Preoperative Sinonasal Computed Tomography Score in Chronic Rhinosinusitis with Nasal Polyps
Abstract
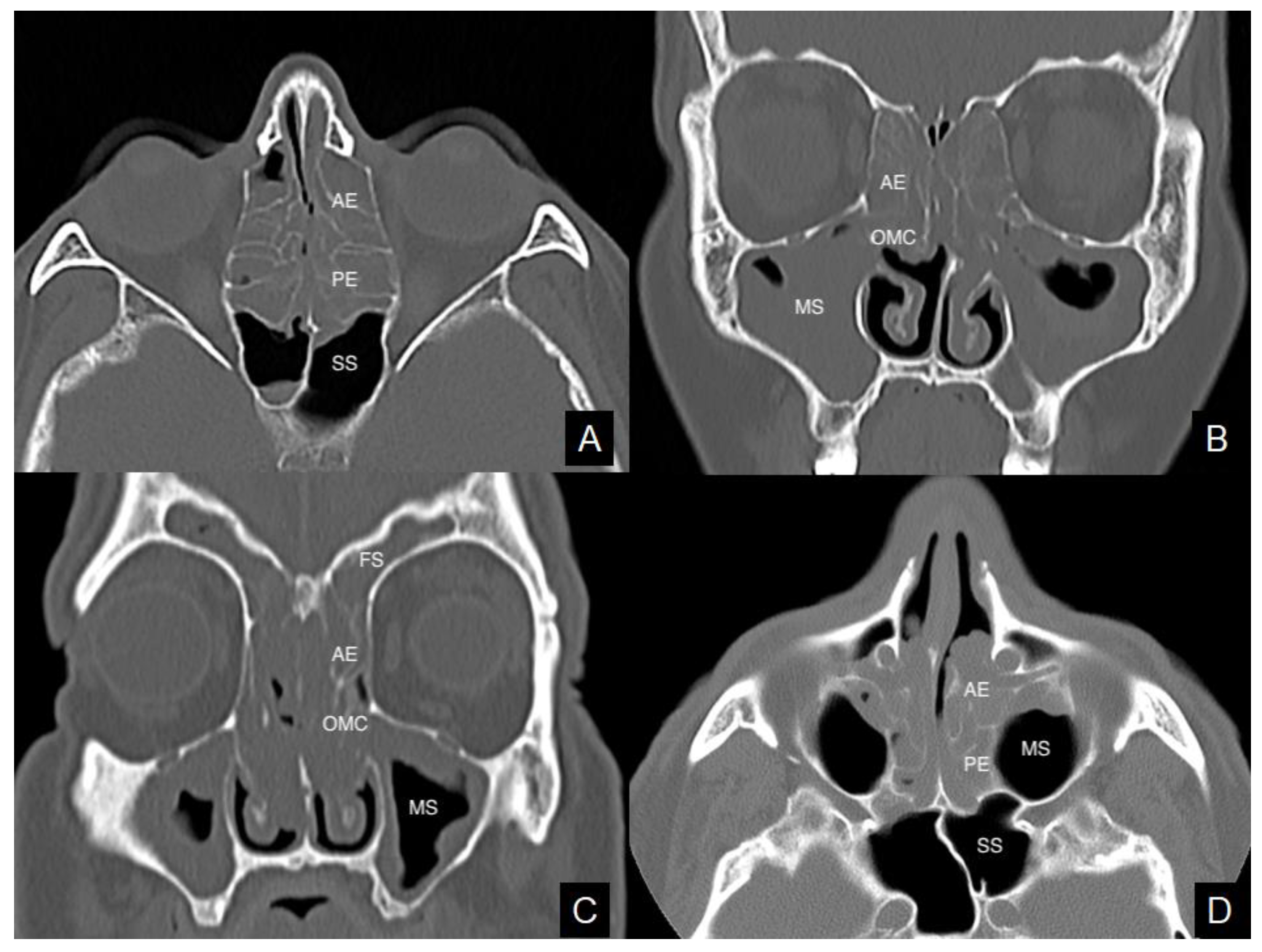
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Statistical Analysis
3. Results
3.1. Sinonasal Computed Tomography (CT) Scores and Clinical Features
3.2. Sinonasal CT Scores and Laboratory Results
3.3. Sinonasal CT Scores and Histopathological Evidence
3.4. CT Scores and Prognosis after Endoscopic Sinus Surgery (ESS)
4. Discussion
4.1. Sinonasal CT Staging System and Prognosis in Chronic Rhinosinusitis with Nasal Polyps (CRSwNPs)
4.2. Sinonasal CT Staging System and Phenotype/Endotype Features in CRSwNPs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brescia, G.; Alessandrini, L.; Marioni, G. Structured histopathology for endotyping and planning rational treatment in chronic rhinosinusitis. Am. J. Otolaryngol. 2021, 42, 102795. [Google Scholar] [CrossRef]
- Brescia, G.; Barion, U.; Zanotti, C.; Giacomelli, L.; Martini, A.; Marioni, G. The prognostic role of serum eosinophil and basophil levels in sinonasal polyposis. Int. Forum Allergy Rhinol. 2017, 7, 261–267. [Google Scholar] [CrossRef]
- Brescia, G.; Marioni, G.; Franchella, S.; Ramacciotti, G.; Pendolino, A.L.; Callegaro, F.; Giacomelli, L.; Marino, F.; Martini, A. Post-operative steroid treatment for eosinophilic-type sinonasal polyposis. Acta Oto-Laryngol. 2015, 135, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58 (Suppl S29), 1–464. [Google Scholar] [CrossRef]
- Orlandi, R.R.; Kingdom, T.T.; Smith, T.L.; Bleier, B.; DeConde, A.; Luong, A.U.; Poetker, D.M.; Soler, Z.; Welch, K.C.; Wise, S.K.; et al. International consensus statement on allergy and rhinology: Rhinosinusitis 2021. Int. Forum Allergy Rhinol. 2021, 11, 213–739. [Google Scholar] [PubMed]
- Huang, B.Y.; Senior, B.A.; Castillo, M. Current trends in sinonasal imaging. Neuroimaging Clin. N. Am. 2015, 25, 507–525. [Google Scholar] [CrossRef] [PubMed]
- Roland, L.T.; Marcus, S.; Schertzer, J.S.; Wise, S.K.; Levy, J.M.; DelGaudio, J.M. Computed tomography findings can help identify different chronic rhinosinusitis with nasal polyp phenotypes. Am. J. Rhinol Allergy 2020, 34, 679–685. [Google Scholar] [CrossRef]
- Lund, V.J.; Mackay, I.S. Staging in rhinosinusitus. Rhinology 1993, 31, 183–184. [Google Scholar] [PubMed]
- Abdullah, B.; Vengathajalam, S.; Daud, M.K.M.; Wan Mohammad, Z.; Hamizan, A.; Husain, S. The clinical and radiological characterizations of the allergic phenotype of chronic rhinosinusitis with nasal polyps. J. Asthma Allergy 2020, 13, 523–531. [Google Scholar] [CrossRef]
- Brooks, S.G.; Trope, M.; Blasetti, M.; Doghramji, L.; Parasher, A.; Glicksman, J.T.; Kennedy, D.W.; Thaler, E.R.; Cohen, N.A.; Palmer, J.N.; et al. Preoperative Lund-Mackay computed tomography score is associated with preoperative symptom severity and predicts quality-of-life outcome trajectories after sinus surgery. Int. Forum Allergy Rhinol. 2018, 8, 668–675. [Google Scholar] [CrossRef]
- Mamat Nasir, M.S.N.; Aziz, M.E.; Tuan Sharif, S.E.; Ibrahim, R.; Abdullah, B. Clinical symptoms of chronic rhinosinusitis with nasal polyps (eosinophilic and non-eosinophilic) are related to sinus computed tomography but not to endoscopic findings. Acta Otorrinolaringol. Esp. (Engl. Ed.) 2021. [Google Scholar] [CrossRef]
- Liu, D.T.; Schwarz-Nemec, U.; Renner, B.; Mueller, C.A.; Besser, G. Radiological markers of the olfactory cleft: Relations to unilateral orthonasal and retronasal olfactory function. Diagnostics 2020, 10, 989. [Google Scholar] [CrossRef]
- Mackay, I.S.; Lund, V.J. Imaging and staging. In Nasal Polyposis: An Inflammatory Disease and Its Treatment; Mygind, N., Lildholdt, T., Eds.; Munksgaard: Copenhagen, Denmark, 1997; pp. 137–144. [Google Scholar]
- Horak, F.; Doberer, D.; Eber, E.; Horak, E.; Pohl, W.; Riedler, J.; Szépfalusi, Z.; Wantke, F.; Zacharasiewicz, A.; Studnicka, M. Diagnosis and management of asthma—Statement on the 2015 GINA Guidelines. Wien. Klin. Wochenschr. 2016, 128, 541–554. [Google Scholar] [CrossRef] [Green Version]
- Gregurić, T.; Trkulja, V.; Baudoin, T.; Grgić, M.V.; Šmigovec, I.; Kalogjera, L. Association between computed tomography findings and clinical symptoms in chronic rhinosinusitis with and without nasal polyps. Eur. Arch. Otorhinolaryngol. 2017, 274, 2165–2173. [Google Scholar] [CrossRef]
- Lu, H.; Liu, Z.; Hu, L.; Feng, X.; Gu, Y.; Li, H.; Li, H.; Wang, D. Are objective ‘findings’ the same as subjective ‘severity’? A study of the relationship between computed tomography findings and subjective severity in preoperative CRSwNP patients. Exp. Ther. Med. 2020, 20, 2985–2992. [Google Scholar] [CrossRef]
- Kim, J.Y.; Han, Y.E.; Seo, Y.; Choe, G.; Kim, M.K.; Huh, G.; Cho, D.; Yang, S.K.; Kang, S.H.; Kim, D.W. Revisiting the clinical scoring system for the prognosis of chronic rhinosinusitis with nasal polyps. Yonsei Med. J. 2019, 60, 578–584. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, L.; Lou, H.; Wang, C. Predictive value of computed tomography in the recurrence of chronic rhinosinusitis with nasal polyps. Int. Forum Allergy Rhinol. 2019, 9, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Kwun, C.; Kim, S.I.; Lee, K.H.; Kim, S.W. Evaluation of aspirin hypersensitivity in patients with chronic rhinosinusitis. Acta Oto-Laryngol. 2016, 136, 633–635. [Google Scholar] [CrossRef] [PubMed]
- Brescia, G.; Sfriso, P.; Marioni, G. Role of blood inflammatory cells in chronic rhinosinusitis with nasal polyps. Acta Oto-Laryngol. 2019, 139, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Sreeparvathi, A.; Kalyanikuttyamma, L.K.; Kumar, M.; Sreekumar, N.; Veerasigamani, N. Significance of Blood Eosinophil Count in Patients with Chronic Rhinosinusitis with Nasal Polyposis. J. Clin. Diagn. Res. 2017, 11, MC08–MC11. [Google Scholar] [CrossRef] [PubMed]
- Brescia, G.; Zanotti, C.; Parrino, D.; Barion, U.; Marioni, G. Nasal polyposis pathophysiology: Endotype and phenotype open issues. Am. J. Otolaryngol. 2018, 39, 441–444. [Google Scholar] [CrossRef]
- Grgić, M.V.; Ćupić, H.; Kalogjera, L.; Baudoin, T. Surgical treatment for nasal polyposis: Predictors of outcome. Eur. Arch. Otorhinolaryngol. 2015, 272, 3735–3743. [Google Scholar] [CrossRef] [PubMed]
- Brescia, G.; Pedruzzi, B.; Barion, U.; Cinetto, F.; Giacomelli, L.; Martini, A.; Marioni, G. Are neutrophil-, eosinophil-, and basophil-to-lymphocyte ratios useful markers for pinpointing patients at higher risk of recurrent sinonasal polyps? Am. J. Otolaryngol. 2016, 37, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Lou, H.; Wang, C.; Zhang, L. Predictive significance of computed tomography in eosinophilic chronic rhinosinusitis with nasal polyps. Int. Forum Allergy Rhinol. 2016, 6, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Rai, G.; Roy, P.; Gupta, N.; Sharma, S.; Dar, S.A.; Ansari, M.A.; Ramachandran, V.G.; Das, S. Computed tomography score an excellent marker: Differentiates eosinophilic and non-eosinophilic variants of chronic rhinosinusitis with nasal polyp. Indian J. Otolaryngol. Head Neck Surg. 2019, 71 (Suppl 3), 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- DelGaudio, J.M.; Loftus, P.A.; Hamizan, A.W.; Harvey, R.J.; Wise, S.K. Central compartment atopic disease. Am. J. Rhinol. Allergy 2017, 31, 228–234. [Google Scholar] [CrossRef]
| NERD | Univariate Logistic Regression | Multivariate Logistic Regression | ||||
|---|---|---|---|---|---|---|
| No. Cases = 78 | No (No. Cases = 68) | Yes (No. Cases = 10) | p Value | OR (95% CI) | p Value | OR (95% CI) |
| OMC CT score | ||||||
| Mean (SD) | 1.60 (1.63) | 3.00 (1.70) | 0.0235 | 1.706 (1.075–2.709) | 0.2592 | 1.381 (0.788–2.419) |
| Median (IQR) | 2.00 (0.00–3.00) | 4.00 (2.00–4.00) | ||||
| Frontal CT score | ||||||
| Mean (SD) | 1.22 (1.40) | 2.50 (1.58) | 0.0164 | 1.742 (1.107–2.742) | 0.3434 | 1.333 (0.735–2.419) |
| Median (IQR) | 1.00 (0.00–2.00) | 2.50 (2.00–4.00) | ||||
| Maxillary CT score | ||||||
| Mean (SD) | 2.07 (1.07) | 2.80 (1.03) | 0.0548 | 2.034 (0.985–4.199) | NC | NC |
| Median (IQR) | 2.00 (1.00–3.00) | 3.00 (3.00–3.00) | ||||
| Ethmoid CT score * | ||||||
| Mean (SD) | 4.49 (2.66) | 6.40 (2.80) | 0.0496 | 1.358 (1.000–1.842) | 0.7974 | 1.054 (0.704–1.580) |
| Median (IQR) | 4.00 (2.00–7.00) | 8.00 (4.00–8.00) | ||||
| Sphenoid CT score | ||||||
| Mean (SD) | 0.54 (0.94) | 1.40 (1.84) | 0.0335 | 1.722 (1.043–2.841) | 0.5947 | 1.174 (0.651–2.116) |
| Median (IQR) | 0.00 (0.00–1.00) | 0.00 (0.00–3.00) | ||||
| Total CT score | ||||||
| Mean (SD) | 9.93 (5.56) | 16.10 (7.00) | 0.0062 | 1.193 (1.051–1.354) | NC | NC |
| Median (IQR) | 9.00 (6.00–14.00) | 19.00 (9.00–22.00) | ||||
| Asthma | Univariate Logistic Regression | Multivariate Logistic Regression | ||||
|---|---|---|---|---|---|---|
| No. Cases = 78 | No (No. Cases = 48) | Yes (No. Cases = 30) | p Value | OR (95% CI) | p Value | OR (95% CI) |
| OMC CT score | ||||||
| Mean (SD) | 1.35 (1.49) | 2.47 (1.80) | 0.0058 | 1.505 (1.125–2.012) | 0.1392 | 1.300 (0.918–1.842) |
| Median (IQR) | 1.50 (0.00–2.00) | 4.00 (0.00–4.00) | ||||
| Frontal CT score | ||||||
| Mean (SD) | 1.04 (1.25) | 1.93 (1.66) | 0.0116 | 1.522 (1.099–2.108) | 0.4218 | 1.198 (0.770–1.864) |
| Median (IQR) | 1.00 (0.00–2.00) | 2.00 (0.00–4.00) | ||||
| Maxillary CT score | ||||||
| Mean (SD) | 1.96 (0.97) | 2.50 (1.20) | 0.0357 | 1.643 (1.034–2.612) | 0.3003 | 1.312 (0.785–2.192) |
| Median (IQR) | 2.00 (1.00–2.50) | 3.00 (2.00–3.00) | ||||
| Ethmoid CT score * | ||||||
| Mean (SD) | 4.19 (2.73) | 5.60 (2.57) | 0.0290 | 1.223 (1.021–1.466) | 0.8921 | 1.017 (0.798–1.296) |
| Median (IQR) | 4.00 (2.00–6.50) | 6.00 (4.00–8.00) | ||||
| Sphenoid CT score | ||||||
| Mean (SD) | 0.44 (0.85) | 1.00 (1.39) | 0.0370 | 1.582 (1.028–2.434) | 0.7068 | 1.104 (0.660–1.846) |
| Median (IQR) | 0.00 (0.00–0.50) | 0.00 (0.00–2.00) | ||||
| Total CT score | ||||||
| Mean (SD) | 8.98 (5.08) | 13.50 (6.56) | 0.0022 | 1.141 (1.049–1.242) | NC | NC |
| Median (IQR) | 8.00 (5.00–13.50) | 13.50 (7.00–20.00) | ||||
| Blood Eosinophil Count (Cells × 109/L) | Univariate Logistic Regression | Multivariate Logistic Regression | ||||
|---|---|---|---|---|---|---|
| No. Cases = 75 | ≤0.24 Cells × 109/L * (No. Cases = 29) | >0.24 Cells × 109/L (No. Cases = 46) | p Value | OR (95% CI) | p Value | OR (95% CI) |
| OMC CT score | ||||||
| Mean (SD) | 1.48 (1.57) | 2.04 (1.76) | 0.1654 | 1.221 (0.921–1.620) | NC | NC |
| Median (IQR) | 2.00 (0.00–2.00) | 2.00 (0.00–4.00) | ||||
| Frontal CT score | ||||||
| Mean (SD) | 1.14 (1.25) | 1.59 (1.61) | 0.2047 | 1.238 (0.890–1.721) | NC | NC |
| Median (IQR) | 1.00 (0.00–2.00) | 1.00 (0.00–3.00) | ||||
| Maxillary CT score | ||||||
| Mean (SD) | 1.79 (0.98) | 2.39 (1.08) | 0.0227 | 1.729 (1.080–2.769) | 0.0700 | 1.574 (0.964–2.573) |
| Median (IQR) | 2.00 (1.00–2.00) | 3.00 (2.00–3.00) | ||||
| Ethmoid CT score ** | ||||||
| Mean (SD) | 4.10 (2.51) | 5.37 (2.69) | 0.0491 | 1.200 (1.001–1.439) | 0.1790 | 1.140 (0.942–1.381) |
| Median (IQR) | 4.00 (2.00–5.00) | 6.00 (4.00–8.00) | ||||
| Sphenoid CT score | ||||||
| Mean (SD) | 0.34 (0.94) | 0.87 (1.20) | 0.0611 | 1.654 (0.977–2.802) | NC | NC |
| Median (IQR) | 0.00 (0.00–0.00) | 0.00 (0.00–2.00) | ||||
| Total CT score | ||||||
| Mean (SD) | 8.86 (5.00) | 12.26 (6.38) | 0.0213 | 1.106 (1.015–1.206) | NC | NC |
| Median (IQR) | 9.00 (6.00–11.00) | 13.00 (7.00–18.00) | ||||
| Histologically Eosinophil CRSwNPs | Univariate Logistic Regression | |||
|---|---|---|---|---|
| No. Cases = 78 | No (No. Cases = 36) | Yes (No. Cases = 42) | p Value | OR (95% CI) |
| OMC CT score | ||||
| Mean (SD) | 1.39 (1.71) | 2.12 (1.63) | 0.0594 | 1.303 (0.990 1.715) |
| Median (IQR) | 0.00 (0.00–3.00) | 2.00 (0.00–4.00) | ||
| Frontal CT score | ||||
| Mean (SD) | 1.11 (1.39) | 1.62 (1.53) | 0.1324 | 1.274 (0.929 1.748) |
| Median (IQR) | 0.50 (0.00–2.00) | 1.00 (0.00–3.00) | ||
| Maxillary CT score | ||||
| Mean (SD) | 1.94 (0.92) | 2.36 (1.19) | 0.0973 | 1.439 (0.936 2.214) |
| Median (IQR) | 2.00 (1.00–3.00) | 2.50 (2.00–3.00) | ||
| Ethmoid CT score * | ||||
| Mean (SD) | 3.53 (2.78) | 5.76 (2.26) | 0.0006 | 1.399 (1.154 1.696) |
| Median (IQR) | 3.00 (1.50–5.50) | 6.00 (4.00–8.00) | ||
| Sphenoid CT score | ||||
| Mean (SD) | 0.42 (0.94) | 0.86 (1.22) | 0.0902 | 1.483 (0.940 2.338) |
| Median (IQR) | 0.00 (0.00–0.00) | 0.00 (0.00–2.00) | ||
| Total CT score | ||||
| Mean (SD) | 8.39 (5.95) | 12.71 (5.50) | 0.0027 | 1.142 (1.047 1.245) |
| Median (IQR) | 6.50 (3.50–12.00) | 12.50 (8.00–18.00) | ||
| Frontal CT score | Total CT score | |||
|---|---|---|---|---|
| Follow-Up (Months) | AUC (Standard Error) | 95% Confidence Interval | AUC (Standard Error) | 95% Confidence Interval |
| 6 | 0.6694 (0.0579) | 0.5558–0.7829 | 0.629 (0.0785) | 0.4751–0.7830 |
| 12 | 0.4131 (0.1422) | 0.1345–0.6918 | 0.5411 (0.0980) | 0.3489–0.7332 |
| 18 | 0.5167 (0.1286) | 0.2647–0.7688 | 0.5389 (0.0883) | 0.3658–0.7121 |
| 24 | 0.6476 (0.0959) | 0.4596–0.8356 | 0.6073 (0.1087) | 0.3943–0.8202 |
| 30 | 0.7104 (0.1015) | 0.5115–0.9093 | 0.6458 (0.1094) | 0.4313–0.8602 |
| 36 | 0.7885 (0.0988) | 0.5948–0.9821 | 0.7475 (0.0972) | 0.5571–0.9380 |
| 42 | 0.7977 (0.0989) | 0.6039–0.9915 | 0.7588 (0.0971) | 0.5686–0.9491 |
| 48 | 0.9759 (0.0279) | 0.9213–1.0000 | 0.8355 (0.1517) | 0.5382–1.0000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brescia, G.; Contro, G.; Ruaro, A.; Frigo, A.C.; Barion, U.; Marioni, G. Preoperative Sinonasal Computed Tomography Score in Chronic Rhinosinusitis with Nasal Polyps. Tomography 2022, 8, 77-88. https://doi.org/10.3390/tomography8010007
Brescia G, Contro G, Ruaro A, Frigo AC, Barion U, Marioni G. Preoperative Sinonasal Computed Tomography Score in Chronic Rhinosinusitis with Nasal Polyps. Tomography. 2022; 8(1):77-88. https://doi.org/10.3390/tomography8010007
Chicago/Turabian StyleBrescia, Giuseppe, Giacomo Contro, Alessandra Ruaro, Anna Chiara Frigo, Umberto Barion, and Gino Marioni. 2022. "Preoperative Sinonasal Computed Tomography Score in Chronic Rhinosinusitis with Nasal Polyps" Tomography 8, no. 1: 77-88. https://doi.org/10.3390/tomography8010007
APA StyleBrescia, G., Contro, G., Ruaro, A., Frigo, A. C., Barion, U., & Marioni, G. (2022). Preoperative Sinonasal Computed Tomography Score in Chronic Rhinosinusitis with Nasal Polyps. Tomography, 8(1), 77-88. https://doi.org/10.3390/tomography8010007







