Assessment of Aortoiliac Atherosclerotic Plaque on CT in Prostate Cancer Patients Undergoing Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Datasets
2.2. Agatston Score Measurement
2.3. Clinical Biomarkers
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Agatston Scores of the Cases and Controls Patients
3.3. Association of Plaque and Cardiovascular Disease (CVD) Risk Factors
3.4. Association of Plaque and Prostate Cancer-Related Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sturgeon, K.M.; Deng, L.; Bluethmann, S.M.; Zhou, S.; Trifiletti, D.M.; Jiang, C.; Kelly, S.P.; Zaorsky, N.G. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur. Heart J. 2019, 40, 3889–3897. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, M.C.; Yeboah, J.; Burke, G.L.; Chen, H.; Klepin, H.D.; Hundley, W.G. Cancer and Its Association With the Development of Coronary Artery Calcification: An Assessment From the Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2015, 4, e002533. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.A., 2nd; Gerber, L.; Banez, L.L.; Moreira, D.M.; Rittmaster, R.S.; Andriole, G.L.; Freedland, S.J. Prostate cancer risk in men with baseline history of coronary artery disease: Results from the REDUCE Study. Cancer Epidemiol. Biomark. Prev. 2012, 21, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Hager, M.; Mikuz, G.; Bartsch, G.; Kolbitsch, C.; Moser, P.L. The association between local atherosclerosis and prostate cancer. BJU Int. 2007, 99, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Davila, J.A.; Johnson, C.D.; Behrenbeck, T.R.; Hoskin, T.L.; Harmsen, W.S. Assessment of cardiovascular risk status at CT colonography. Radiology 2006, 240, 110–115. [Google Scholar] [CrossRef]
- O’Connor, S.D.; Graffy, P.M.; Zea, R.; Pickhardt, P.J. Does Nonenhanced CT-based Quantification of Abdominal Aortic Calcification Outperform the Framingham Risk Score in Predicting Cardiovascular Events in Asymptomatic Adults? Radiology 2019, 290, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Chuang, M.L.; Massaro, J.M.; Levitzky, Y.S.; Fox, C.S.; Manders, E.S.; Hoffmann, U.; O’Donnell, C.J. Prevalence and distribution of abdominal aortic calcium by gender and age group in a community-based cohort (from the Framingham Heart Study). Am. J. Cardiol. 2012, 110, 891–896. [Google Scholar] [CrossRef]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- D’Agostino, R.B.S.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef] [PubMed]
- lassoan. Cardiac Agatston Scoring Function for 3D Slicer. Available online: https://gist.github.com/lassoan/d85be45b7284a3b4e580688fccdb1d02/revisions (accessed on 30 December 2021).
- O’Farrell, S.; Garmo, H.; Holmberg, L.; Adolfsson, J.; Stattin, P.; Van Hemelrijck, M. Risk and timing of cardiovascular disease after androgen-deprivation therapy in men with prostate cancer. J. Clin. Oncol. 2015, 33, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, B.; Kantert, C.; Meyer, T.; Hadamitzky, M.; Martinoff, S.; Schömig, A.; Hausleiter, J. Cardiovascular risk assessment based on the quantification of coronary calcium in contrast-enhanced coronary computed tomography angiography. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 468–475. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Groen, J.M.; Greuter, M.J.; Schmidt, B.; Suess, C.; Vliegenthart, R.; Oudkerk, M. The influence of heart rate, slice thickness, and calcification density on calcium scores using 64-slice multidetector computed tomography: A systematic phantom study. Investig. Radiol. 2007, 42, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Komen, N.; Klitsie, P.; Hermans, J.J.; Niessen, W.J.; Kleinrensink, G.J.; Jeekel, J.; Lange, J.F. Calcium scoring in unenhanced and enhanced CT data of the aorta-iliacal arteries: Impact of image acquisition, reconstruction, and analysis parameter settings. Acta Radiol. 2011, 52, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Mühlenbruch, G.; Thomas, C.; Wildberger, J.E.; Koos, R.; Das, M.; Hohl, C.; Katoh, M.; Günther, R.W.; Mahnken, A.H. Effect of varying slice thickness on coronary calcium scoring with multislice computed tomography in vitro and in vivo. Investig. Radiol. 2005, 40, 695–699. [Google Scholar] [CrossRef]
- McCollough, C.H.; Ulzheimer, S.; Halliburton, S.S.; Shanneik, K.; White, R.D.; Kalender, W.A. Coronary artery calcium: A multi-institutional, multimanufacturer international standard for quantification at cardiac CT. Radiology 2007, 243, 527–538. [Google Scholar] [CrossRef]
- Bell, K.J.; Del Mar, C.; Wright, G.; Dickinson, J.; Glasziou, P. Prevalence of incidental prostate cancer: A systematic review of autopsy studies. Int. J. Cancer 2015, 137, 1749–1757. [Google Scholar] [CrossRef]

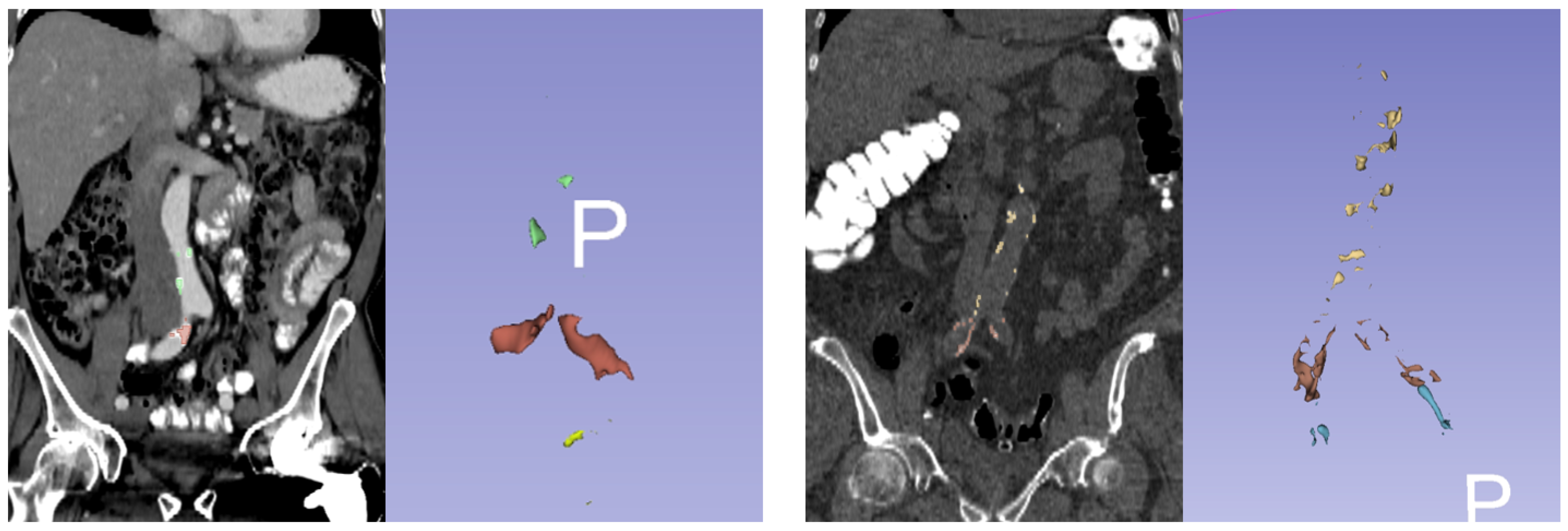
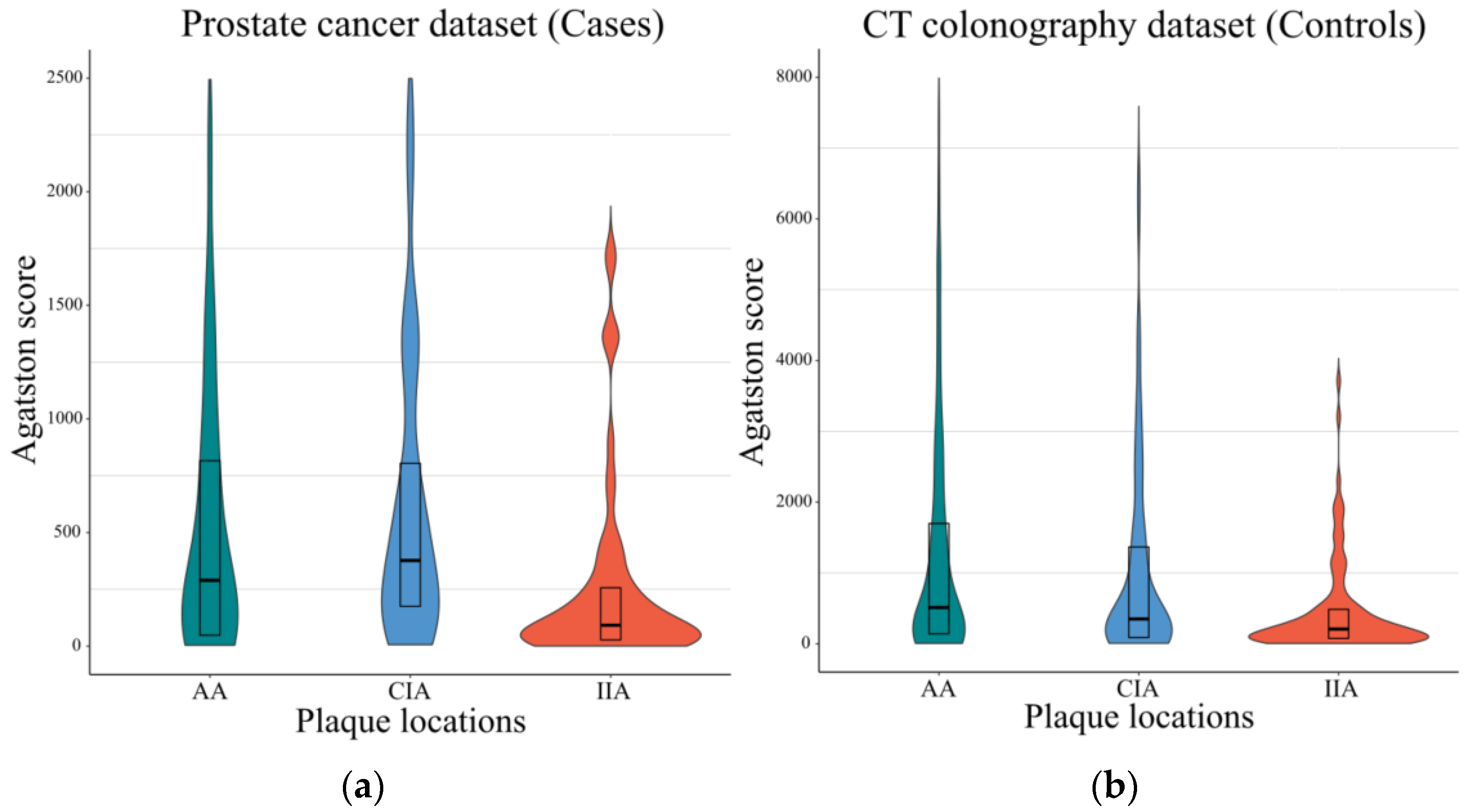

| Cases Dataset (n = 93) | Controls Dataset (n = 186) | |
|---|---|---|
| Age | 63 (IQR, 57–70) | 62 (IQR, 58–70) |
| Gender | Male 100% | Male 100% |
| Framingham score | 11 ± 5.3 (mean ± 2SD) | 12 ± 5.2 (mean ± 2SD) |
| Prior CVD event | 7 (7.5%) | 21 (11.3%) |
| PSA | 16.02 (IQR, 6.5–61.1) | - |
| Gleason score | 6(3 + 3) 4.3%, 7(3 + 4) 24.7%, 7(4 + 3) 2.2%, 8(3 + 5) 2.2%, 8(4 + 4) 17.2%, 8(unknown individual score) 2.2%, 9(4 + 5) 41.9%, 9(5 + 4) 2.2%, 10(5 + 5) 3.2% | - |
| Mortality | 8.6% | - |
| Bone metastasis | Present (54.8%), Absent (45.2%) | - |
| ADT use | Used (54.8%), Not used (45.2%) | - |
| ADT use term | 3 (IQR, 2–5) months | - |
| Tumor location | PZ (34.4%), TZ (7.5%), PZ + TZ (9.7%), Unknown (49.5%) | - |
| Prostatectomy | 24 (25.8%) | - |
| Time after prostatectomy | 39 months (IQR, 8–88) | - |
| Cases Dataset | Controls Dataset | |
|---|---|---|
| Original reconstruction | 1 mm ST (0.8 pitch) Soft kernel (BR40d) | 1.25 mm (0.8 pitch) Standard-plus |
| Resampled for Agatston measurement | 3 mm ST | 3 mm ST |
| Tube voltage | 120 kVp | 120 kVp |
| Tube current | Automated tube current modulation | 30–300 mA |
| Time delay technique | Fixed time delay (80 s after injection) | - |
| Scanner type | SIEMENS SOMATOM Force SIEMENS SOMATOM Definition Flash TOSHIBA Aquilion ONE | GE Lightspeed series |
| Channel | 8- to 128-MDCT | 16- to 64-MDCT |
| Agatston Scores of Cases | Agatston Scores of Controls | |||
|---|---|---|---|---|
| p-Value | Spearman’s Rho | p-Value | Spearman’s Rho | |
| Total (AA + CIA + IIA) plaques | ||||
| Age | <0.001 | 0.39 | <0.001 | 0.67 |
| Framingham scores | <0.001 | 0.41 | <0.001 | 0.49 |
| Abdominal aortic (AA) plaques | ||||
| Age | <0.001 | 0.38 | <0.001 | 0.69 |
| Framingham scores | <0.001 | 0.39 | <0.001 | 0.51 |
| Common iliac artery (CIA) plaques | ||||
| Age | 0.001 | 0.35 | <0.001 | 0.58 |
| Framingham scores | 0.002 | 0.35 | <0.001 | 0.39 |
| Internal iliac artery (IIA) plaques | ||||
| Age | 0.002 | 0.34 | <0.001 | 0.58 |
| Framingham scores | <0.001 | 0.38 | <0.001 | 0.37 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| β | 95% CI for β | p-Value | β | 95% CI for β | p-Value | |
| Total (AA + CIA + IIA) plaques | ||||||
| Age | 0.07 | (0.06, 0.09) | <0.001 | 0.06 | (0.04, 0.07) | <0.001 |
| Framingham scores | 0.17 | (0.12, 0.21) | <0.001 | 0.08 | (0.03, 0.13) | 0.002 |
| Prostate cancer | −0.01 | (−0.31, 0.29) | 0.95 | - | - | - |
| Abdominal aortic (AA) plaques | ||||||
| Age | 0.08 | (0.06, 0.09) | <0.001 | 0.06 | (0.04, 0.08) | <0.001 |
| Framingham scores | 0.18 | (0.13, 0.23) | <0.001 | 0.09 | (0.04, 0.15) | <0.001 |
| Prostate cancer | −0.24 | (−0.56, 0.09) | 0.16 | |||
| Common iliac artery (CIA) plaques | ||||||
| Age | 0.07 | (0.06, 0.09) | <0.001 | 0.06 | (0.04, 0.08) | <0.001 |
| Framingham scores | 0.15 | (0.09, 0.21) | <0.001 | 0.06 | (0, 0.12) | 0.057 |
| Prostate cancer | −0.06 | (−0.41, 0.28) | 0.72 | |||
| Internal iliac artery (IIA) plaques | ||||||
| Age | 0.06 | (0.05, 0.08) | <0.001 | 0.05 | (0.04, 0.07) | <0.001 |
| Framingham scores | 0.13 | (0.08, 0.18) | <0.001 | 0.05 | (0, 0.1) | 0.064 |
| Prostate cancer | −0.06 | (−0.36, 0.24) | 0.68 | |||
| Univariate Analysis | |||||
|---|---|---|---|---|---|
| β | 95% CI for β | p-Value | Method | p-Value | |
| PSA | −0.00 | (−0.00, 0.00) | 0.43 | Spearman’s correlation | 0.73 |
| Gleason score | 0.08 | (−0.16, 0.33) | 0.50 | Spearman’s correlation | 0.36 |
| Mortality | −0.35 | (−1.22, 0.53) | 0.43 | Mann–Whitney test | 0.36 |
| Bone metastasis | 0.15 | (−0.34, 0.65) | 0.55 | Mann–Whitney test | 0.62 |
| ADT use | −0.00 | (−0.50, 0.49) | 0.98 | Mann–Whitney test | 0.98 |
| Tumor location | −0.25 | (−0.67, 0.17) | 0.24 | Kruskal–Wallis test | 0.43 |
| Prostatectomy | 0.00 | (−0.55, 0.57) | 0.98 | Mann–Whitney test | 0.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Elton, D.C.; Gulley, J.L.; Pickhardt, P.J.; Dahut, W.L.; Madan, R.A.; Pinto, P.A.; Citrin, D.E.; Summers, R.M. Assessment of Aortoiliac Atherosclerotic Plaque on CT in Prostate Cancer Patients Undergoing Treatment. Tomography 2022, 8, 607-616. https://doi.org/10.3390/tomography8020050
Lee S, Elton DC, Gulley JL, Pickhardt PJ, Dahut WL, Madan RA, Pinto PA, Citrin DE, Summers RM. Assessment of Aortoiliac Atherosclerotic Plaque on CT in Prostate Cancer Patients Undergoing Treatment. Tomography. 2022; 8(2):607-616. https://doi.org/10.3390/tomography8020050
Chicago/Turabian StyleLee, Sungwon, Daniel C. Elton, James L. Gulley, Perry J. Pickhardt, William L. Dahut, Ravi A. Madan, Peter A. Pinto, Deborah E. Citrin, and Ronald M. Summers. 2022. "Assessment of Aortoiliac Atherosclerotic Plaque on CT in Prostate Cancer Patients Undergoing Treatment" Tomography 8, no. 2: 607-616. https://doi.org/10.3390/tomography8020050
APA StyleLee, S., Elton, D. C., Gulley, J. L., Pickhardt, P. J., Dahut, W. L., Madan, R. A., Pinto, P. A., Citrin, D. E., & Summers, R. M. (2022). Assessment of Aortoiliac Atherosclerotic Plaque on CT in Prostate Cancer Patients Undergoing Treatment. Tomography, 8(2), 607-616. https://doi.org/10.3390/tomography8020050







