Acute Pulmonary Embolism: Prognostic Role of Computed Tomography Pulmonary Angiography (CTPA)
Abstract
1. Introduction
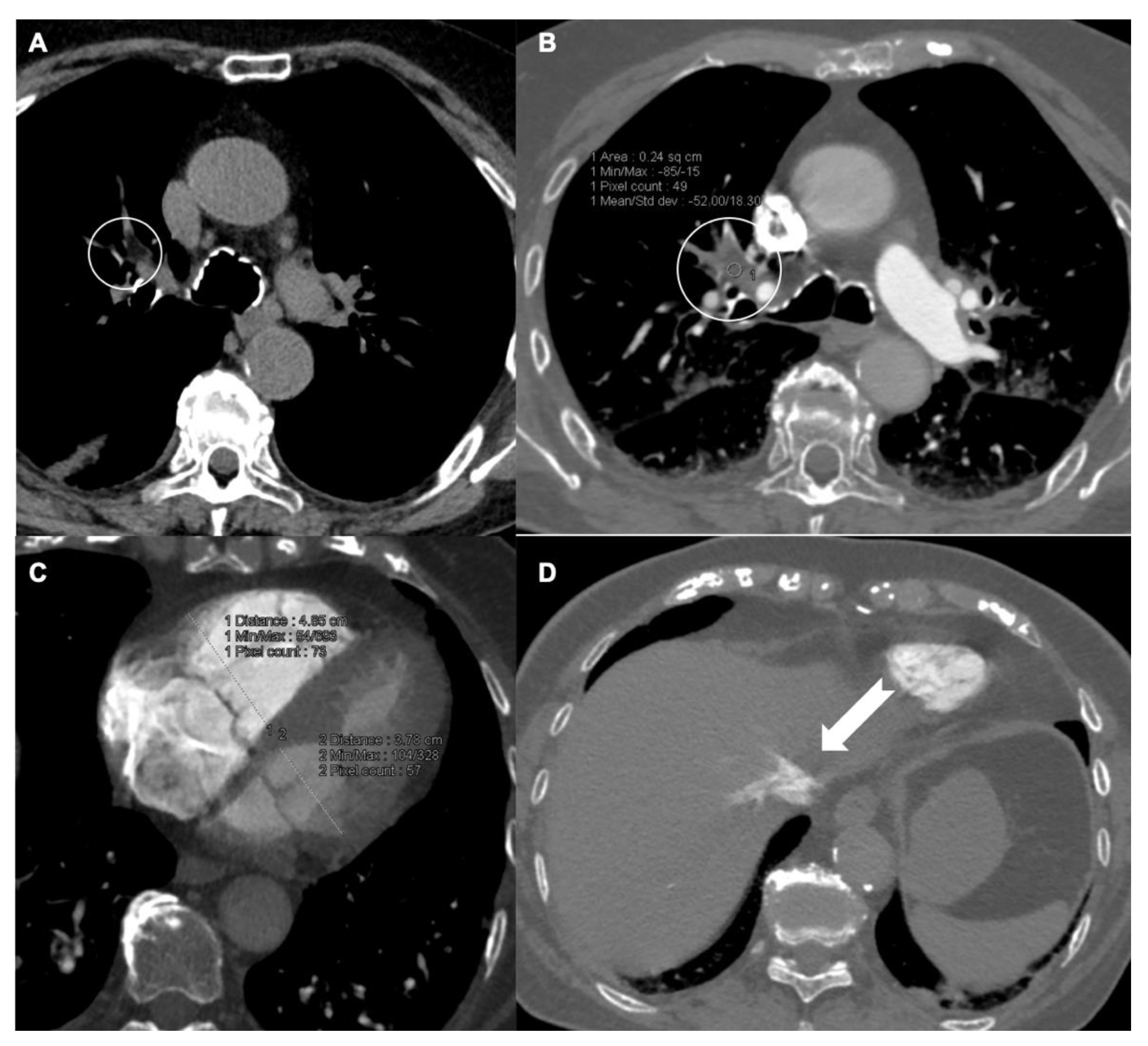
2. Pulmonary Artery Clots Burden Indexes
3. Predictor Findings
3.1. RV/LV Ratio
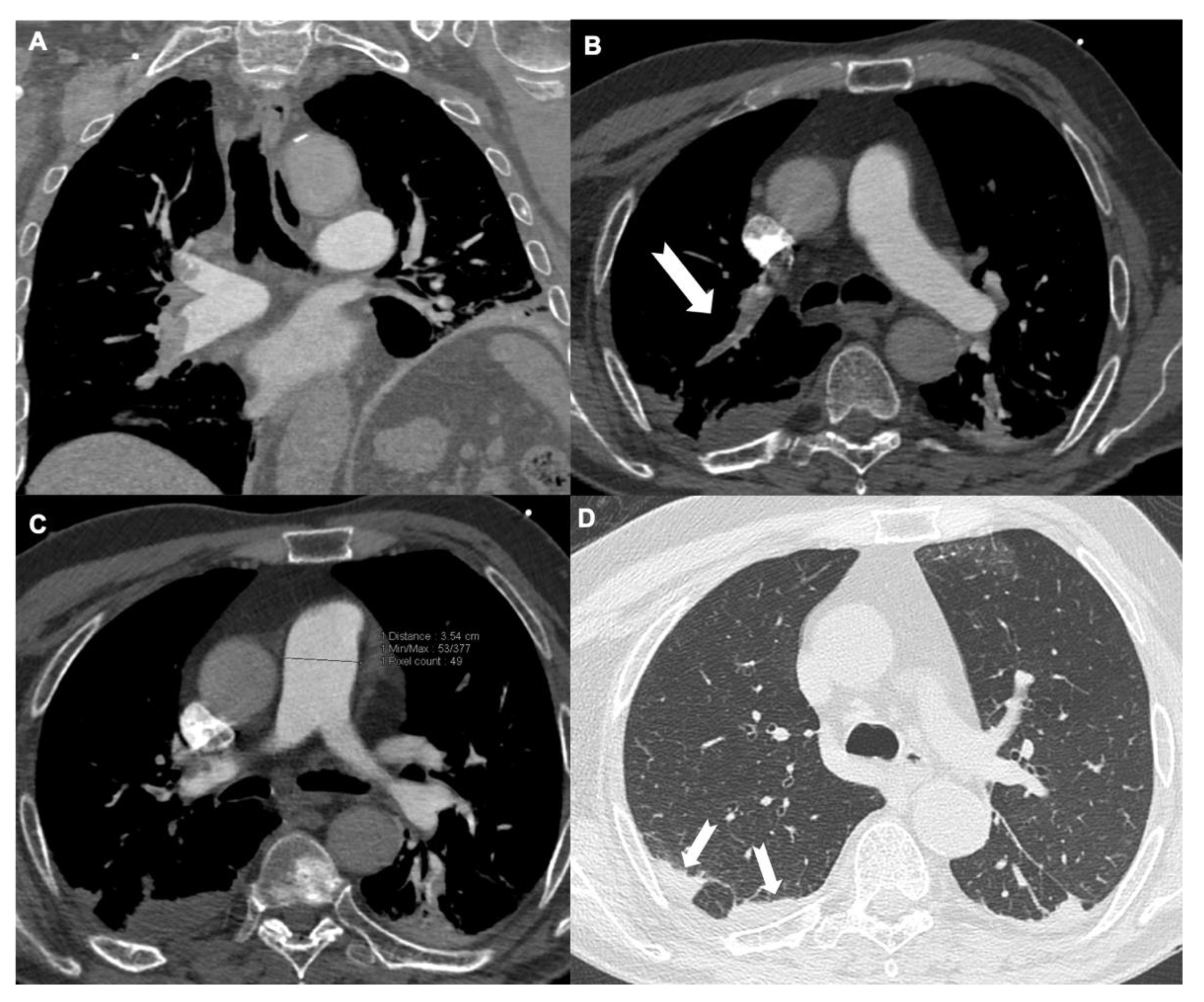
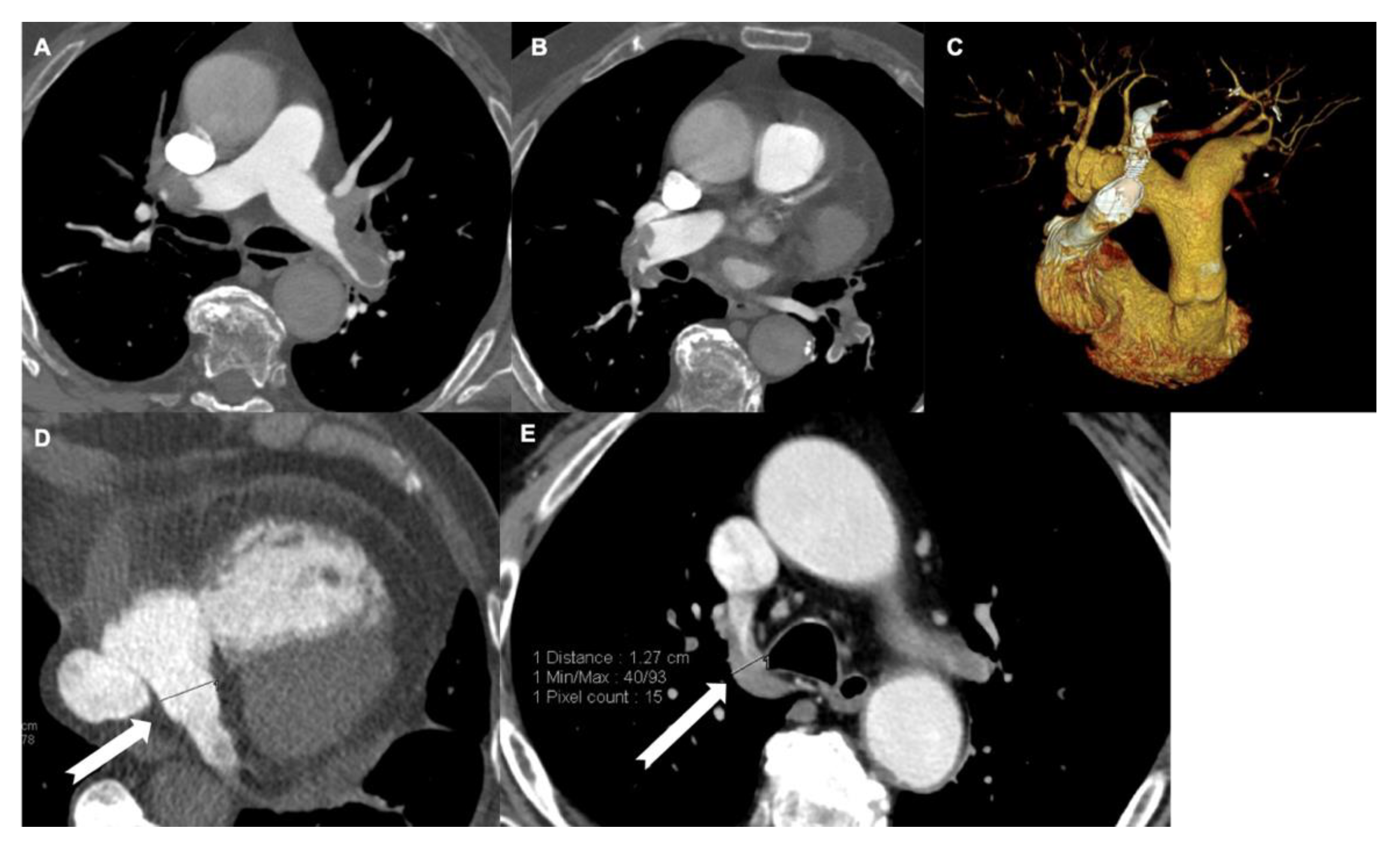
3.2. Diameter of Pulmonary Artery
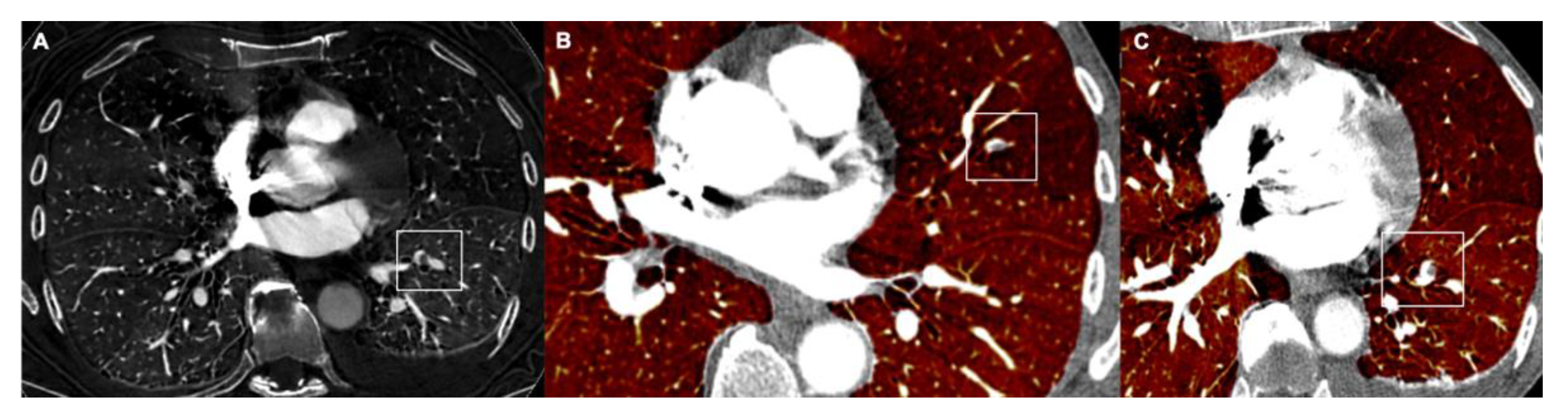
3.3. Diameter of Coronary Sinus
3.4. Inferior Vena Cava Reflux
3.5. Displacement of the Interventricular Septum
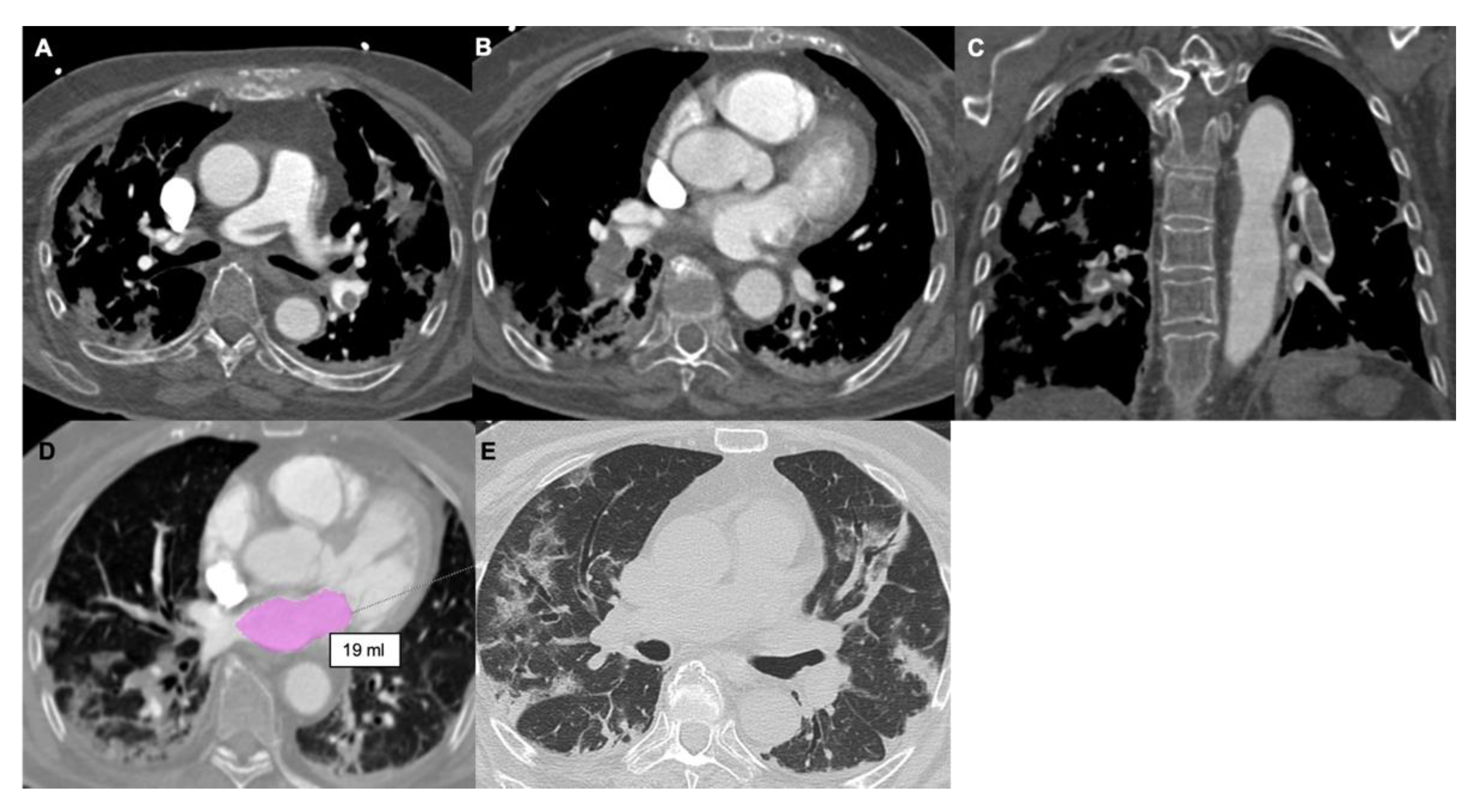
3.6. Left Atrial Size
3.7. Pulmonary Artery Distensibility
3.8. Coronary Artery Calcifications
4. COVID-19 Pneumonia
5. Dual Energy CT
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APE | Acute pulmonary embolism |
| CTOI | CT Obstruction Index |
| CTPA | Computed Tomography Pulmonary Angiography |
| IVC | Inferior vena cava |
| LA | Left atrium |
| LV | Left ventricle |
| PA | Pulmonary artery |
| PAD | Pulmonary artery distensibility |
| PE | Pulmonary embolism |
| RA | Right atrium |
| RV | Right ventricle |
| RVD | Right ventricular dysfunction |
References
- Raskob, G.E.; Angchaisuksiri, P.; Blanco, A.N.; Buller, H.; Gallus, A.; Hunt, B.J.; Hylek, E.M.; Kakkar, A.; Konstantinides, S.V.; McCumber, M.; et al. Thrombosis: A major contributor to global disease burden. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2363–2371. [Google Scholar] [CrossRef] [PubMed]
- Wendelboe, A.M.; Raskob, G.E. Global Burden of Thrombosis: Epidemiologic Aspects. Circ. Res. 2016, 118, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Harjola, V.P.V.; Mebazaa, A.; Čelutkiene, J.J.; Bettex, D.D.; Bueno, H.; Chioncel, O.O.; Crespo-Leiro, M.G.; Falk, V.; Filippatos, G.; Gibbs, S.S.; et al. Contemporary management of acute right ventricular failure: A statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur. J. Hear. Fail. 2016, 18, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.D.; Fowler, S.E.; Goodman, L.R.; Gottschalk, A.; Hales, C.A.; Hull, R.D.; Leeper, K.V., Jr.; Popovich, J., Jr.; Quinn, D.A.; Sos, T.A.; et al. Multidetector Computed Tomography for Acute Pulmonary Embolism. N. Engl. J. Med. 2006, 354, 2317–2327. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.-J.; Harjola, V.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European respiratory society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef]
- Kline, J.A.; Garrett, J.S.; Sarmiento, E.J.; Strachan, C.C.; Courtney, D.M. Over-testing for suspected pulmonary embolism in American emergency departments: The continuing epidemic. Circ. Cardiovasc. Qual. Outcomes 2020, 13, e005753. [Google Scholar] [CrossRef]
- Raji, H.; Moosavi, S.A.J.; Dastoorpoor, M.; Mohamadipour, Z.; Ghanavati, P.M. Overuse and underuse of pulmonary CT angiography in patients with suspected pulmonary embolism. Med. J. Islam. Repub. Iran 2018, 32, 13–17. [Google Scholar] [CrossRef]
- Mirabile, A.; Lucarelli, N.M.; Sollazzo, E.P.; Stabile Ianora, A.A.; Sardaro, A.; Mirabile, G.; Lorusso, F.; Racanelli, V.; Maggialetti, N.; Scardapane, A. CT pulmonary angiography appropriateness in a single emergency department: Does the use of revised Geneva score matter? Radiol. Med. 2021, 126, 1544–1552. [Google Scholar] [CrossRef]
- Cozzi, D.; Moroni, C.; Cavigli, E.; Bindi, A.; Caviglioli, C.; Nazerian, P.; Vanni, S.; Miele, V.; Bartolucci, M. Prognostic value of CT pulmonary angiography parameters in acute pulmonary embolism. Radiol. Med. 2021, 126, 1030–1036. [Google Scholar] [CrossRef]
- Shayganfar, A.; Hajiahmadi, S.; Astaraki, M.; Ebrahimian, S. The assessment of acute pulmonary embolism severity using CT angiography features. Int. J. Emerg. Med. 2020, 13, 1–5. [Google Scholar] [CrossRef]
- Wells, P.; Peacock, W.F.; Fermann, G.J.; Coleman, C.I.; Wang, L.; Baser, O.; Schein, J.; Crivera, C. The value of sPESI for risk stratification in patients with pulmonary embolism. J. Thromb. Thrombolysis 2019, 48, 149–157. [Google Scholar] [CrossRef]
- Apfaltrer, P.; Henzler, T.; Meyer, M.; Roeger, S.; Haghi, D.; Gruettner, J.; Süselbeck, T.; Wilson, R.; Schoepf, U.; Schoenberg, S.; et al. Correlation of CT angiographic pulmonary artery obstruction scores with right ventricular dysfunction and clinical outcome in patients with acute pulmonary embolism. Eur. J. Radiol. 2012, 81, 2867–2871. [Google Scholar] [CrossRef]
- Bankier, A.A.; Janata, K.; Fleischmann, D.; Kreuzer, S.; Mallek, R.; Frossard, M.; Domanovits, H.; Herold, C.J. Severity Assessment of Acute Pulmonary Embolism with Spiral CT. J. Thorac. Imaging 1997, 12, 150–158. [Google Scholar] [CrossRef]
- Qanadli, S.D.; El Hajjam, M.; Vieillard-Baron, A.; Joseph, T.; Mesurolle, B.; Oliva, V.L.; Barré, O.; Bruckert, F.; Dubourg, O.; Lacombe, P. New CT Index to Quantify Arterial Obstruction in Pulmonary Embolism. Am. J. Roentgenol. 2001, 176, 1415–1420. [Google Scholar] [CrossRef]
- Mastora, I.; Remy-Jardin, M.; Masson, P.; Galland, E.; Delannoy, V.; Bauchart, J.-J.; Remy, J. Severity of acute pulmonary embolism: Evaluation of a new spiral CT angiographic score in correlation with echocardiographic data. Eur. Radiol. 2003, 13, 29–35. [Google Scholar] [CrossRef]
- Faghihi Langroudi, T.; Sheikh, M.; Naderian, M.; Sanei Taheri, M.; Ashraf-ganjouei, A.; Khaheshi, I. The Association between the Pulmonary Arterial Obstruction Index and Atrial Size in Patients with Acute Pulmonary Embolism. Radiol. Res. Pract. 2019, 2019, 1–6. [Google Scholar] [CrossRef]
- Praveen Kumar, B.S.; Rajasekhar, D.; Vanajakshamma, V. Study of clinical, radiological and echocardiographic features and correlation of Qanadli CT index with RV dysfunction and outcomes in pulmonary embolism. Indian Heart J. 2014, 66, 629–634. [Google Scholar] [CrossRef]
- Rotzinger, D.C.; Knebel, J.-F.; Jouannic, A.-M.; Adler, G.; Qanadli, S.D. CT Pulmonary Angiography for Risk Stratification of Patients with Nonmassive Acute Pulmonary Embolism. Radiol. Cardiothorac. Imaging 2020, 2, e190188. [Google Scholar] [CrossRef]
- Sun, Z.-T.; Hao, F.-E.; Guo, Y.-M.; Liu, A.-S.; Zhao, L. Assessment of Acute Pulmonary Embolism by Computer-Aided Technique: A Reliability Study. Med. Sci. Monit. 2020, 26, e920239. [Google Scholar] [CrossRef]
- Aribas, A.; Keskin, S.; Akilli, H.; Kayrak, M.; Erdogan, H.I.; Guler, I.; Yildirim, O.; Bekci, T.T. The use of axial diameters and CT obstruction scores for determining echocardiographic right ventricular dysfunction in patients with acute pulmonary embolism. Jpn. J. Radiol. 2014, 32, 451–460. [Google Scholar] [CrossRef]
- Cho, S.-U.; Cho, Y.-D.; Choi, S.-H.; Yoon, Y.-H.; Park, J.-H.; Park, S.-J.; Lee, E.-S. Assessing the severity of pulmonary embolism among patients in the emergency department: Utility of RV/LV diameter ratio. PLoS ONE 2020, 15, e0242340. [Google Scholar] [CrossRef]
- Ayöz, S.; Erol, S.; Kul, M.; Kaya, A.G.; Çoruh, A.G.; Savaş, I.; Aydın, Ö.; Kaya, A. Using RV/LV ratio and cardiac biomarkers to define the risk of mortality from pulmonary embolism. Tuberk. Toraks 2021, 69, 297–306. [Google Scholar] [CrossRef]
- Lyhne, M.D.; Schultz, J.G.; MacMahon, P.J.; Haddad, F.; Kalra, M.; Tso, D.M.K.; Muzikansky, A.; Lev, M.H.; Kabrhel, C. Septal bowing and pulmonary artery diameter on computed tomography pulmonary angiography are associated with short-term outcomes in patients with acute pulmonary embolism. Emerg. Radiol. 2019, 26, 623–630. [Google Scholar] [CrossRef]
- Groves, A.; Win, T.; Charman, S.; Wisbey, C.; Pepke-Zaba, J.; Coulden, R. Semi-quantitative assessment of tricuspid regurgitation on contrast-enhanced multidetector CT. Clin. Radiol. 2004, 59, 715–719. [Google Scholar] [CrossRef]
- Aviram, G.; Cohen, D.; Steinvil, A.; Shmueli, H.; Keren, G.; Banai, S.; Berliner, S.; Rogowski, O. Significance of Reflux of Contrast Medium Into the Inferior Vena Cava on Computerized Tomographic Pulmonary Angiogram. Am. J. Cardiol. 2012, 109, 432–437. [Google Scholar] [CrossRef]
- Bach, A.G.; Nansalmaa, B.; Kranz, J.; Taute, B.-M.; Wienke, A.; Schramm, D.; Surov, A. CT pulmonary angiography findings that predict 30-day mortality in patients with acute pulmonary embolism. Eur. J. Radiol. 2015, 84, 332–337. [Google Scholar] [CrossRef]
- Seon, H.J.; Kim, K.H.; Lee, W.S.; Choi, S.; Yoon, H.J.; Ahn, Y.; Kim, Y.-H.; Jeong, M.H.; Cho, J.G.; Park, J.C.; et al. Usefulness of Computed Tomographic Pulmonary Angiography in the Risk Stratification of Acute Pulmonary Thromboembolism—Comparison With Cardiac Biomarkers. Circ. J. 2011, 75, 428–436. [Google Scholar] [CrossRef]
- Meinel, F.; Nance, J.W.; Schoepf, U.J.; Hoffmann, V.S.; Thierfelder, K.M.; Costello, P.; Goldhaber, S.Z.; Bamberg, F. Predictive Value of Computed Tomography in Acute Pulmonary Embolism: Systematic Review and Meta-analysis. Am. J. Med. 2015, 128, 747–759.e2. [Google Scholar] [CrossRef]
- Zuin, M.; Rigatelli, G.; Turchetta, S.; Zonzin, P.; Zuliani, G.; Roncon, L. Left atrial size measured on CT pulmonary angiography: Another parameter of pulmonary embolism severity? A systematic review. J. Thromb. Thrombolysis 2019, 50, 181–189. [Google Scholar] [CrossRef]
- Aviram, G.; Soikher, E.; Bendet, A.; Shmueli, H.; Ziv-Baran, T.; Amitai, Y.; Friedensohn, L.; Berliner, S.; Meilik, A.; Topilsky, Y. Prediction of Mortality in Pulmonary Embolism Based on Left Atrial Volume Measured on CT Pulmonary Angiography. Chest 2016, 149, 667–675. [Google Scholar] [CrossRef]
- Guo, Z.-J.; Liu, H.-T.; Bai, Z.-M.; Lin, Q.; Zhao, B.-H.; Xu, Q.; Zeng, Y.-H.; Feng, W.-Q.; Zhou, H.-T.; Liang, F.; et al. A new method of CT for the cardiac measurement: Correlation of computed tomography measured cardiac parameters and pulmonary obstruction index to assess cardiac morphological changes in acute pulmonary embolism patients. J. Thromb. Thrombolysis 2018, 45, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-K.; Hsiao, S.-H.; Lin, S.-K.; Lee, C.-Y.; Yang, S.-H.; Chang, S.-M.; Chiou, K.-R. Main Pulmonary Arterial Distensibility Different Presentation Between Chronic Pulmonary Hypertension and Acute Pulmonary Embolism. Circ. J. 2008, 72, 1454–1459. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, D.; Cui, S.; Zhu, Y.; Liu, L.; Ji, M.; Zou, D.; Zhao, R.; Liu, Q. Decreased pulmonary artery distensibility as a marker for severity in acute pulmonary embolism patients undergoing ECG-gated CTPA. J. Thromb. Thrombolysis 2021, 51, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Kasai, H.; Sugiura, T.; Tanabe, N.; Sakurai, Y.; Yahaba, M.; Matsuura, Y.; Shigeta, A.; Kawata, N.; Sakao, S.; Kasahara, Y.; et al. Electrocardiogram-Gated 320-Slice Multidetector Computed Tomography for the Measurement of Pulmonary Arterial Distensibility in Chronic Thromboembolic Pulmonary Hypertension. PLoS ONE 2014, 9, e111563. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liang, W.; Chen, D.; Chen, W.; Cheng, G. The ascending aortic elasticity feature in normotensive subjects: Evaluation with coronary CT angiography. Clin. Imaging 2014, 38, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Yuan, M.; Zhang, Q.; Shi, H.; Wang, D. Evaluation of computed tomography obstruction index in guiding therapeutic decisions and monitoring percutanous catheter fragmentation in massive pulmonary embolism. J. Biomed. Res. 2011, 25, 431–437. [Google Scholar] [CrossRef]
- Mori, H.; Torii, S.; Kutyna, M.; Sakamoto, A.; Finn, A.V.; Virmani, R. Coronary Artery Calcification and its Progression. JACC Cardiovasc. Imaging 2018, 11, 127–142. [Google Scholar] [CrossRef]
- Kiryu, S.; Raptopoulos, V.; Baptista, J.; Hatabu, H. Increased prevalence of coronary artery calcification in patients with suspected pulmonary embolism1. Acad. Radiol. 2003, 10, 840–845. [Google Scholar] [CrossRef]
- Williams, M.; Morley, N.; Muir, K.; Reid, J.; van Beek, E.; Murchison, J. Coronary artery calcification is associated with mortality independent of pulmonary embolism severity: A retrospective cohort study. Clin. Radiol. 2019, 74, 973.e7–973.e14. [Google Scholar] [CrossRef]
- Heidinger, B.H.; DaBreo, D.; Kirkbride, R.R.; Santos, M.; Carroll, B.J.; Feldman, S.A.; Mohebali, D.; McCormick, I.; Matos, J.D.; Manning, W.J.; et al. Risk assessment of acute pulmonary embolism utilizing coronary artery calcifications in patients that have undergone CT pulmonary angiography and transthoracic echocardiography. Eur. Radiol. 2021, 31, 2809–2818. [Google Scholar] [CrossRef]
- Ng, A.C.C.; Chung, T.; Yong, A.S.C.; Wong, H.S.P.; Chow, V.; Celermajer, D.S.; Kritharides, L. Long-Term Cardiovascular and Noncardiovascular Mortality of 1023 Patients with Confirmed Acute Pulmonary Embolism. Circ. Cardiovasc. Qual. Outcomes 2011, 4, 122–128. [Google Scholar] [CrossRef]
- Ippolito, D.; Giandola, T.; Maino, C.; Pecorelli, A.; Capodaglio, C.; Ragusi, M.; Porta, M.; Gandola, D.; Masetto, A.; Drago, S.; et al. Acute pulmonary embolism in hospitalized patients with SARS-CoV-2-related pneumonia: Multicentric experience from Italian endemic area. Radiol. Med. 2021, 126, 669–678. [Google Scholar] [CrossRef]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Gandet, F.F.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef]
- Ierardi, A.M.; Gaibazzi, N.; Tuttolomondo, D.; Fusco, S.; La Mura, V.; Peyvandi, F.; Aliberti, S.; Blasi, F.; Cozzi, D.; Carrafiello, G.; et al. Deep vein thrombosis in COVID-19 patients in general wards: Prevalence and association with clinical and laboratory variables. La Radiol. Med. 2021, 126, 722–728. [Google Scholar] [CrossRef]
- Masselli, G.; Almberger, M.; Tortora, A.; Capoccia, L.; Dolciami, M.; D’Aprile, M.R.; Valentini, C.; Avventurieri, G.; Bracci, S.; Ricci, P. Role of CT angiography in detecting acute pulmonary embolism associated with COVID-19 pneumonia. Radiol. Med. 2021, 126, 1553–1560. [Google Scholar] [CrossRef]
- Weidman, E.K.; Plodkowski, A.J.; Halpenny, D.F.; Hayes, S.A.; Perez-Johnston, R.; Zheng, J.; Moskowitz, C.; Ginsberg, M.S. Dual-Energy CT Angiography for Detection of Pulmonary Emboli: Incremental Benefit of Iodine Maps. Radiology 2018, 289, 546–553. [Google Scholar] [CrossRef]
- Abdellatif, W.; Ebada, M.A.; Alkanj, S.; Negida, A.; Murray, N.; Khosa, F.; Nicolaou, S. Diagnostic Accuracy of Dual-Energy CT in Detection of Acute Pulmonary Embolism: A Systematic Review and Meta-Analysis. Can. Assoc. Radiol. J. 2020, 72, 285–292. [Google Scholar] [CrossRef]
- Meinel, F.G.; Graef, A.; Bamberg, F.; Thieme, S.F.; Schwarz, F.; Sommer, W.H.; Neurohr, C.; Kupatt, C.; Reiser, M.F.; Johnson, T.R.C. Effectiveness of Automated Quantification of Pulmonary Perfused Blood Volume Using Dual-Energy CTPA for the Severity Assessment of Acute Pulmonary Embolism. Investig. Radiol. 2013, 48, 563–569. [Google Scholar] [CrossRef]
- Tao, S.M.; Li, X.; Schoepf, U.J.; Nance, J.W.; Jacobs, B.E.; Zhou, C.S.; Gu, H.F.; Lu, M.J.; Lu, G.M.; Zhang, L.J. Comparison of the effect of radiation exposure from dual-energy CT versus single-energy CT on double-strand breaks at CT pulmonary angiography. Eur. J. Radiol. 2018, 101, 92–96. [Google Scholar] [CrossRef]
- Lenga, L.; Trapp, F.; Albrecht, M.H.; Wichmann, J.L.; Johnson, A.A.; Yel, I.; D’Angelo, T.; Booz, C.; Vogl, T.J.; Martin, S.S. Single- and dual-energy CT pulmonary angiography using second- and third-generation dual-source CT systems: Comparison of radiation dose and image quality. Eur. Radiol. 2019, 29, 4603–4612. [Google Scholar] [CrossRef]
- Petritsch, B.; Kosmala, A.; Gassenmaier, T.; Weng, A.; Veldhoen, S.; Kunz, A.; Bley, T.A. Diagnosis of Pulmonary Artery Embolism: Comparison of Single-Source CT and 3rd Generation Dual-Source CT using a Dual-Energy Protocol Regarding Image Quality and Radiation Dose. Rofo 2017, 189, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Chae, E.J.; Seo, J.B.; Jang, Y.M.; Krauss, B.; Lee, C.W.; Lee, H.J.; Song, K.-S. Dual-Energy CT for Assessment of the Severity of Acute Pulmonary Embolism: Pulmonary Perfusion Defect Score Compared With CT Angiographic Obstruction Score and Right Ventricular/Left Ventricular Diameter Ratio. Am. J. Roentgenol. 2010, 194, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Dournes, G.; Verdier, D.; Montaudon, M.; Bullier, E.; Rivière, A.; Dromer, C.; Picard, F.; Billes, M.-A.; Corneloup, O.; Laurent, F.; et al. Dual-energy CT perfusion and angiography in chronic thromboembolic pulmonary hypertension: Diagnostic accuracy and concordance with radionuclide scintigraphy. Eur. Radiol. 2013, 24, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Thieme, S.F.; Becker, C.R.; Hacker, M.; Nikolaou, K.; Reiser, M.F.; Johnson, T.R. Dual energy CT for the assessment of lung perfusion—Correlation to scintigraphy. Eur. J. Radiol. 2008, 68, 369–374. [Google Scholar] [CrossRef]
- Monti, C.B.; Zanardo, M.; Cozzi, A.; Schiaffino, S.; Spagnolo, P.; Secchi, F.; De Cecco, C.N.; Sardanelli, F. Dual-energy CT performance in acute pulmonary embolism: A meta-analysis. Eur. Radiol. 2021, 31, 6248–6258. [Google Scholar] [CrossRef]
- Carrascosa, P.; Capunay, C.; Rodriguez-Granillo, G.A.; Deviggiano, A.; Vallejos, J.; Leipsic, J.A. Substantial iodine volume load reduction in CT angiography with dual-energy imaging: Insights from a pilot randomized study. Int. J. Cardiovasc. Imaging 2014, 30, 1613–1620. [Google Scholar] [CrossRef]
- Johansen, C.; Martinsen, A.; Enden, T.; Svanteson, M. The potential of iodinated contrast reduction in dual-energy CT thoracic angiography; an evaluation of image quality. Radiography 2021, 28, 2–7. [Google Scholar] [CrossRef]
- Tabari, A.; Gee, M.S.; Singh, R.; Lim, R.; Nimkin, K.; Primak, A.; Schmidt, B.; Kalra, M.K. Reducing Radiation Dose and Contrast Medium Volume With Application of Dual-Energy CT in Children and Young Adults. Am. J. Roentgenol. 2020, 214, 1199–1205. [Google Scholar] [CrossRef]
- Nance, J.W.; Henzler, T.; Meyer, M.; Apfaltrer, P.; Braunagel, M.; Krissak, R.; Schoepf, U.J.; Schoenberg, S.O.; Fink, C. Optimization of Contrast Material Delivery for Dual-Energy Computed Tomography Pulmonary Angiography in Patients With Suspected Pulmonary Embolism. Investig. Radiol. 2012, 47, 78–84. [Google Scholar] [CrossRef]
- Kumar, G.; Sakhuja, A.; Taneja, A.; Majumdar, T.; Patel, J.; Whittle, J.; Nanchal, R. Pulmonary Embolism in Patients with CKD and ESRD. Clin. J. Am. Soc. Nephrol. 2012, 7, 1584–1590. [Google Scholar] [CrossRef]
- Li, X.; Ni, Q.Q.; Schoepf, U.J.; Wichmann, J.L.; Felmly, L.M.; Qi, L.; Kong, X.; Zhou, C.S.; Luo, S.; Zhang, L.J.; et al. 70-kVp High-pitch Computed Tomography Pulmonary Angiography with 40 mL Contrast Agent. Acad. Radiol. 2015, 22, 1562–1570. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, Y.E.; Qi, L.; Li, X.; Zhou, C.S.; Zhang, L.J.; Lu, G.M. Prospectively ECG-triggered high-pitch coronary CT angiography at 70 kVp with 30 mL contrast agent: An intraindividual comparison with sequential scanning at 120 kVp with 60 mL contrast agent. Eur. J. Radiol. 2017, 90, 97–105. [Google Scholar] [CrossRef]
- Suntharalingam, S.; Mikat, C.; Stenzel, E.; Erfanian, Y.; Wetter, A.; Schlosser, T.; Forsting, M.; Nassenstein, K. Submillisievert standard-pitch CT pulmonary angiography with ultra-low dose contrast media administration: A comparison to standard CT imaging. PLoS ONE 2017, 12, e0186694. [Google Scholar] [CrossRef]
- Alobeidi, H.; Alshamari, M.; Widell, J.; Eriksson, T.; Lidén, M. Minimizing contrast media dose in CT pulmonary angiography with high-pitch technique. Br. J. Radiol. 2020, 93, 20190995. [Google Scholar] [CrossRef]
- Silva, M.; Milanese, G.; Cobelli, R.; Manna, C.; Rasciti, E.; Poggesi, S.; Sverzellati, N. CT angiography for pulmonary embolism in the emergency department: Investigation of a protocol by 20 ml of high-concentration contrast medium. Radiol. Med. 2019, 125, 137–144. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zantonelli, G.; Cozzi, D.; Bindi, A.; Cavigli, E.; Moroni, C.; Luvarà, S.; Grazzini, G.; Danti, G.; Granata, V.; Miele, V. Acute Pulmonary Embolism: Prognostic Role of Computed Tomography Pulmonary Angiography (CTPA). Tomography 2022, 8, 529-539. https://doi.org/10.3390/tomography8010042
Zantonelli G, Cozzi D, Bindi A, Cavigli E, Moroni C, Luvarà S, Grazzini G, Danti G, Granata V, Miele V. Acute Pulmonary Embolism: Prognostic Role of Computed Tomography Pulmonary Angiography (CTPA). Tomography. 2022; 8(1):529-539. https://doi.org/10.3390/tomography8010042
Chicago/Turabian StyleZantonelli, Giulia, Diletta Cozzi, Alessandra Bindi, Edoardo Cavigli, Chiara Moroni, Silvia Luvarà, Giulia Grazzini, Ginevra Danti, Vincenza Granata, and Vittorio Miele. 2022. "Acute Pulmonary Embolism: Prognostic Role of Computed Tomography Pulmonary Angiography (CTPA)" Tomography 8, no. 1: 529-539. https://doi.org/10.3390/tomography8010042
APA StyleZantonelli, G., Cozzi, D., Bindi, A., Cavigli, E., Moroni, C., Luvarà, S., Grazzini, G., Danti, G., Granata, V., & Miele, V. (2022). Acute Pulmonary Embolism: Prognostic Role of Computed Tomography Pulmonary Angiography (CTPA). Tomography, 8(1), 529-539. https://doi.org/10.3390/tomography8010042





