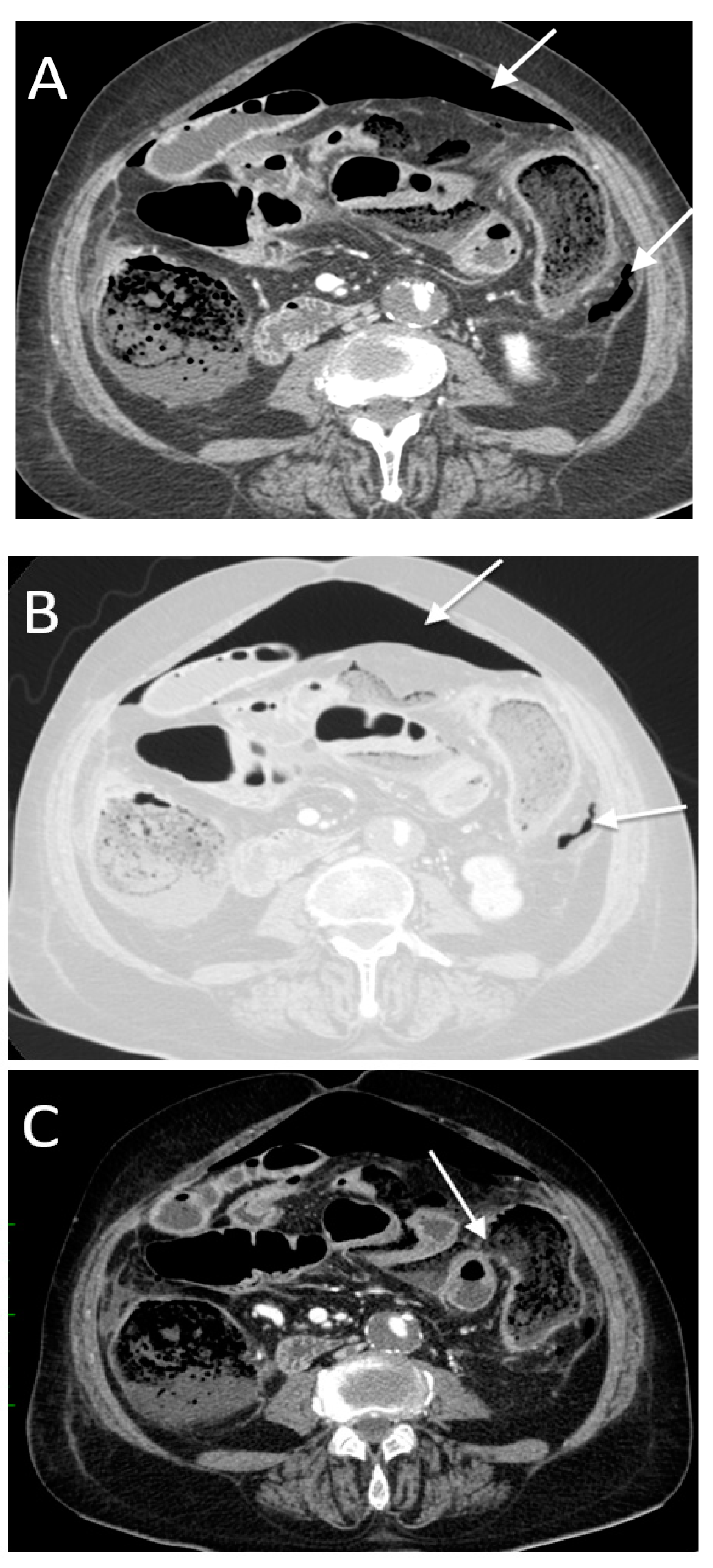
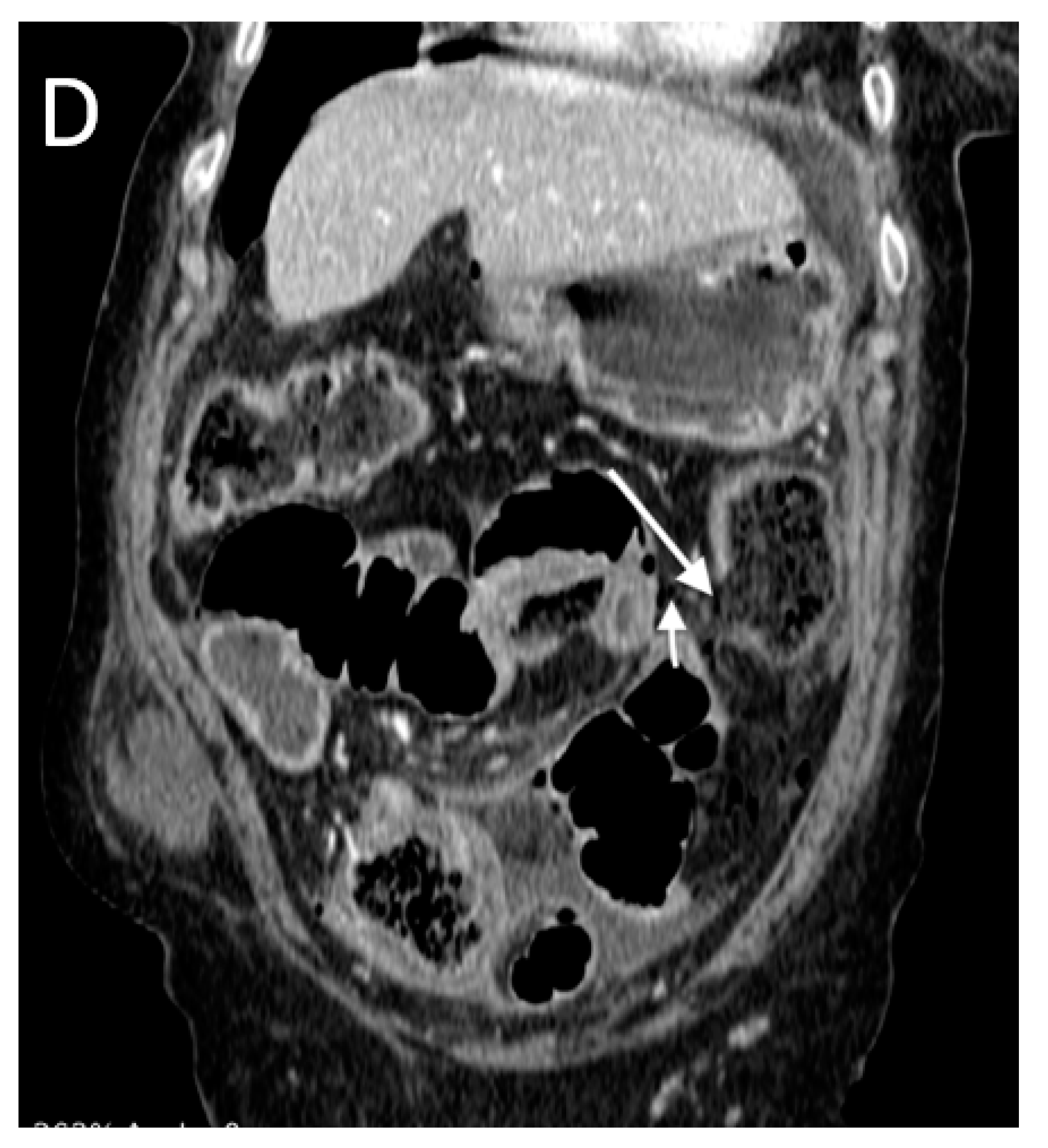
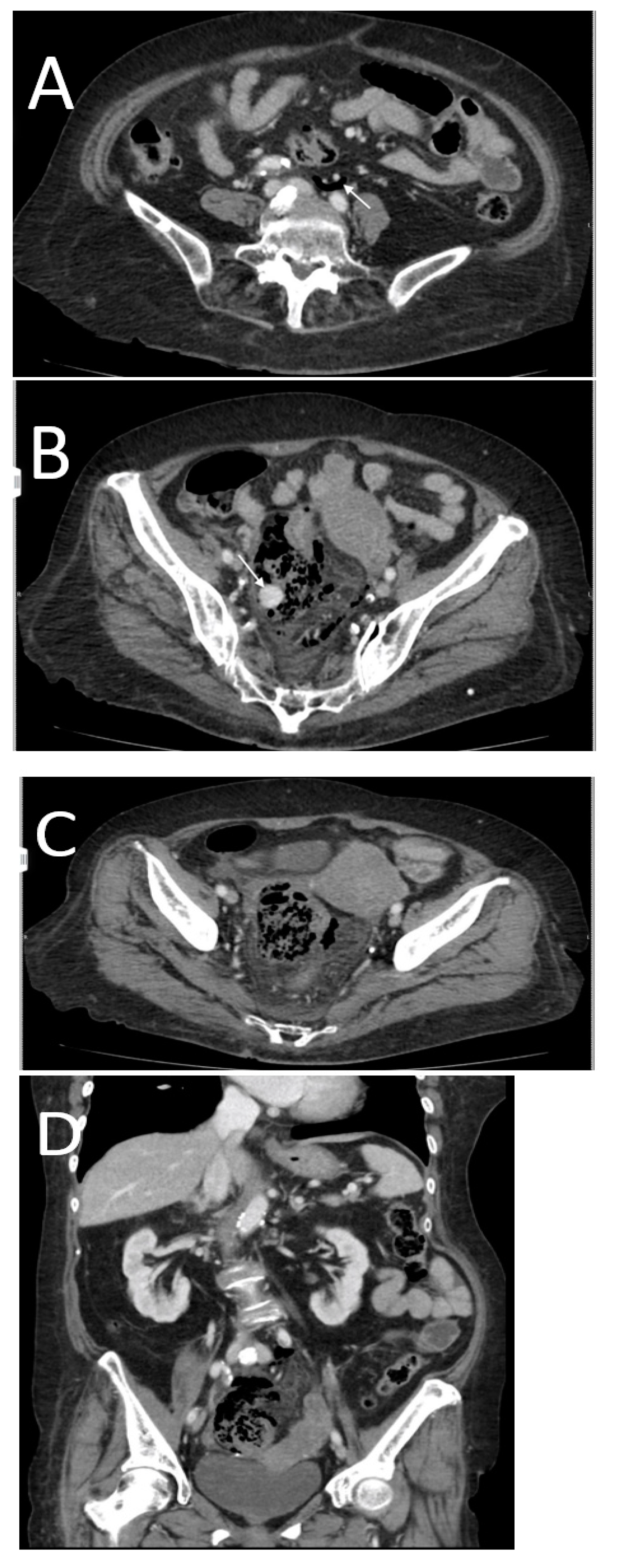
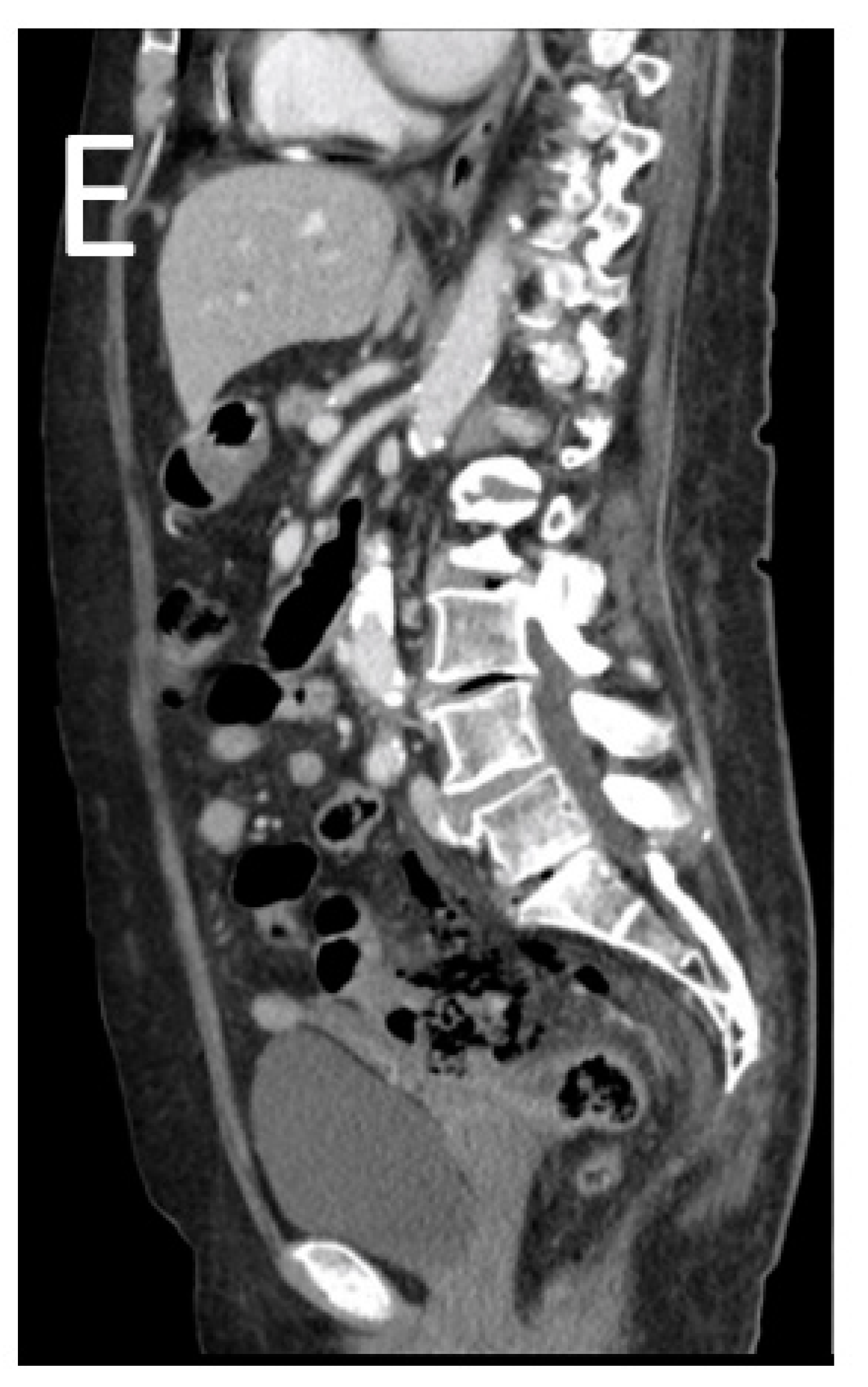
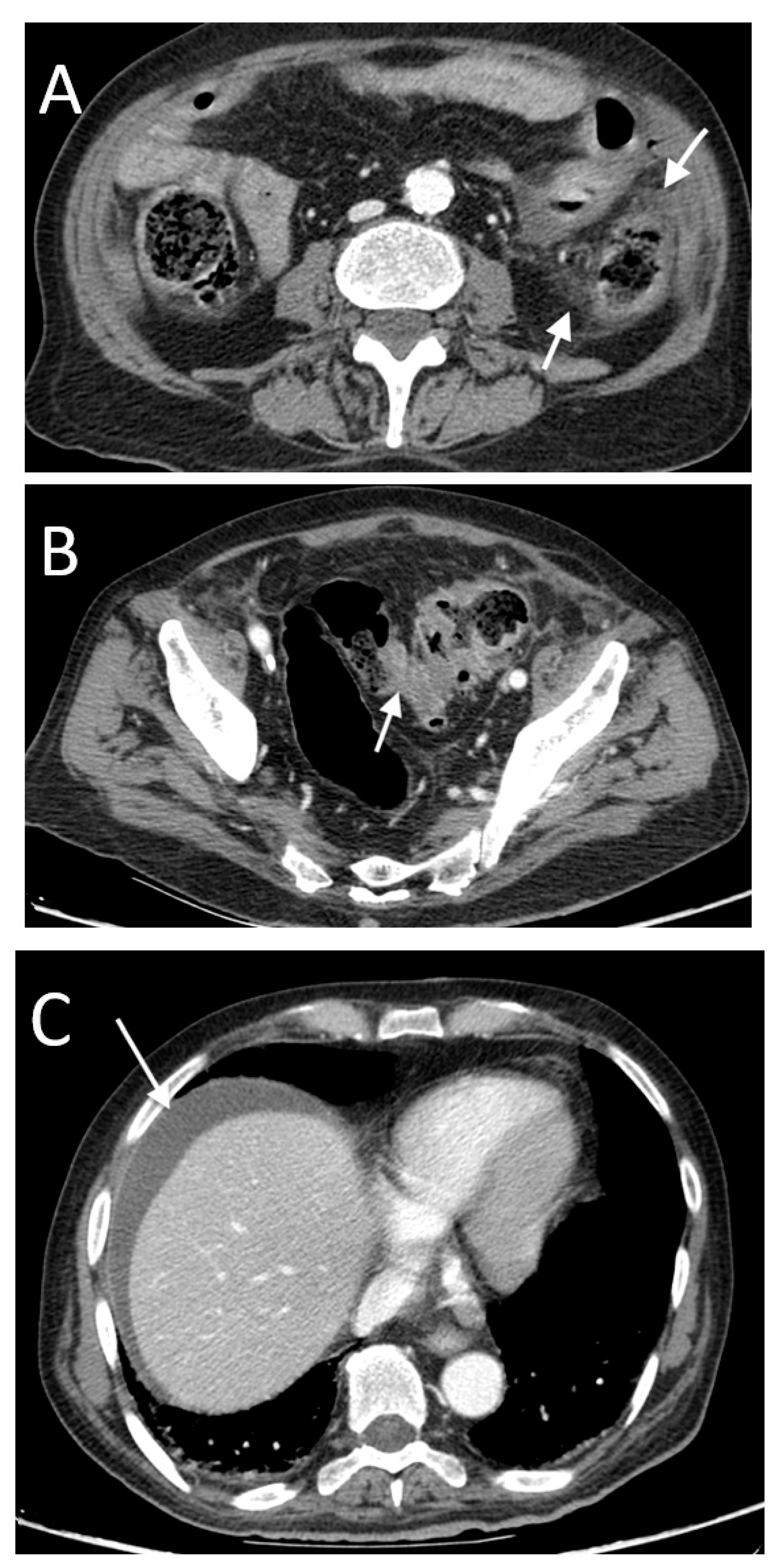
3. Results
- -
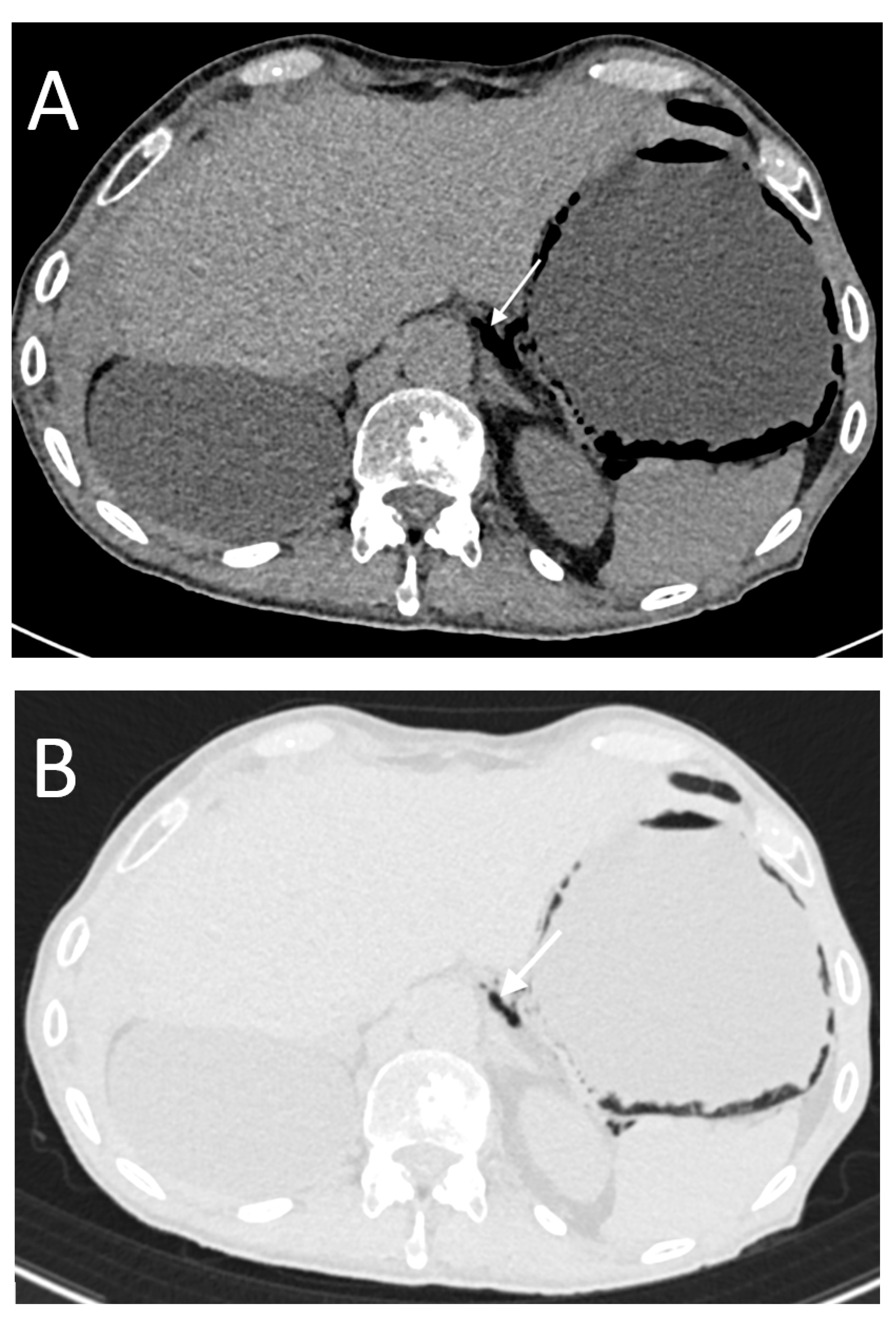
- High occurrence of perforation site D;
- -
- Low occurrence of perforation sites A and B.
- -
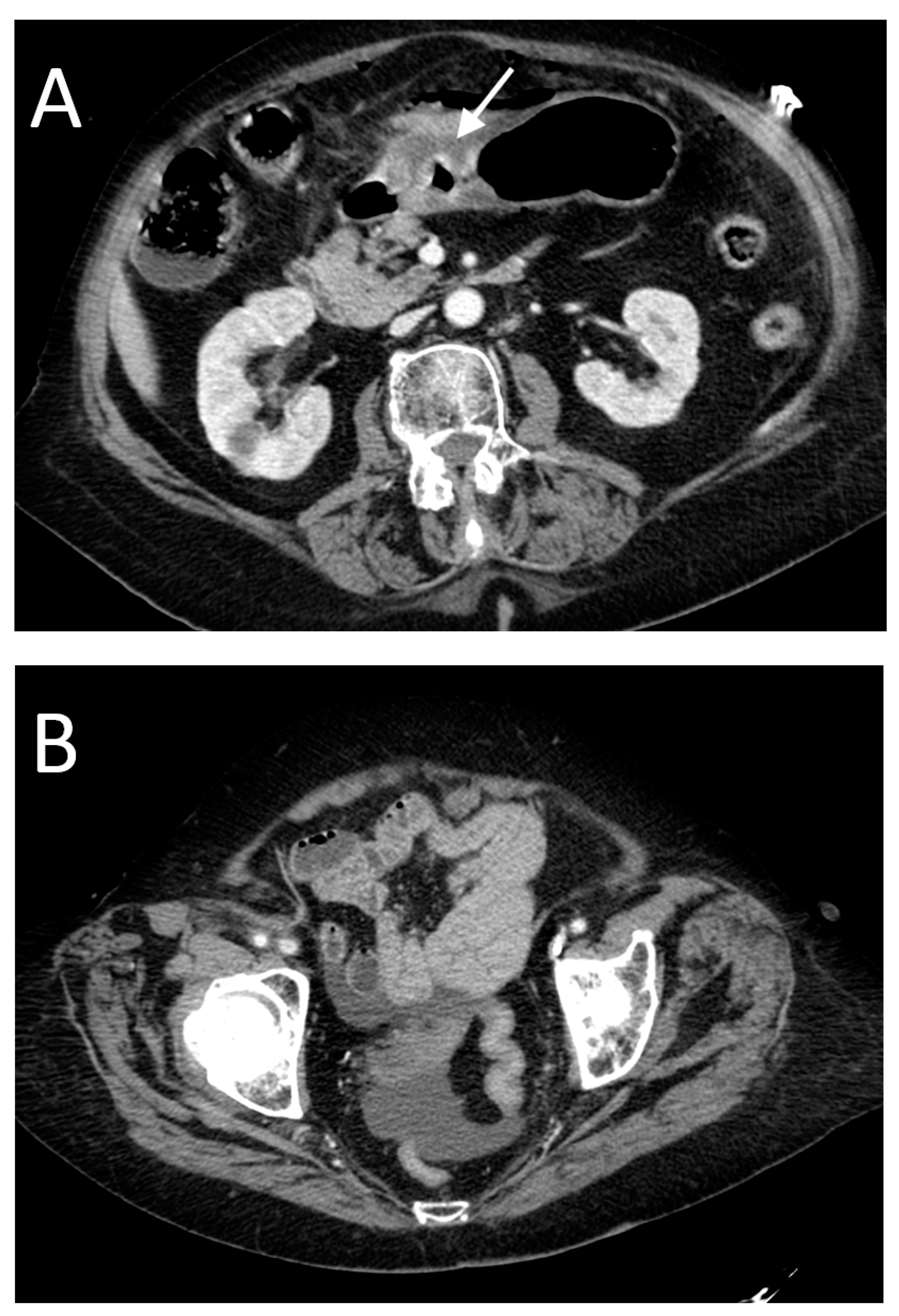
- High occurrence of perforation site A;
- -
- Low occurrence of perforation site B.
- -
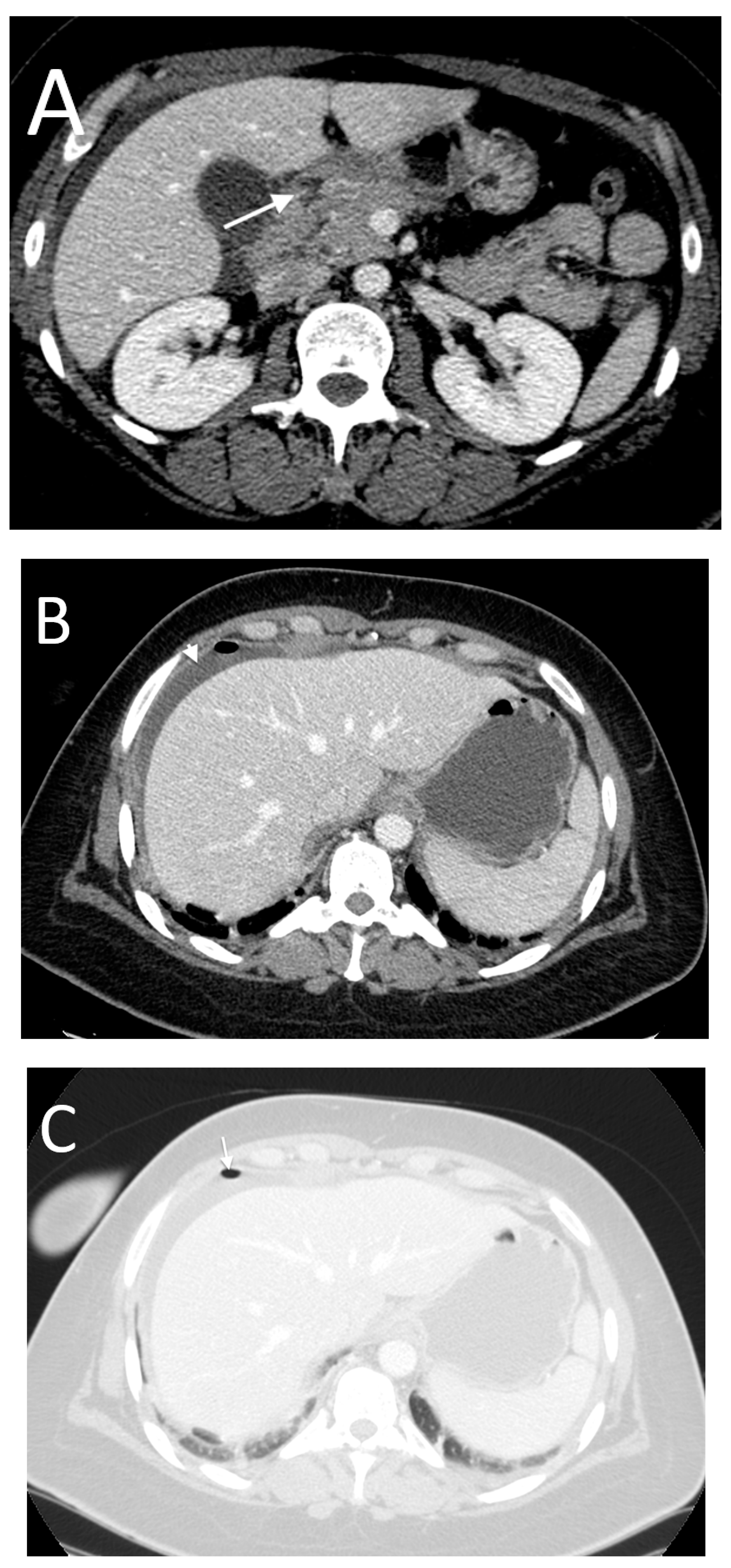
- High occurrence of perforation site A;
- -
- Low occurrence of perforation site B.
- -
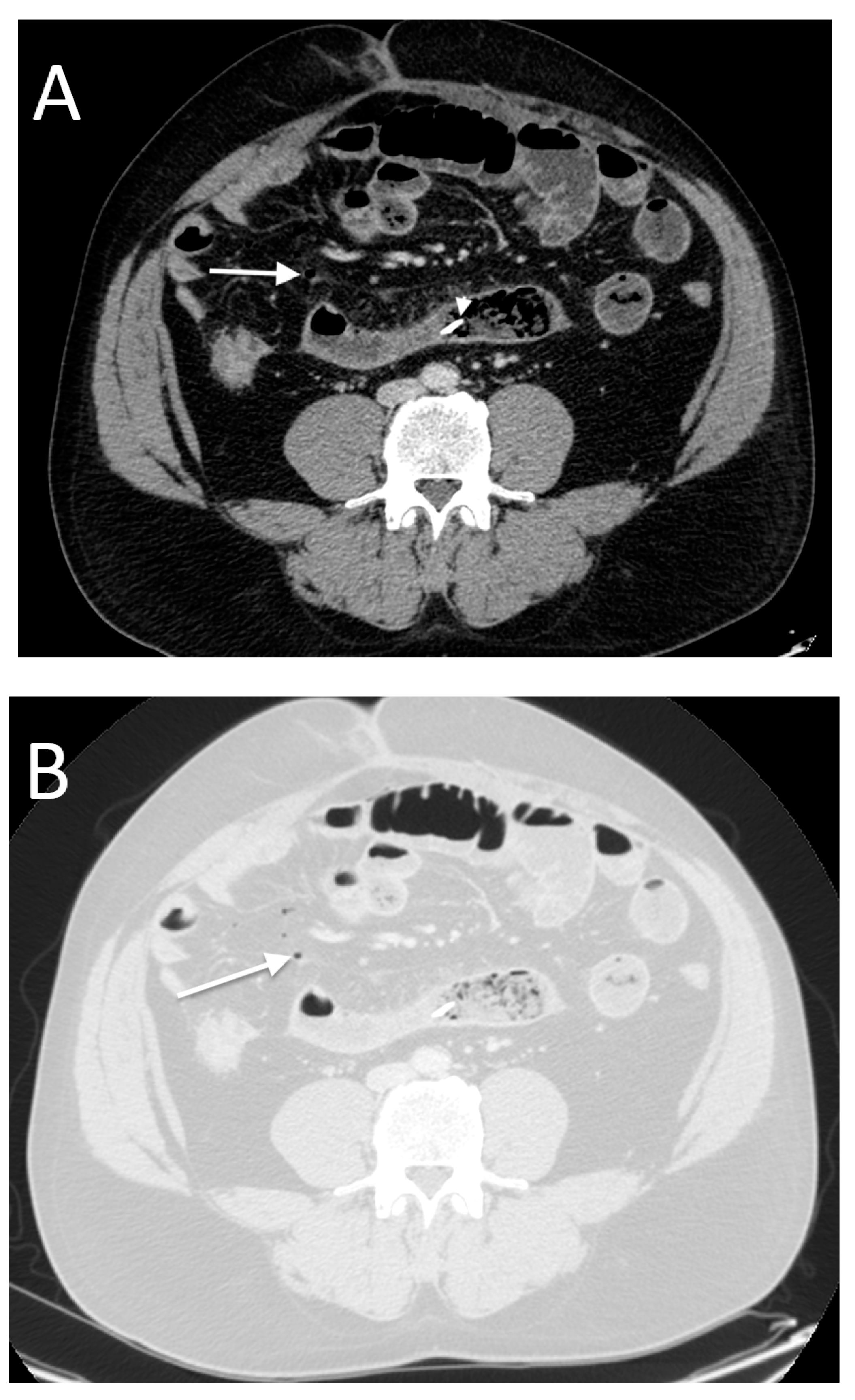
- High occurrence of perforation site C.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taourel, P.; Baert, A.L.; Reiser, M.F.; Hricak, H.; Knauth, M. Medical Radiology Diagnostic Imaging, CT of the Acute Abdomen, Bowel Perforations; Springer: Berlin/Heidelberg, Germany, 2011; pp. 309–327. [Google Scholar]
- Hines, J.; Rosenblat, J.; Duncan, D.R.; Friedman, B.; Katz, D.S. Perforation of the mesenteric small bowel: Etiologies and CT findings. Emerg. Radiol. 2012, 20, 155–161. [Google Scholar] [CrossRef]
- Kothari, K.; Friedman, B.; Grimaldi, G.M.; Hines, J.J. Nontraumatic large bowel perforation: Spectrum of etiologies and CT findings. Abdom. Radiol. 2017, 6, 177–2608. [Google Scholar] [CrossRef] [PubMed]
- Zissin, R.; Osadchy, A.; Gayer, G. Abdominal CT findings in small bowel perforation. Br. J. Radiol. 2009, 82, 162–171. [Google Scholar] [CrossRef]
- Simonetti, I.; Puglia, M.; Tarotto, L.; Palumbo, F.; Esposito, F.; Sciuto, A.; Ragozzino, A. When traditions become dangerous: Intestinal perforation from unusual foreign body—Case report and short literature review. Eur. J. Radiol. Open 2019, 6, 152–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siu, W.T.; Leong, H.T.; Law, B.K.B.; Chau, C.H.; Li, A.C.N.; Fung, K.H.; Tai, Y.P.; Li, M.K.W. Laparoscopic Repair for Perforated Peptic Ulcer: A randomized controlled trial. Ann. Surg. 2002, 235, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Re, G.L.; La Mantia, F.; Picone, D.; Salerno, S.; Vernuccio, F.; Midiri, M. Small Bowel Perforations: What the Radiologist Needs to Know. Semin. Ultrasound CT MRI 2016, 37, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Grassi, R.; Romano, S.; Pinto, A.; Romano, L. Gastro-duodenal perforations: Conventional plain film, US and CT findings in 166 consecutive patients. Eur. J. Radiol. 2004, 50, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.-W.; Chang, M.-S.; Hsiao, C.-P.; Huang, J.-F. CT evaluation of gastrointestinal tract perforation. Clin. Imaging 2004, 28, 329–333. [Google Scholar] [CrossRef]
- Hainaux, B.; Agneessens, E.; Bertinotti, R.; De Maertelaer, V.; Rubesova, E.; Capelluto, E.; Moschopoulos, C. Accuracy of MDCT in Predicting Site of Gastrointestinal Tract Perforation. Am. J. Roentgenol. 2006, 187, 1179–1183. [Google Scholar] [CrossRef]
- Kim, S.H.; Shin, S.S.; Jeong, Y.Y.; Heo, S.H.; Kim, J.W.; Kang, H.K. Gastrointestinal Tract Perforation: MDCT Findings according to the Perforation Sites. Korean J. Radiol. 2009, 10, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, A.; Sakoda, M.; Yamasaki, M.; Kono, N.; Tanaka, T.; Nitta, N.; Kanasaki, S.; Imoto, K.; Takahashi, M.; Murata, K.; et al. Gastrointestinal tract perforation: CT diagnosis of presence, site, and cause. Gastrointest. Radiol. 2005, 30, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.S.; Yoon, S.E.; Park, S.H.; Kim, H.; Lee, Y.-H.; Yoon, K.-H. Distinction between upper and lower gastrointestinal perforation: Usefulness of the periportal free air sign on computed tomography. Eur. J. Radiol. 2009, 69, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.P.; Steward, M.J.; Booth, T.C.; Mukhtar, H.; Murray, D. Evolution of imaging for abdominal perforation. Ann. R. Coll. Surg. Engl. 2010, 92, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Kunin, J.R.; Korobkin, M.; Ellis, J.H.; Francis, I.R.; Kane, N.M.; Siegel, S.E. Duodenal injuries caused by blunt abdominal trauma: Value of CT in differentiating perforation from hematoma. Am. J. Roentgenol. 1993, 160, 1221–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghekiere, O.; Lesnik, A.; Hoa, D.; Laffargue, G.; Uriot, C.; Taourel, P. Value of Computed Tomography in the Diagnosis of the Cause of Nontraumatic Gastrointestinal Tract Perforation. J. Comput. Assist. Tomogr. 2007, 31, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Burling, D.; Halligan, S.; Slater, A.; Noakes, M.J.; Taylor, S.A. Potentially Serious Adverse Events at CT Colonography in Symptomatic Patients: National Survey of the United Kingdom. Radiology 2006, 239, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Rozenblit, A.; Cohen-Schwartz, D.; Wolf, E.; Foxx, M.; Brenner, S. Stercoral Perforation of the Sigmoid Colon: Computed Tomography Findings. Clin. Radiol. 2000, 55, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Cirocchi, R.; Søreide, K.; Di Saverio, S.; Rossi, E.; Arezzo, A.; Zago, M.; Abraha, I.; Vettoretto, N.; Chiarugi, M. Meta-analysis of perioperative outcomes of acute laparoscopic versus open repair of perforated gastroduodenal ulcers. J. Trauma Acute Care Surg. 2018, 85, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.M.; Jiang, J.-K.; Lake, J.P.; Ault, G.; Artinyan, A.; Gonzalez-Ruiz, C.; Essani, R.; Beart, R.W. The Management of Complicated Diverticulitis and the Role of Computed Tomography. Am. J. Gastroenterol. 2005, 100, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Francis, N.K.; Sylla, P.; Abou-Khalil, M.; Arolfo, S.; Berler, D.; Curtis, N.J.; Dolejs, S.C.; Garfinkle, R.; Gorter-Stam, M.; Hashimoto, D.A.; et al. EAES and SAGES 2018 consensus conference on acute diverticulitis management: Evidence-based recommendations for clinical practice. Surg. Endosc. 2019, 33, 2726–2741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tochigi, T.; Kosugi, C.; Shuto, K.; Mori, M.; Hirano, A.; Koda, K. Management of complicated diverticulitis of the colon. Ann. Gastroenterol. Surg. 2018, 2, 22–27. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Riddle, E.W. Minimally Invasive Surgery for Complicated Diverticulitis. J. Gastrointest. Surg. 2017, 21, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-I.; Siewert, B.; Raptopoulos, V.; Hodin, R.A. Factors associated with conversion to laparotomy in patients undergoing laparoscopic appendectomy. J. Am. Coll. Surg. 2002, 194, 298–305. [Google Scholar] [CrossRef]
| GROUP A (n = 31) | GROUP B (n = 18) | GROUP C (n = 16) | GROUP D (n = 28) | |||||
|---|---|---|---|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | |
| FREE AIR(FA) | 27 | 87 (70–96) | 6 | 33 (13–59) | 10 | 62 (35–85) | 18 | 64 (44–81) |
| FA_INTRA | 25 | 81 (63–93) | 4 | 22 (6–48) | 10 | 62 (35–85) | 15 | 54 (34–72) |
| FA_SUPRA | 16 | 52 (33–70) | 2 | 11 (1–35) | 3 | 19 (4–46) | 7 | 25 (11–45) |
| FA_INFRA | 0 | 0 (-) | 0 | 0 (-) | 2 | 13 (2–38) | 0 | 0 (-) |
| FA_SUPRA+INFRA | 9 | 29 (14–48) | 2 | 11 (1–35) | 5 | 31 (11–59) | 8 | 29 (13–49) |
| FA_RETRO | 5 | 16 (5–34) | 1 | 6 (0–27) | 0 | 0 (-) | 7 | 25 (11–45) |
| FA_INTRA+RETRO | 3 | 10 (2–26) | 0 | 0 (-) | 0 | 0 (-) | 4 | 14 (4–33) |
| AIR BUBBLES(AB) | 27 | 87 (70–96) | 11 | 61 (36–83) | 10 | 62 (35–85) | 23 | 82 (63–94) |
| AB_INTRA | 25 | 81 (63–93) | 10 | 56 (31–78) | 10 | 62 (35–85) | 22 | 79 (59–92) |
| AB_SUPRA | 15 | 48 (30–67) | 3 | 17 (4–41) | 2 | 13 (2–38) | 7 | 25 (11–45) |
| AB_INFRA | 0 | 0 (-) | 6 | 33 (13–59) | 2 | 13 (2–38) | 4 | 14 (4–33) |
| AB_SUPRA+INFRA | 10 | 32 (17–51) | 1 | 6 (0–27) | 6 | 38 (15–65) | 11 | 39 (22–59) |
| AB_RETRO | 3 | 10 (2–26) | 1 | 6 (0–27) | 3 | 19 (4–46) | 9 | 14 (41–79) |
| AB_INTRA+RETRO | 1 | 3 (0–17) | 0 | 0 (-) | 4 | 25 (7–52) | 7 | 25 (11–45) |
| FREE FLUID(FF) | 26 | 84 (66–95) | 13 | 72 (47–90) | 10 | 62 (35–85) | 19 | 68 (48–84) |
| FF_INTRA | 26 | 84 (66–95) | 13 | 72 (47–90) | 8 | 50 (25–75) | 17 | 61 (41–79) |
| FF_SUPRA | 2 | 6 (0–21) | 0 | 0 (-) | 2 | 13 (2–38) | 0 | 0 (-) |
| FF_INFRA | 2 | 6 (0–21) | 2 | 11 (1–35) | 2 | 13 (2–38) | 6 | 21 (8–41) |
| FF_SUPRA+INFRA | 22 | 71 (52–86) | 11 | 61 (36–83) | 4 | 25 (7–52) | 11 | 39 (22–59) |
| FF_RETRO | 3 | 10 (2–26) | 1 | 6 (0–27) | 2 | 13 (2–38) | 7 | 25 (11–45) |
| FF_INTRA+RETRO | 3 | 10 (2–26) | 1 | 6 (0–27) | 2 | 13 (2–38) | 6 | 21 (8–41) |
| WALL THICKNESS | 26 | 84 (66–95) | 11 | 61 (36–83) | 11 | 69 (41–89) | 21 | 75 (55–89) |
| WALL ENHANCEMENT | 26 | 84 (66–95) | 14 | 78 (52–94) | 4 | 25 (7–52) | 12 | 43 (24–63) |
| WALL DEFECT | 13 | 42 (25–61) | 8 | 44 (22–69) | 4 | 25 (7–52) | 9 | 32 (16–52) |
| n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | |
| FAT STRANDING | 18 | 58 (39–75) | 9 | 50 (26–74) | 14 | 88 (62–98) | 24 | 86 (67–96) |
| NEXT TO PERF. LOOP | 18 | 58 (39–75) | 9 | 50 (26–74) | 14 | 88 (62–98) | 11 | 39 (22–59) |
| NEXT TO UPSTREAM LOOP | 0 | 0 (-) | 0 | 0 (-) | 0 | 0 (-) | 13 | 46 (28–66) |
| FLUID COLLECTIONS | 3 | 10 (2–26) | 2 | 11 (1–35) | 4 | 25 (7–52) | 13 | 46 (28–66) |
| AIR BUBBLES CLOSE TO PERF. LOOP | 24 | 77 (59–90) | 9 | 50 (26–74) | 8 | 50 (25–75) | 17 | 61 (41–79) |
| UPSTREAM LOOPS | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) |
| DISTENSION | 19 | 61 (42–78) | 7 | 39 (17–64) | 8 | 50 (25–75) | 8 | 29 (13–49) |
| COLLAPSE | 4 | 13 (4–30) | 5 | 28 (10–53) | 2 | 13 (2–38) | 13 | 46 (28–66) |
| NORMAL | 8 | 26 (12–45) | 6 | 33 (13–59) | 6 | 38 (15–65) | 7 | 25 (11–45) |
| WALL THICKNESS | 12 | 39 (22–58) | 5 | 28 (10–53) | 4 | 25 (7–52) | 15 | 54 (34–72) |
| ENHANCEMENT+ | 14 | 45 (27–64) | 7 | 39 (17–64) | 5 | 31 (11–59) | 7 | 25 (11–45) |
| DOWNSTREAM LOOPS | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) |
| DISTENSION | 6 | 19 (7–37) | 3 | 17 (4–41) | 3 | 19 (4–46) | 7 | 25 (11–45) |
| COLLAPSE | 18 | 58 (39–75) | 9 | 50 (26–74) | 8 | 50 (25–75) | 11 | 39 (22–59) |
| NORMAL | 7 | 23 (10–41) | 6 | 33 (13–59) | 5 | 31 (11–59) | 10 | 36 (19–56) |
| WALL THICKNESS | 12 | 39 (22–58) | 7 | 39 (17–64) | 2 | 13 (2–38) | 11 | 39 (22–59) |
| ENHANCEMENT+ | 13 | 42 (25–61) | 7 | 39 (17–64) | 3 | 19 (4–46) | 7 | 25 (11–45) |
| GROUP A PPV (%) | GROUP B PPV (%) | GROUP C PPV (%) | GROUP D PPV (%) | |
| FREE AIR(FA) | 44 | 10 | 16 | 30 |
| FA_INTRA | 46 | 7 | 19 | 28 |
| FA_SUPRA | 57 | 7 | 11 | 25 |
| FA_INFRA | 0 | 0 | 100 | 0 |
| FA_SUPRA+INFRA | 38 | 8 | 21 | 33 |
| FA_RETRO | 38 | 8 | 0 | 54 |
| FA_INTRA+RETRO | 43 | 0 | 0 | 57 |
| AIR BUBBLES(AB) | 38 | 15 | 14 | 32 |
| AB_INTRA | 37 | 15 | 15 | 33 |
| AB_SUPRA | 56 | 11 | 7 | 26 |
| AB_INFRA | 0 | 50 | 17 | 33 |
| AB_SUPRA+INFRA | 36 | 4 | 21 | 39 |
| AB_RETRO | 19 | 6 | 19 | 56 |
| AB_INTRA+RETRO | 8 | 0 | 33 | 58 |
| FREE FLUID(FF) | 38 | 19 | 15 | 28 |
| FF_INTRA | 41 | 20 | 13 | 27 |
| FF_SUPRA | 50 | 0 | 50 | 0 |
| FF_INFRA | 17 | 17 | 17 | 50 |
| FF_SUPRA+INFRA | 46 | 23 | 8 | 23 |
| FF_RETRO | 23 | 8 | 15 | 54 |
| FF_INTRA+RETRO | 25 | 8 | 17 | 50 |
| WALL THICKNESS | 38 | 16 | 16 | 30 |
| WALL ENHANCEMENT | 46 | 25 | 7 | 21 |
| WALL DEFECT | 38 | 24 | 12 | 26 |
| GROUP A PPV (%) | GROUP B PPV (%) | GROUP C PPV (%) | GROUP D PPV (%) | |
| FAT STRANDING | 28 | 14 | 22 | 37 |
| NEXT TO PERF. LOOP | 35 | 17 | 27 | 21 |
| NEXT TO UPSTREAM LOOP | 0 | 0 | 0 | 100 |
| FLUID COLLECTIONS | 14 | 9 | 18 | 59 |
| AIR BUBBLES CLOSE TO PERF. LOOP | 41 | 16 | 14 | 29 |
| UPSTREAM LOOPS | GROUP A PPV (%) | GROUP B PPV (%) | GROUP C PPV (%) | GROUP D PPV (%) |
| DISTENSION | 45 | 17 | 19 | 19 |
| COLLAPSE | 17 | 21 | 8 | 54 |
| NORMAL | 30 | 22 | 22 | 26 |
| WALL THICKNESS | 33 | 14 | 11 | 42 |
| ENHANCEMENT+ | 42 | 21 | 15 | 21 |
| DOWNSTREAM LOOPS | GROUP A PPV (%) | GROUP B PPV (%) | GROUP C PPV (%) | GROUP D PPV (%) |
| DISTENSION | 32 | 16 | 16 | 37 |
| COLLAPSE | 39 | 20 | 17 | 24 |
| NORMAL | 25 | 21 | 18 | 36 |
| WALL THICKNESS | 38 | 22 | 6 | 34 |
| ENHANCEMENT+ | 43 | 23 | 10 | 23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, S.; Somma, C.; Sciuto, A.; Jutidamrongphan, W.; Pacella, D.; Esposito, F.; Puglia, M.; Mauriello, C.; Khanungwanitkul, K.; Pirozzi, F. MDCT Findings in Gastrointestinal Perforations and the Predictive Value according to the Site of Perforation. Tomography 2022, 8, 667-687. https://doi.org/10.3390/tomography8020056
Romano S, Somma C, Sciuto A, Jutidamrongphan W, Pacella D, Esposito F, Puglia M, Mauriello C, Khanungwanitkul K, Pirozzi F. MDCT Findings in Gastrointestinal Perforations and the Predictive Value according to the Site of Perforation. Tomography. 2022; 8(2):667-687. https://doi.org/10.3390/tomography8020056
Chicago/Turabian StyleRomano, Stefania, Carmela Somma, Antonio Sciuto, Warissara Jutidamrongphan, Daniela Pacella, Francesco Esposito, Marta Puglia, Claudio Mauriello, Khanin Khanungwanitkul, and Felice Pirozzi. 2022. "MDCT Findings in Gastrointestinal Perforations and the Predictive Value according to the Site of Perforation" Tomography 8, no. 2: 667-687. https://doi.org/10.3390/tomography8020056
APA StyleRomano, S., Somma, C., Sciuto, A., Jutidamrongphan, W., Pacella, D., Esposito, F., Puglia, M., Mauriello, C., Khanungwanitkul, K., & Pirozzi, F. (2022). MDCT Findings in Gastrointestinal Perforations and the Predictive Value according to the Site of Perforation. Tomography, 8(2), 667-687. https://doi.org/10.3390/tomography8020056






