Identification of Pelvic Congestion Syndrome Using Transvaginal Ultrasonography. A Useful Tool
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Data Collection
2.3. Ultrasound Assessment
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Taylor, H.C. Vascular congestion and hyperemia. Am. J. Obstet. Gynecol. 1949, 57, 211–230. [Google Scholar] [CrossRef]
- Beard, R.; Pearce, S.; Highman, J.; Reginald, P. Diagnosis of pelvic varicosities in women with chronic pelvic pain. Lancet 1984, 324, 946–949. [Google Scholar] [CrossRef]
- Meissner, M.H.; Khilnani, N.M.; Labropoulos, N.; Gasparis, A.P.; Gibson, K.; Greiner, M.; Learman, L.A.; Atashroo, D.; Lurie, F.; Passman, M.A. The Symp-toms-Varices-Pathophysiology classification of pelvic venous disorders: A report of the American Vein & Lymphatic Society International Working Group on Pelvic Venous Disorders. J. Vasc. Surg. Venous Lymphat. Disorder. 2021, 9, 568–584. [Google Scholar]
- Park, S.J.; Lim, J.W.; Ko, Y.T.; Lee, D.H.; Yoon, Y.; Oh, J.H.; Lee, H.K.; Huh, C.Y. Diagnosis of Pelvic Congestion Syndrome Using Transabdominal and Transvaginal Sonography. Am. J. Roentgenol. 2004, 182, 683–688. [Google Scholar] [CrossRef]
- Harris, R.D.; Holtzman, S.R.; Poppe, A.M. Clinical outcome in female patients with pelvic pain and normal pelvic US findings. Radiology 2000, 216, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Arnoldussen, C.W.K.P.; Wolf, M.A.F.D.; Wittens, C.H.A. Diagnostic imaging of pelvic congestive syndrome. Phlebol. J. Venous Dis. 2015, 30, 67–72. [Google Scholar] [CrossRef]
- Ganeshan, A.; Upponi, S.; Hon, L.-Q.; Uthappa, M.C.; Warakaulle, D.R.; Uberoi, R. Chronic Pelvic Pain due to Pelvic Congestion Syndrome: The Role of Diagnostic and Interventional Radiology. Cardiovasc. Interv. Radiol. 2007, 30, 1105–1111. [Google Scholar] [CrossRef]
- Steenbeek, M.P.; van der Vleuten, C.J.M.; Schultze Kool, L.J.; Nieboer, T.E. Noninvasive diagnostic tools for pelvic congestion syndrome: A systematic review. Acta Obstet. Gynecol. Scand. 2018, 97, 776–786. [Google Scholar] [CrossRef]
- Gloviczki, P.; Comerota, A.J.; Dalsing, M.C.; Eklof, B.G.; Gillespie, D.; Gloviczki, M.L.; Lohr, J.M.; McLafferty, R.B.; Meissner, M.H.; Murad, M.H.; et al. The care of patients with varicose veins and associated chronic venous diseases: Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J. Vasc. Surg. 2011, 53, 2S–48S. [Google Scholar] [CrossRef] [Green Version]
- Malgor, R.D.; Adrahtas, D.; Spentzouris, G.; Gasparis, A.P.; Tassiopoulos, A.K.; Labropoulos, N. The role of duplex ultrasound in the workup of pelvic congestion syndrome. J. Vasc. Surg. Venous Lymphat. Disord. 2014, 2, 34–38. [Google Scholar] [CrossRef]
- Freedman, J.; Ganeshan, A.; Crowe, P.M. Pelvic congestion syndrome: The role of interventional radiology in the treatment of chronic pelvic pain. Postgrad. Med. J. 2010, 86, 704–710. [Google Scholar] [CrossRef]
- Smith, M. Sonographic View of Pelvic Congestion Syndrome. J. Diagn. Med. Sonogr. 2017, 33, 193–198. [Google Scholar] [CrossRef]
- Whiteley, M.S.; Dos Santos, S.; Harrison, C.C.; Holdstock, J.M.; Lopez, A.J. Transvaginal duplex ultrasonography appears to be the gold standard investigation for the haemodynamic evaluation of pelvic venous reflux in the ovarian and internal iliac veins in women. Phlebol. J. Venous Dis. 2014, 30, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Knuttinen, M.-G.; Xie, K.; Jani, A.; Palumbo, A.; Carrillo, T.; Mar, W. Pelvic Venous Insufficiency: Imaging Diagnosis, Treatment Approaches, and Therapeutic Issues. Am. J. Roentgenol. 2015, 204, 448–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, M.; Sauerbrei, E.E.; Cooperberg, P.L. Medical implications of ultrasonically detected polycystic ovaries. J. Clin. Ultrasound 1981, 9, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Coakley, F.V.; Varghese, S.L.; Hricak, H. CT and MRI of Pelvic Varices in Women. J. Comput. Assist. Tomogr. 1999, 23, 429–434. [Google Scholar] [CrossRef]
- Geier, B.; Barbera, L.; Mumme, A.; Köster, O.; Marpea, B.; Kaminsky, C.; Asciutto, G. Reflux patterns in the ovarian and hypogastric veins in patients with varicose veins and signs of pelvic venous incompetence. Chir. Ital. 2007, 59, 481–488. [Google Scholar]
- Corrêa, M.P.; Bianchini, L.; Saleh, J.N.; Noel, R.S.; Bajerski, J.C. Síndrome da congestão pélvica e embolização de varizes pélvicas. J. Vasc. Bras. 2019, 18, 20190061. [Google Scholar] [CrossRef]
- Leiber, L.; Thouveny, F.; Bouvier, A.; Labriffe, M.; Berthier, E.; Aube, C.; Willoteaux, S. MRI and venographic aspects of pelvic venous insufficiency. Diagn. Interv. Imaging 2014, 95, 1091–1102. [Google Scholar] [CrossRef] [Green Version]
- Díaz- Reyes, C.G. Várices pélvicas y síndrome de congestión pélvica en la mujer. Rev. CES Med. 2012, 26, 57–69. [Google Scholar]
- Ahlberg, N.E.; Bartley, O.; Chidekel, N. Right and left gonadal veins. An anatomical and statistical study: An anatomical and statistical study. Acta Radiol. Diagn. 1966, 4, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Amin, T.N.; Wong, M.; Foo, X.; Pointer, S.-L.; Goodhart, V.; Jurkovic, D. The effect of pelvic pathology on uterine vein diameters. Ultrasound J. 2021, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Bora, M.K.; Varghese, J.; Malik, G.; Kuruvilla, R. Role of trans vaginal ultrasound and Doppler in diagnosis of pelvic congestion syndrome. J. Clin. Diagn. Res. 2014, 8, OD05–OD07. [Google Scholar] [CrossRef] [PubMed]
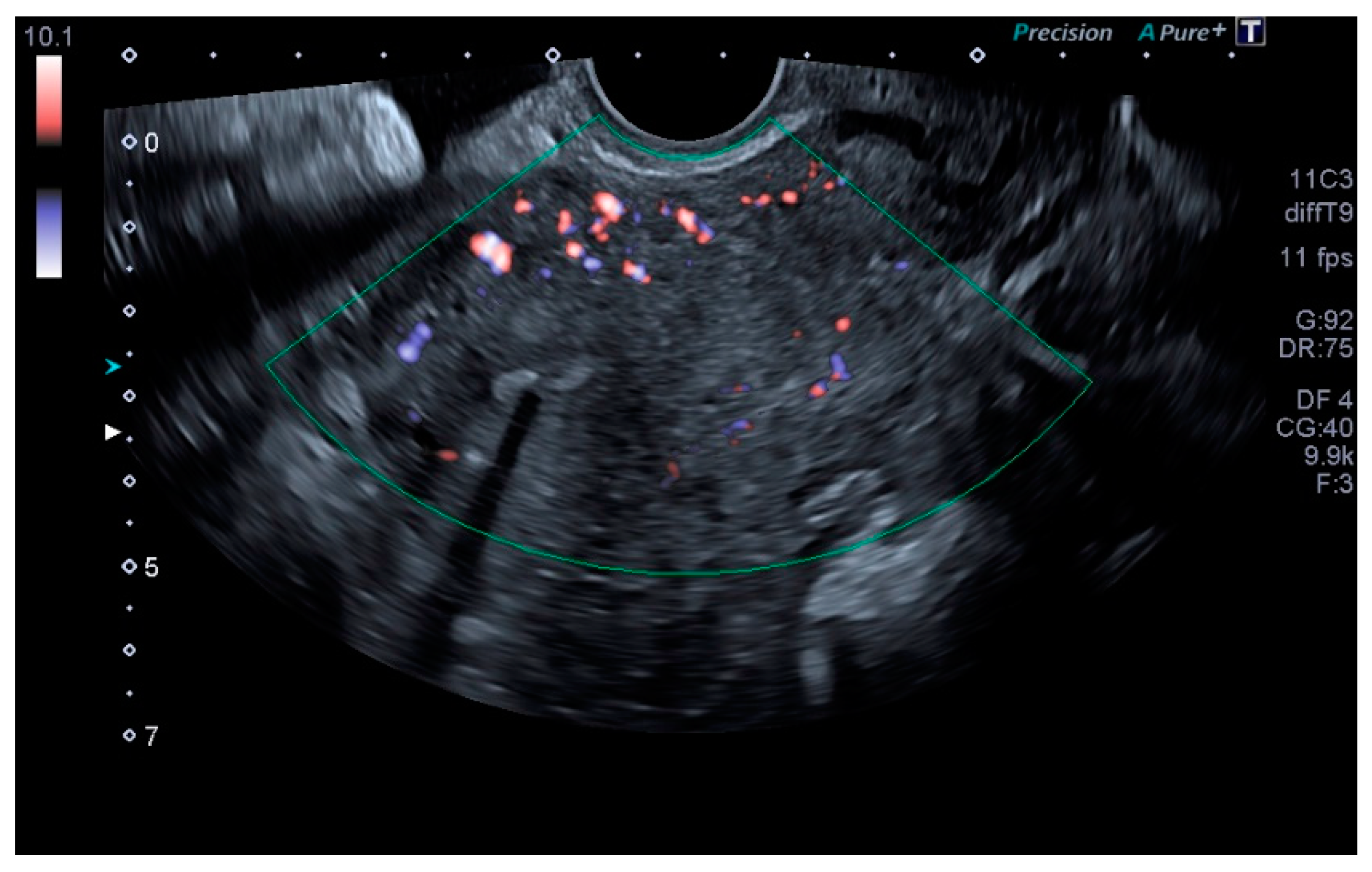

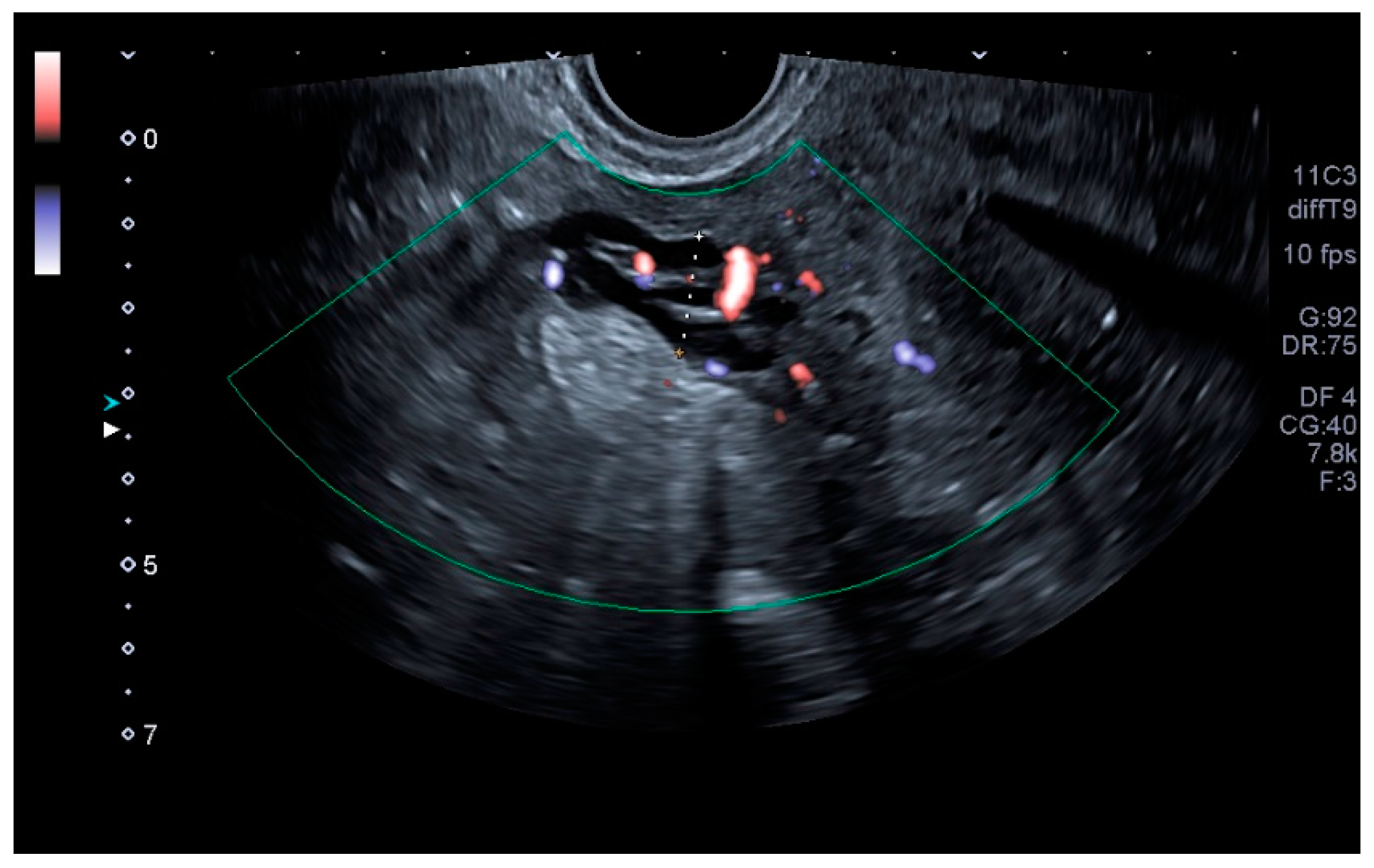


| Variables | PCS Group | Normal Group | All Patients | p |
|---|---|---|---|---|
| Age | 41.5 ± 6.95 | 44.85 ± 9.92 | 42.2 ± 7.8 | 0.212 |
| Multiparity | 29 (74.3%) | 11 (91.6%) | 40 (80%) | 0.197 |
| Maximum newborn birth weight | 3565.56 ± 546.7 | 3665 ± 442.14 | 3591.9 ± 518.5 | 0.476 |
| Menopausal | 3 (7.69%) | 3 (25%) | 6 (11.8%) | 0.165 |
| Age of the on-set of symptoms | 31.5 ± 8.5 | 37.25 ± 12.7 | 32.96 ± 9.9 | 0.847 |
| Worsening of symptoms during pregnancy | 27 (69.2%) | 5 (41.6%) | 32 (64%) | 0.119 |
| Vulvar varicosities during pregnancy | 24 (61.5%) | 8 (66.6%) | 32 (64%) | 0.83 |
| Medical history | 33 (84.6%) | 10 (83.3%) | 43 (84.3%) | 0.396 |
| Endometriosis | 1 (2.5%) | 1 (8.3%) | 2 (3.9%) | 0.449 |
| Adenomyosis | 1 (2.5%) | 2 (16.6%) | 3 (5.9%) | 0.156 |
| Urologic disorders | 3 (7.69%) | 0 (0%) | 3 (5.9%) | 0.405 |
| Gastrointestinal disorders | 0 (0%) | 1 (8.3%) | 1 (2%) | 0.255 |
| Varicosities in lower extremities | 27 (69.2%) | 6 (50%) | 33 (64.7%) | 0.105 |
| Prior pelvic surgery | 3 (7.9%) | 2 (16.6%) | 5 (9.8%) | 0.378 |
| Fibroids | 4 (10.2%) | 3 (25%) | 7 (13.7%) | 0.256 |
| Presence of varicosities (vulva, perineum, buttocks, lower extremities) | 29 (74.3%) | 7 (58.3%) | 36 (70.6%) | 0.14 |
| Pain (VAS score ≥ 7) | ||||
| Walking | 21 (53.8%) | 7 (58.3%) | 28 (56%) | 0.856 |
| Sitting | 19 (48.7%) | 4 (33.3%) | 23 (46%) | 0.2 |
| Supine | 15 (28.4%) | 5 (41.6%) | 20 (40%) | 0.895 |
| Dysmenorrhea | 23 (58.9%) | 7 (58.3%) | 30 (60%) | 0.599 |
| Dyspareunia | 16 (41%) | 4 (33.3%) | 20 (40%) | 0.43 |
| Postcoital pain | 22 (56.5%) | 9 (75%) | 31 (62%) | 0.532 |
| Lumbar pain | 13 (33.3%) | 4 (33.3%) | 17 (34%) | 0.775 |
| Variables | PCS Group | Normal Group | All Patients | p |
|---|---|---|---|---|
| Uterine volume | 80 ± 30.8 | 73 ± 66.8 | 79.7 ± 41.4 | 0.626 |
| Right ovarian volume | 9.94 ± 6.2 | 13.6 ± 13.1 | 10.65 ± 8 | 0.514 |
| Left ovarian volume | 12.64 ± 8.5 | 15.2 ± 14.4 | 13.26 ± 9.9 | 0.5 |
| PCO | 6 (15.8%) | 3 (23.1%) | 9 (17.6%) | 0.552 |
| Largest pelvic vein Ø | 6.3 ± 4.5 | 4.8 ± 1.3 | 5.9 ± 2.8 | 0.308 |
| Right side | 6.8 ± 12.4 | 3.9 ± 1.7 | 5.9 ± 10.5 | 0.411 |
| Left side | 6.1 ± 3.2 | 4.7 ± 2.1 | 5.7 ± 2.9 | 0.187 |
| Largest venous plexus Ø | 15.1 ± 6.4 | 12 ± 5.2 | 16.9 ± 12.1 | 0.009 |
| Right side | 14.3 ± 7.9 | 10.5 ± 5.3 | 13.4 ± 7.4 | 0.185 |
| Left side | 18.4 ± 13.1 | 9.5 ± 4.3 | 15.9 ± 12.03 | 0.155 |
| Reverse of altered flow during Valsalva | 23 (58.9%) | 3 (25%) | 26 (51%) | 0.04 |
| Crossing veins in the myometrium | 29 (74.35%) | 4 (33.3%) | 33 (64.7%) | 0.009 |
| Crossing veins in the myometrium Ø | 3.5 ± 1.99 | 6 ± 4.2 | 3.75 ± 2.25 | 0.141 |
| Pelvic vein Ø ≥ 8 mm | 36 (92.3%) | 3 (25%) | 39 (76.5%) | ˂0.000 |
| Variables | Left Side | Right Side | p |
|---|---|---|---|
| Ovarian volume | 8 ± 15.4 | 7.4 ± 7.99 | 0.646 |
| Largest pelvic vein Ø | 6 ± 3.6 | 4.5 ± 1.88 | 0.011 |
| Largest venous plexus Ø | 15.2 ± 8.75 | 15.5 ± 9.75 | 0.359 |
| Venography (Gold Standard) | |||||
|---|---|---|---|---|---|
| Normal | PCS | Total | p | ||
| Transvaginal Ultrasound | Normal | 9 | 3 | 12 | ˂0.005 |
| PCS | 3 | 36 | 39 | ||
| Total | 12 | 39 | 51 | ||
| Transvaginal Ultrasound | Value | CI 95% |
|---|---|---|
| Sensitivity | 92.31% | 78.03–97.99% |
| Specificity | 75% | 42.84–93.31% |
| Positive predictive value | 92.28% | 81.71–96.97% |
| Negative predictive value | 75.07% | 49.18–90.36% |
| False positive rate | 7.69% | 2.01–21.97% |
| False negative rate | 25% | 6.7–57.16% |
| Transvaginal Ultrasound | Nulliparous | Multiparous | p |
|---|---|---|---|
| Largest pelvic vein Ø | 5.6 ± 2.3 | 6.2 ± 2.9 | 0.25 |
| Largest venous plexus Ø | 16 ± 4.55 | 17.2 ± 12.6 | 0.75 |
| Premenopause | Menopause | p | |
| Largest pelvic vein Ø | 6.2 ± 2.8 | 4.9 ± 2.1 | 0.43 |
| Largest venous plexus Ø | 16 ± 7.3 | 27.5 ± 35.2 | 0.33 |
| No adenomyosis | Adenomyosis | p | |
| Largest pelvic vein Ø | 5.8 ± 2.8 | 6.9 ± 0.21 | 0.33 |
| Largest venous plexus Ø | 15.9 ± 11.6 | - | - |
| No fibroids | Fibroids | p | |
| Largest pelvic vein Ø | 6.1 ± 2.9 | 6.2 ± 0.61 | 0.7 |
| Largest venous plexus Ø | 17.1 ± 12.4 | 16.5 ± 5.7 | 0.17 |
| Normal ovaries | PCO | p | |
| Largest pelvic vein Ø | 6.11 ± 2.9 | 6.2 ± 1.8 | 0.44 |
| Largest venous plexus Ø | 17.7 ± 12.8 | 14 ± 4.8 | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valero, I.; Garcia-Jimenez, R.; Valdevieso, P.; Garcia-Mejido, J.A.; Gonzalez-Herráez, J.V.; Pelayo-Delgado, I.; Fernandez-Palacin, A.; Sainz-Bueno, J.A. Identification of Pelvic Congestion Syndrome Using Transvaginal Ultrasonography. A Useful Tool. Tomography 2022, 8, 89-99. https://doi.org/10.3390/tomography8010008
Valero I, Garcia-Jimenez R, Valdevieso P, Garcia-Mejido JA, Gonzalez-Herráez JV, Pelayo-Delgado I, Fernandez-Palacin A, Sainz-Bueno JA. Identification of Pelvic Congestion Syndrome Using Transvaginal Ultrasonography. A Useful Tool. Tomography. 2022; 8(1):89-99. https://doi.org/10.3390/tomography8010008
Chicago/Turabian StyleValero, Irene, Rocio Garcia-Jimenez, Pamela Valdevieso, Jose A. Garcia-Mejido, Jose V. Gonzalez-Herráez, Irene Pelayo-Delgado, Ana Fernandez-Palacin, and Jose A. Sainz-Bueno. 2022. "Identification of Pelvic Congestion Syndrome Using Transvaginal Ultrasonography. A Useful Tool" Tomography 8, no. 1: 89-99. https://doi.org/10.3390/tomography8010008
APA StyleValero, I., Garcia-Jimenez, R., Valdevieso, P., Garcia-Mejido, J. A., Gonzalez-Herráez, J. V., Pelayo-Delgado, I., Fernandez-Palacin, A., & Sainz-Bueno, J. A. (2022). Identification of Pelvic Congestion Syndrome Using Transvaginal Ultrasonography. A Useful Tool. Tomography, 8(1), 89-99. https://doi.org/10.3390/tomography8010008






